Abstract
5-Fluorouracil (5-FU)-based chemotherapy is widely used for the treatment of colorectal cancer (CRC). While optimal doses of 5-FU are generally established based on a patient's estimated body surface area, the plasma concentrations of 5-FU vary among patients. In addition, hyperglycemia in patients with CRC has been reported as a risk factor in poor prognosis. The aim of the present study was to investigate whether hyperglycemia affects antiproliferative effect of 5-FU on the human colon cancer cells (SW480, SW620, LoVo, and HCT116). Growth inhibition of 5-FU was accessed by WST-8 assay. The effect of high glucose (HG, 15 mM) and 5-FU on the cellular proliferation was evaluated by flow cytometry analysis using 5-ethynyl-2′-deoxy-uridine (EdU) incorporation plus 7-AAD. Cell death was determined by flow cytometry using Annexin V-FITC and PI. The results showed that HG, compared to physiological normal glucose (NG) concentration (5 mM), leads to increased cell proliferation and increased GI50 of 5-FU in the four colon cancer cell lines. When the cells were pretreated with a low-dose 5-FU in NG condition, subsequent HG treatment eliminated inhibitory effect of 5-FU in cancer cell growth. In the presence of 5-FU (0.5 μg/mL for LoVo and HCT116; 1 μg/mL for SW480 and SW620), culture with HG for 72 h does not significantly altered cell cycle profile in the four cell lines but significantly increased DNA replication in SW620 (21%) and LoVo (17%). Flow cytometric analysis showed that HG protects cells against 5-FU-induced cell death in SW480. Finally, HG did not alter intracellular level of reactive oxygen species (ROS), although 5-FU indeed induced higher intracellular level of ROS. In conclusion, HG attenuates growth inhibition of 5-FU and our results indicate that decreased cell death and increased DNA replication may account for the attenuating effect of a HG environment on 5-FU-induced tumor growth inhibition.
Introduction
Although surgical resection is the cornerstone treatment of early-stage colorectal cancer (CRC), chemotherapy remains the preference for either preventing local recurrence or treating locally advanced/unresectable metastatic cancer. Among patients with metastatic CRC receiving 5-fluorouracil (5-FU) as the first-line chemotherapy, their mean survival prolonged from 10–12 months to more than 20 months when new chemotherapeutic or biological agents, such as irinotecan, oxaliplatin, cetuximab, and bevacizumab, are administrated in combination (Meyerhardt and Mayer, 2005). The goal of such a therapeutic chemotherapy is the disruption of cellular pathways critical for tumor growth, survival, and metastasis.
Clinically, the dosing of 5-FU is given as the backbone of standard protocol and is limited by the safety profile. The most frequent side effects of 5-FU are due to its myelotoxicity and gastrointestinal toxicity. The severity of these depends on dosage, patients' metabolism and performance status, other drugs given as a part of combination therapy, or scheduling and duration of the treatment. While 5-FU dosing is typically based on body surface area of the individual patient, a considerable variability in plasma 5-FU levels may reach as much as 100-fold (Peters et al., 1993). Toxicity and treatment failure therefore become the consequences of such interpatient or intrapatient pharmacokinetic variability. On the other hand, chemoresistance remains a major obstacle for successful 5-FU-based chemotherapy. The underlying mechanisms have been intensively investigated but still many details remain unidentified.
Epidemiological evidence suggests that patients with diabetes mellitus are at a significantly higher risk of developing many types of cancers. Diabetes has also become a risk factor for the incidence and mortality of CRC, although contradictions regarding the prognosis of patients still remain (Flood et al., 2007; Jullumstro et al., 2009; Noh et al., 2010; Stein et al., 2010). In comparison with normal cells, aerobic glycolytic activity is enhanced in cancer cells by increasing the glucose transportation into the cytoplasm and limiting downstream mitochondrial respiration (Warburg, 1956). The major regulatory pathways of glucose metabolism are largely mediated through insulin receptor signaling and the AMP-activated protein kinase signaling pathways (Barnes and Zierath, 2005; Tomas et al., 2012). Activation of Ras, PI3K/Akt, and c-Myc will increase gene expression of glucose transporter 1 (GLUT1) and further facilitate glucose importation (Fritz and Fajas, 2010). In an in vitro study, diabetogenic concentration of glucose may lead to altered expression of genes that promote cell proliferation, migration, and adhesion in various tumor cell lines, including the colon (Masur et al., 2011; Tomas et al., 2012). In CRC, elevated GLUT1 expression (Chung et al., 2009; Saigusa et al., 2012), high insulin, and low IGFBP-1 levels (Wolpin et al., 2009) have been associated with CRC stage or poor prognosis. These findings highlight the importance of well-controlling blood sugar in cancer patients.
It has been proposed that patients whose blood glucose is poorly controlled are at high risk for severe 5-FU cytotoxicity (Sadoff, 1998). A recent study demonstrated that hyperglycemia may alter 5-FU cytotoxicity in MCF-7 breast cancer cells (Pandey et al., 2011). Therefore, it is interesting to know whether high glucose (HG) concentration may influence the efficacy of 5-FU in colon cancer cells. In the present study, we determined the effect of HG on colon cancer cell proliferation and on the cytotoxicity of 5-FU in four human colon cancer cell lines to elucidate the potential effect of HG condition on the 5-FU efficacy.
Materials and Methods
Cell culture
Human colonic carcinoma cell line SW480, SW620, LoVo, and HCT116 were purchased from Bioresource Collection and Research Center (BCRC, Hsinchu City, Taiwan) and were cultured in DMEM (Cat. No: 11885; Gibco-BRL) supplemented with 10% fetal calf serum (Gibco-BRL), 100 units/mL penicillin, and 100 μg streptomycin. The tumorigenic SW480 and highly metastatic SW620 cells originated from the same donor but had different metastatic potential (Duranton et al., 2003). The concentration of glucose in the DMEM was 5 mM (normal glucose [NG]) for general culture condition (control group) or was 15 mM as referring to the group of HG treatment. HG medium was prepared by mixing the LG medium with DMEM containing 4.5 g/L glucose (Cat. No: 11995; Gibco-BRL). The cells were maintained at 37°C in a humidified atmosphere of 5% CO2. 5-FU was purchased from Sigma-Aldrich. The stock solution (5 mg/mL) was prepared in phosphate-buffered saline and then sterilized by passing through a disk filter unit (0.22 μm).
Cell proliferation assay
For the analysis of growth inhibition of the cells by 5-FU, WST-8 Cell Proliferation Assay (Sigma-Aldrich) was performed as per the manufacturer's instructions. WST-8 reagent is more stable, less cytotoxic, and higher sensitive than the other currently available tetrazolium-based salts (Failli et al., 2013). Cells were seeded at a concentration of 2.5×103 cells/100 μL/well into 96-well plates in at least triplicate and were incubated at 37°C for 24 h. Cells were treated with various concentrations of 5-FU (0.1–100 μg/mL) for 3 days. Then, the culture medium were replaced with 100 μL of assay medium containing 1/10 volume of cell proliferation reagent WST-8. After incubation for 2–3 h at 37°C, absorbance at 450 nm was measured using the Benchmark Plus Microplate Spectrophotometer (Bio-Rad). Oversaturated reading of OD450 (>3) was prevented by optimizing plating density of cells as mentioned above. The cell viability was analyzed as a percentage of background-subtracted absorbance measured using the following equation: [(T − T0)/(C − T0)]×100, according to the OD450 measurements at time zero (T0), control growth (C), and treated sample (T). Linear regression analysis of the log concentration versus percentage of control growth was used to calculate the concentration of 5-FU that inhibited the growth rate by 50% (GI50) (Monks et al., 1991). GI50 is calculated from [(T − T0)/(C − T0)]×100=50, which is the 5-FU concentration resulting in a 50% reduction in the net OD450 increase in control cells during the drug incubation.
EdU incorporation assay
Cell proliferation was also carried out by Click-iT® EdU Alexa Fluor 488 Flow Cytometry Assay Kit (Molecular Probes) in combination with DNA dye 7-AAD (BD Pharmingen). Cells were inoculated in six-well plates under standard culture conditions for 24 h. The medium was then replaced with either NG or HG medium, and a dose of 5-FU (1 μg/mL for SW480 and SW620; 0.5 μg/mL for LoVo and HCT116) was added. After 3 days, cells were pulsed for 1 h with 20 μM 5-ethynyl-2′-deoxy-uridine (EdU) in growth media. Subsequently, the cells were harvested by accutase (Lonza) and then fixed, washed, and stained according to the manufacturer's instruction. Cells were analyzed using a FACScan cytofluorimeter (Becton Dickinson) equipped with the CellQuest software (BD Biosciences) and at least 10,000 events were collected. Data analysis was carried out with the FlowJo software. The intensity of EdU Alexa Fluor 488 fluorescence of mid-S phase cells was normalized with that of unlabeled cells (G0/G1 and G2/M).
Apoptosis assay
Cytotoxicity was analyzed by flow cytometry with FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen). Cells were seeded into six-well plates. After a 72-h incubation with or without 5-FU, cells were harvested by accutase (Lonza) and stained with Annexin V-FITC and PI according to the manufacturer's instruction. Cells were analyzed by FACScan cytofluorimeter (Becton Dickinson) in three independent experiments.
Statistical analyses
Data evaluation was performed using Microsoft Excel 2007. All data were statistically analyzed using the JMP software version 9.0.0 (SAS Institute, Inc.). Data are presented as mean±standard deviation. Student's t-test (two-tailed) was applied to analyze the data. p<0.05 was considered as statistically significant.
Results
HG promoted proliferation of colon cancer cells and increased GI50 of 5-FU
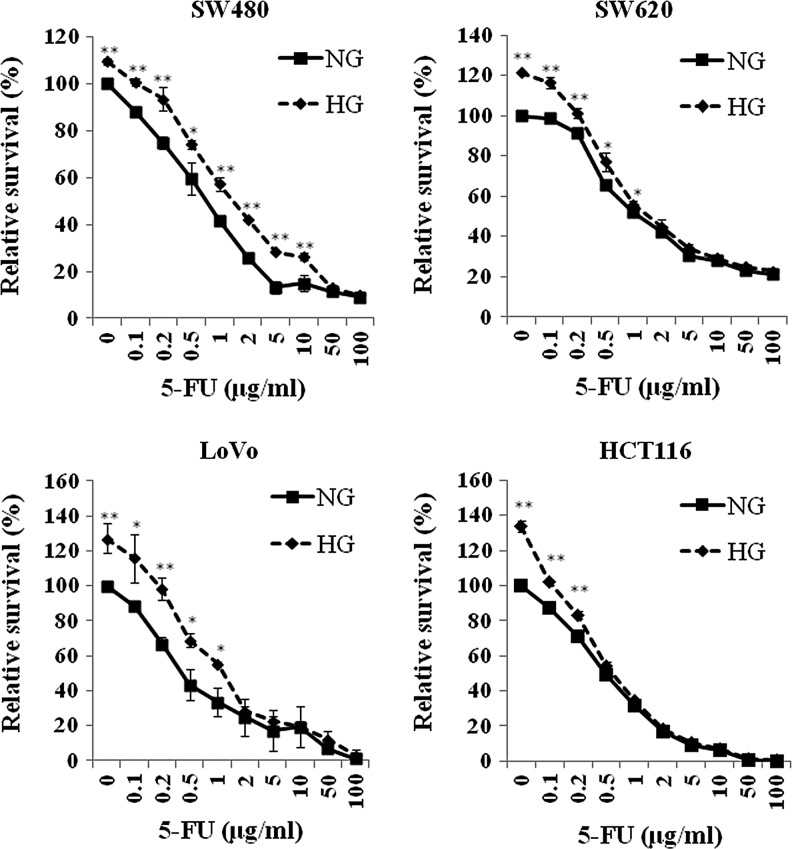
We first tested the proliferation of the four human colon cancer cell lines SW480, SW620, LoVo, and HCT116 in response to a 3-day incubation of HG (15 mM) by using the WST-8 cell proliferation kit. When 104 cells were plated and assayed after 2.5-h incubation with WST-8 reagent, the OD450 (blank subtracted) was 0.376, 0.210, 0.123, and 0.199 for SW480, SW620, LoVo, and HCT116, respectively. Thus, the OD450 of each sample was expressed as relative percentage of that in control samples of corresponding cell line to overcome the differential intrinsic cellular redox property as well as the err raised from cell number counting. As shown in Figure 1, the percentage of proliferation increased without drug treatment in SW480, SW620, LoVo, and HCT116 were 9.3±1.3 (p<0.001), 21.5±0.2 (p<0.001), 27.2±8.6 (p=0.005), and 33.8±3.3 (p<0.001) respectively. Generally switching from 5 mM (normal plasma glucose level) to HG significantly increased cellular proliferation in all cell lines, although a lesser content of increase was observed in SW480. The data also indicated that metastatic colon cancer cells proliferated faster under hyperglycemic conditions (SW480 vs. SW620, p<0.001). We then assayed the growth inhibition of 5-FU to four colon cancer cell lines by WST-8 assay. The cells were plated initially in the NG or HG medium, and the growth inhibition of 5-FU in each cell lines were determined by treating with various concentrations of 5-FU (0–100 μg/mL) to evaluate the impact of HG on GI50 of 5-FU. The relative proliferation of each condition was normalized with the OD450 of the well cultured in the NG medium without 5-FU (Fig. 1). The GI50 of 5-FU (72 h) in each cell line was calculated and is shown in Table 1. In NG condition, lower GI50 values in LoVo and HCT116 showed higher 5-FU sensitivity in relation to that in SW480 and SW620. This increase of GI50 was more significant in SW480 (120%, p=0.0002) and LoVo cells (140%, p=0.0024) than in those of SW620 (27%, p=0.040) and HCT116 (20%, p=0.021). As it was shown in the Figure 1, the two survival curves (NG vs. HG) are getting close at near GI50 region in SW620 and HCT116. Therefore, it is possible that GI50 may not very significantly whatever the growth rate of the cells. In this study, we saw that HG significantly lead to increased GI50 of 5-FU in all four cell lines (Table 1). When GI50 was individually determined in HG condition (Table 1), higher growth rate did increase the measure of control growth (C) in the formulation of GI50 calculation and subsequently lead to decreased GI50. These results show the importance of reporting the data for the comparison of reported GI50 among different study. Nevertheless, the impact of HG on relieving of 5-FU-induced growth inhibition was significant when the dose of 5-FU was in the range of the clinically relevant concentrations (0.13–0.65 μg/mL) (Seifert et al., 1975; Fraile et al., 1980).
FIG. 1.
Proliferation of four colon cancer cells under the treatment of high glucose and 5-FU. Cells were plated in DMEM with normal glucose concentration (NG, 5 mM, —■—) or high glucose concentration (HG, 15 mM,  ) for 24 h. Then, the cells were treated with various concentration of 5-fluorouracil (5-FU) for 3 days. The cellular proliferation was assessed by WST-8 assay as described. Cellular proliferation of each colon cancer cell was significantly enhanced by the treatment of high glucose. The proliferation diminished with the increase of 5-FU dose. High glucose eliminated the growth inhibition of 5-FU when the dose of 5-FU was at least higher than GI50 for each cells. These analyses were mean±standard deviation (SD) of three independent experiments from at least three replications. **p<0.01, *p<0.05.
) for 24 h. Then, the cells were treated with various concentration of 5-fluorouracil (5-FU) for 3 days. The cellular proliferation was assessed by WST-8 assay as described. Cellular proliferation of each colon cancer cell was significantly enhanced by the treatment of high glucose. The proliferation diminished with the increase of 5-FU dose. High glucose eliminated the growth inhibition of 5-FU when the dose of 5-FU was at least higher than GI50 for each cells. These analyses were mean±standard deviation (SD) of three independent experiments from at least three replications. **p<0.01, *p<0.05.
Table 1.
GI50 of 5-FU in NG and HG in the Colon Cancer Cells
| GI50 Cell | ||||
|---|---|---|---|---|
| Cell | SW480 | SW620 | LoVo | HCT116 |
| NG | 0.70±0.09 | 1.40±0.07 | 0.47±0.14 | 0.49±0.04 |
| HGa | 1.54±0.07c | 1.78±0.21c | 1.13±0.09d | 0.59±0.02d |
| HGb | 1.23±0.06c | 0.96±0.11c | 0.65±0.06 | 0.34±0.04d |
Three independent experiments were carried out in at least three replications, and the data were represented as the mean±standard deviation.
Control growth (C) in the formulation of calculating GI50 was derived from OD450 measurement of control samples in NG.
Control growth (C) in the formulation of calculating GI50 was derived from OD450 measurement of control samples in HG.
p<0.01 compared to that in NG.
p<0.05 compared to that in NG.
NG, normal glucose concentration (5 mM); HG, high glucose concentration (15 mM).
HG abolished the growth inhibition of pretreated low-dose 5-FU
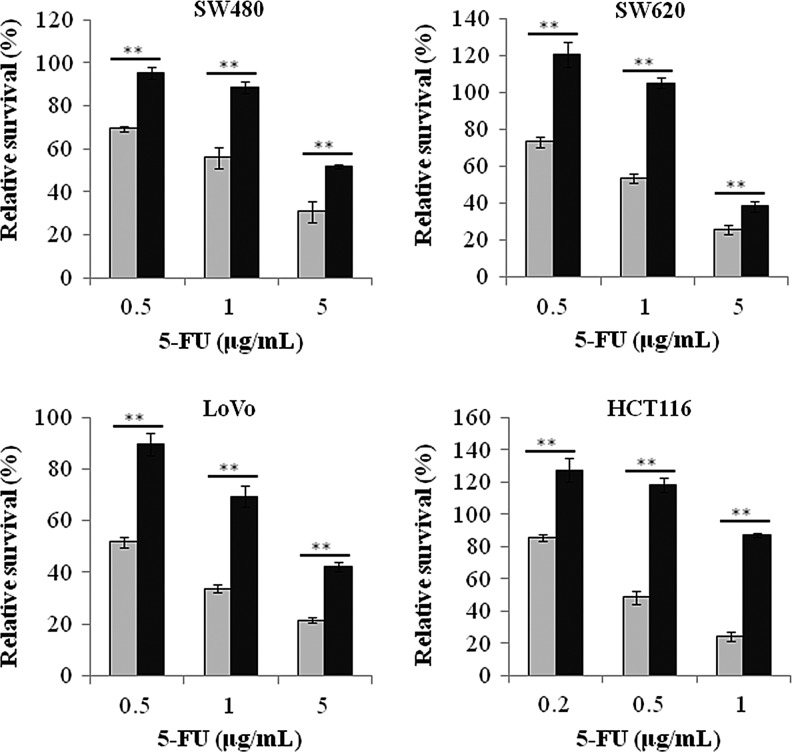
5-FU and HG operate cell proliferation in opposite directions or antagonistically. It is thus interesting to know whether a subsequent HG has an effect on the cytotoxicity of 5-FU when the cells are pretreated with 5-FU. It has been demonstrated that LoVo cells treated with 5 μM 5-FU for 12 h arrested at S phase, and such S-phase arrest persisted for a long time (Xu et al., 2002). Therefore, we pretreated the cells with ∼1×GI25 (72 h) dose of 5-FU in the NG medium for 24 h. Then, the medium were replaced with the HG medium with 5-FU and cultured for 3 days as described in Figure 2. The results indicated that HG significantly quenches growth inhibition of 5-FU (NG vs. HG) in all cell lines at three different doses of 5-FU. The result also indicates that the cytotoxicity of 5-FU may overcome proliferation effect of HG when dosage of 5-FU is over GI75 (72 h).
FIG. 2.
High glucose quenched the growth inhibition of short pretreated 5-FU. Cells were plated with the NG medium after 24 h, a dose of 5-FU lower than GI50 for each cell (0.5 μg/mL for SW480, SW620, and LoVo; 0.2 μg/mL for HCT116) was added and cultured for another 24 h. Then, the medium were replaced with either the NG (5 mM, gray column) or the HG (15 mM, black column) medium with 5-FU of three different concentration and incubated for another 3 days as indicated in horizontal axis. The survival fraction was assessed by WST-8 assay. The data were normalized with the OD450 read of the well in which the cells were pretreated with 5-FU for 24 h in the NG medium but without 5-FU in the NG medium during subsequent 3-day incubation. The data indicated that high glucose may abolish the cytotoxicity of 5-FU when the cells were pretreated with a lower concentration of 5-FU. The data were represented as the mean±SD from three independent experiments in at least four replications. **p<0.01.
HG increased EdU incorporation
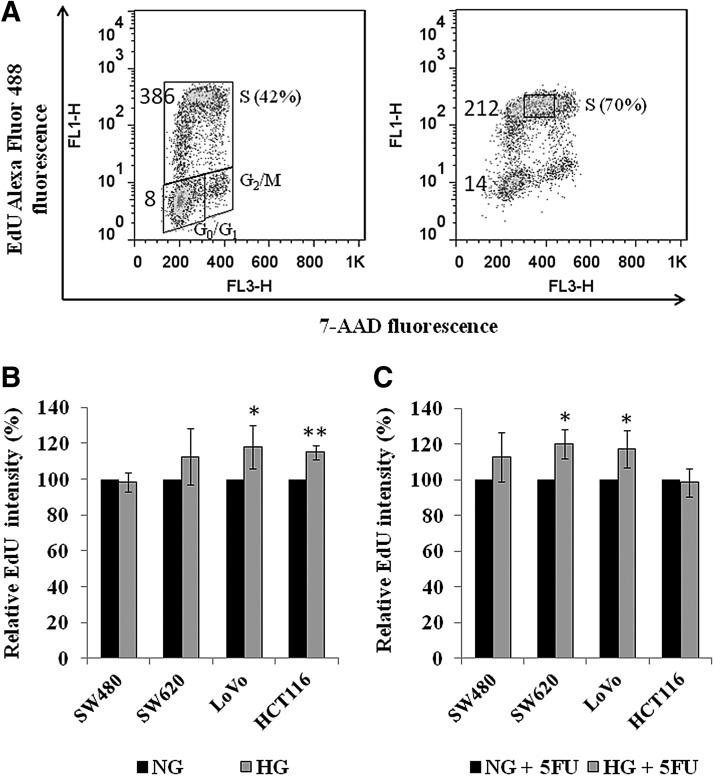
It has been documented that 5-FU usually arrests tumor cells at the G1–S phase of the cell cycle and cause apoptosis (Yoshikawa et al., 2001; Guo et al., 2008). Besides, we have also demonstrated that HG promotes cell proliferation. We next examined the effect of HG on DNA replication and cell cycle perturbation by analyzing the incorporation of EdU into cellular DNA (Buck et al., 2008). A represented plot of SW620 labeled with EdU and 7-AAD counterstain was shown in Figure 3A. The incubation of 5-FU significantly increased the proportion of cells in S-phase of the cell cycle from 48.7%±6.7% to 75.0%±4.4% (p=0.005) in SW620 (Fig. 3A). A comparable trend was also observed in the other three cells (data not shown). However, HG does not alter the proportion of S-phase cells compared to that in NG condition, no matter with or without the treatment of 5-FU (data not shown). Separately, the mean fluorescence intensity of EdU-labeled mid-S phase cells was normalized with that of EdU-unlabeled G0/G1 plus G2/M cells. We found that the normalized EdU intensity in 5-FU-treated SW620 cells was only 32% of that in the control (Fig. 3A). Similar results were obtained from the other three cell lines (data not shown). It is likely that the decrease of incorporated EdU caused by 5-FU represents decreased DNA replication factory (Cseresnyes et al., 2009). When the cells were cultured in HG media, a significant increase of normalized EdU intensity was in LoVo (p=0.045) and HCT116 (p=0.002) but a less significant increase in SW620 (p=0.243) (Fig. 3B). In the presence of 5-FU, however, a significant increase of EdU intensity by HG was found in SW620 (p=0.013) and LoVo (p=0.048) (Fig. 3C).
FIG. 3.
High glucose increased DNA replication. (A) Detection of DNA replication by EdU click chemistry in SW620. Unsynchronized cells either untreated (left) or treated (right) with 1 μg/mL of 5-FU in the NG medium for 3 days. The incorporated EdU was detected with Alexa Fluor 488 tagged azide using Click-it kit (Molecular Probes). Bivariate distributions demonstrated DNA content (7-AAD fluorescence) versus incorporation of EdU. The mean fluorescence intensities of EdU-labeled mid-S phase cells (gating was shown in the right plot) as well as unlabeled cells were also shown. A comparable plot was also obtained from SW480, LoVo, and HCT116. (B) High glucose significantly increased the fluorescence intensity of EdU in LoVo and HCT116. The mean fluorescence intensity of EdU-labeled mid-S phase cells was normalized with that of the EdU-unlabeled cells. (C) In the presence of 5-FU, high glucose significantly increased the fluorescence intensity of EdU in SW620 and LoVo. The data represent the mean±SD of three independent experiments. *p<0.05. **p<0.01. EdU, 5-ethynyl-2′-deoxy-uridine.
HG dissipated 5-FU-induced apoptosis
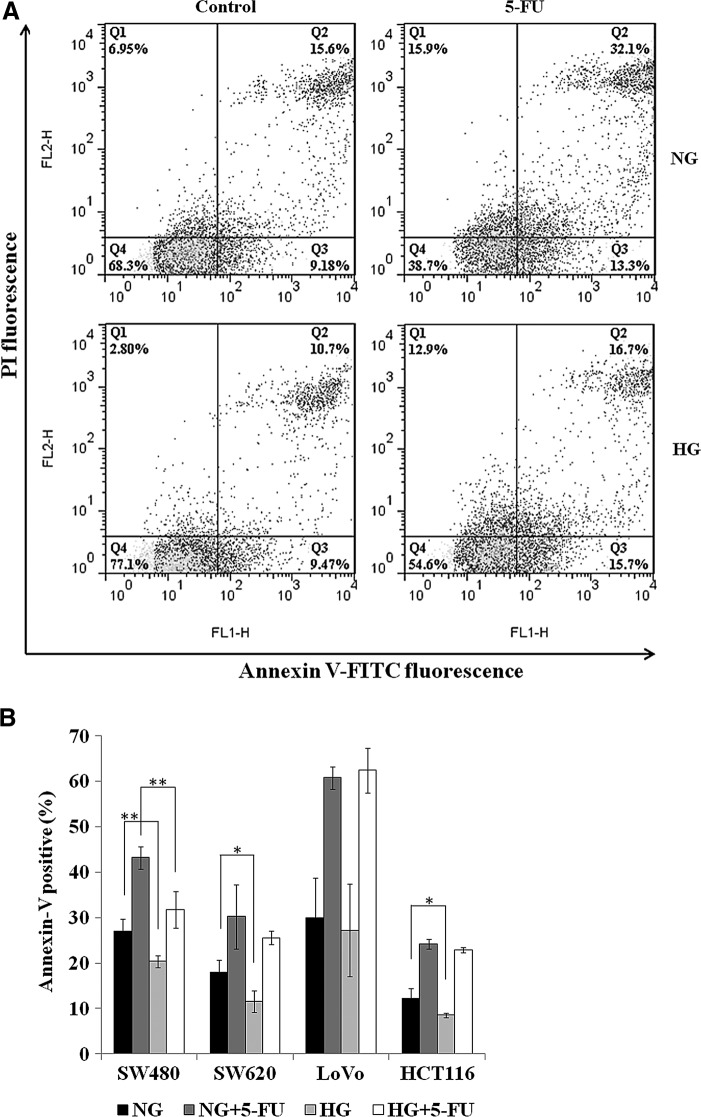
To investigate if HG dissipates 5-FU-induced apoptosis in colon cancer cells, we next performed FACS analysis using Annexin-V-FITC and PI. Annexin-V has a high affinity for phosphatidylserine, which translocates from inner to the outer layer of cell membrane during apoptosis. The use of the cell impermeable probe PI could detect plasma-membrane integrity and distinguish late apoptotic/necrotic cells with leaky plasma membrane. Thus, early apoptotic cells stain with Annexin-V but exclude PI, whereas late apoptotic and necrotic cells are both Annexin-V and PI positive (Wlodkowic et al., 2011). Analysis by flow cytometry showed that incubation for 72 h with 5-FU (0.5 μg/mL for LoVo and HCT116; 1.0 μg/mL for SW480 and SW620) did trigger cell death in the four colon cancer cells in NG condition (Fig. 4A, B). The proportion of Annexin V-positive cells significantly increased from 27% to 43% (p=0.002) in SW480, 18% to 30% (p=0.018) in SW620, 30% to 61% (p=0.004) in LoVo, and 12% to 24% (p=0.001) in HCT116. The data also indicated the protection effect of HG on preventing cell death in SW480 (27% to 20%, p=0.005), SW620 (18% to 12%, p=0.011), and HCT116 (12% to 9%, p=0.049) compared to that in NG condition. Moreover, in the presence of 5-FU, the proportion of apoptotic cells in HG condition compared to that in NG condition was significantly decreased only in SW480 (43% to 32%, p=0.003). However, no significant change of apoptotic population between NG and HG condition was observed in LoVo and HCT116 when 5-FU was presented. To test if reactive oxygen species (ROS) is involved in the modulation of the effect of 5-FU by HG in colon cancer cells, we treated the colon cancer cells with nearly GI50 dose of 5-FU for 3 days and assayed intracellular level of ROS by FACS analysis. The data were presented in the Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/dna). No significant change of intracellular ROS was modulated by HG in the colon cancer cells but only in HCT116 (p=0.030), indicating that the underlying mechanism may not similar with the finding in the breast cancer cells (Pandey et al., 2011). It is of interest to note that the concentration of 5-FU (450 μM) used in the work of Pandey et al. is extremely high for colon cancer cells.
FIG. 4.
Effect of high glucose on 5-FU-induced cell death in the colon cancer cells. Cells were treated with 5-FU (1 μg/mL for SW480 and SW620; 0.5 μg/mL for LoVo and HCT116) for 3 days. Cells were then stained with Annexin V-FITC in combination with PI and analyzed by flow cytometry. (A) Represented bivariate plot of Annexin V-FITC and PI for SW480 were shown. Percentages show Annexin-positive/PI-positive cells (late apoptotic/necrotic, top right quadrant) and Annexin-positive/PI-negative cells (early apoptotic, bottom right quadrant). (B) Annexin V-positive cells were counted for the representation of apoptotic cells. Each bar represents the mean±SD of three independent experiments. *p<0.05; **p<0.01.
Discussion
It has been shown that hyperglycemia increases cellular proliferation in colon cancer cells (Tomas et al., 2012). Nevertheless, little is known about how hyperglycemia plays a role in drug response in colon cancer cells. In this study, we showed that HG increased the proliferation of four human colon cancer cell lines. We presented the data illustrating that the antiproliferative effect of 5-FU to colon cancer cells was diminished by HG treatment. When a lower dose of 5-FU or a clinical recommended dose of 5-FU was administrated, a following HG treatment succeeds 5-FU-elicited growth inhibition of colon cancer cells. From the results, it might be proposed that in CRC patients with hyperglycemia, a higher dosage and duration of 5-FU treatment is required to inhibit the growth of tumor cells adequately.
5-FU is widely used in treating colorectal, breast, gastrointestinal, head and neck, and ovarian cancers. It has been shown that a sustained steady-state level of 5-FU (1–5 μM or 0.13–0.65 μg/mL) in plasma is adequate to achieve therapeutic effects with continuous intravenous infusion at a dosage of 1000–2000 mg/m2/day (Seifert et al., 1975). The result of another study also indicated that a similar plasma concentration of 5-FU (0.160–0.490 μg/mL) was reached when following continuous infusion of 1100 mg/m2/day for 4 days (Fraile et al., 1980). Interestingly, the concentrations of 5-FU in colon tumor tissues were largely variable but higher than those in normal tissues and were inversely associated with the expression of dihydropyrimidine dehydrogenase (DPD), which is the initial key enzyme in 5-FU catabolism (Tanaka-Nozaki et al., 2001). Mutations or polymorphisms of DPD and polymorphisms in the promoter of thymidylate synthase (TS) gene may account for (substantially, but not all) the 5-FU clearance rate and cytotoxicity (Saif et al., 2009). Accordingly, conventional BSA dosing is associated with high pharmacokinetic variability and is an unreliable indicator of optimal drug exposure. The expression of genes involved in 5-FU sensitivity such as DPD and TS is also a concern for such variability (Yamagishi et al., 2005; Kline et al., 2011; Li et al., 2013), indicating that the chemotherapy regimen should be modified. Dosing of 5-FU, which was evaluated by the area under the time/concentration curve of 5-FU, certainly predicts disease-free survival in patients administered adjuvant chemotherapy for CRC (Di Paolo et al., 2008). With the concern to eliminate the effect of pharmacokinetic heterogeneity, pharmacokinetically adjusted dosing has been widely applied clinically by the concept of target concentration intervention for the optimization of therapy (reviewed in Saif et al., 2009). Moreover, for obese patients with cancer, BSA-based chemotherapy dosing is less than that for the weight-based one, and this reduction of dose may document higher motility rate in overweight patients. A recent published guideline of chemotherapy dosing suggests that a weight-based dose for obese patients with cancer is preferred (Griggs et al., 2012). No significant deleterious effect of overdosing is found in obese cancer patients using the dose based on real body weight. Nevertheless, 5-FU-based adjuvant chemotherapy may not increase survival in the tumors with defective DNA mismatch repair and the CpG island methylator phenotype (Sargent et al., 2010; Jover et al., 2011).
5-FU principally works as a TS inhibitor to block the synthesis of the thymidine, and direct incorporation of 5-FU metabolites into DNA and RNA may cause strand breaks and apoptosis (Xu et al., 2002; Longley et al., 2003). A work of Yoshikawa et al. (2001) showed a dose-dependent biphasic pathway of 5-FU in colon cancer cells. A lower dose of 5-FU confers to G2–M phase cell cycle arrest and mitotic catastrophe, whereas a relatively higher dose of 5-FU causes G1–S phase cell cycle arrest and apoptosis. Our data clearly confirmed that 5-FU blocked G1–S cells cycle transition and also accumulated the cells in S-phase with a less degree of DNA replication (Fig. 3A). Prolonged G1 and S phases may also allow cancer cells to repair 5-FU-induced damage. Thus, forcing resistant cancer cells into a cell cycle might be another potential strategy to reverse 5-FU chemoresistance (Guo et al., 2008). We also found that HG not only enhances cellular proliferation but also diminishes 5-FU-induced apoptosis as well as the cell death in regular cell culture condition (Figs. 1 and 4). The decrease in cell death and/or increase of DNA replication, by some extent due to genetic and phenotypic heterogeneity within subpopulation of tumor cells, may account for the attenuating effect of a HG environment on 5-FU-induced tumor growth inhibition. The combination of oncogenic and/or tumor suppressor mutations and the local tumor microenvironment determines the metabolic response of an individual tumor cells. The dynamic environmental conditions, such as nutrient, oxygen, acidity, specific stromal cell components, and extracellular matrix, play important role in cancer development, progress, and drug resistance (Lu et al., 2012). Those intrinsic and extrinsic factors may explain why the response of HG on 5-FU cytotoxicity is different between the colon (this study) and breast cancer cells (Pandey et al., 2011). The underlying molecular mechanism, regarding the role of HG in attenuating or diminishing the cytotoxicity of 5-FU, needs further study for clarification.
Cytotoxicity of 5-FU to colon cancer cells has been widely investigated, but glucose concentration in the culture medium has received less attention and varies from 1.0 g/L (NG DMEM), 2.0 g/L (RPMI) to 4.5 g/L (HG DMEM) (Xu et al., 2002; De Angelis et al., 2006; Zhou et al., 2010). The differences observed for GI50 of 5-FU at the same incubation time in different studies are likely owing to different culture conditions, frozen subclones, and assay protocols. Alternatively, in those studies where only one glucose concentration was applied in culture condition, the control growth for normalization to 100% survival was varied due to the higher cell proliferation in a HG concentration as we have demonstrated. The current data suggest that hyperglycemia dominates cytotoxic effect of 5-FU when the dose of 5-FU is equivalent to clinical relevant plasma concentration of 5-FU. A recent clinical study conducted by Meyerhardt and his colleges demonstrated that foods with high glycemic load are most closely correlated with higher recurrence of colon cancer (Meyerhardt et al., 2012). Besides, hyperglycemia elevates the levels of blood insulin and free IGF1, which can drive antiapoptotic and proliferation signaling pathways for most cancer cells (Pollak, 2008).
In summary, our results indicate that blood glucose control is significantly correlated to optimized dose of 5-FU, and the optimal dosing of 5-FU is relevant for those colon cancer cells under hyperglycemia, especially when the tumors are more aggressive in a HG environment. When 5-FU is combined with other drugs as an adjunct or concurrent therapy, however, the effect of insufficient dose of 5-FU in diabetic patients with CRC on the efficacy of therapy remain to be elucidated. A further larger scale clinical trial is mandatory for the confirmation.
Supplementary Material
Acknowledgments
This work was supported by grants from the Kaohsiung Medical University Hospital (KMUH1M-29) and Kaohsiung Municipal Hsiao-Kang Hospital (KMHK101-025); Biosignature in Colorectal Cancers, Academia Sinica, Taiwan; and an Excellence for Cancer Research Center Grant funded by the Department of Health, Executive Yuan, Taiwan, Republic of China (DOH102-TD-C-111-002).
Disclosure Statement
No competing financial interests exist.
References
- Barnes B.R., and Zierath J.R. (2005). Role of AMP-activated protein kinase in the control of glucose homeostasis. Curr Mol Med 5,341–348 [DOI] [PubMed] [Google Scholar]
- Buck S.B., Bradford J., Gee K.R., Agnew B.J., Clarke S.T., and Salic A. (2008). Detection of S-phase cell cycle progression using 5-ethynyl-2′-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2′-deoxyuridine antibodies. Biotechniques 44,927–929 [DOI] [PubMed] [Google Scholar]
- Chung F.Y., Huang M.Y., Yeh C.S., Chang H.J., Cheng T.L., Yen L.C., Wang J.Y., and Lin S.R. (2009). GLUT1 gene is a potential hypoxic marker in colorectal cancer patients. BMC Cancer 9,241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseresnyes Z., Schwarz U., and Green C.M. (2009). Analysis of replication factories in human cells by super-resolution light microscopy. BMC Cell Biol 10,88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis P.M., Svendsrud D.H., Kravik K.L., and Stokke T. (2006). Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer 5,20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo A., Lencioni M., Amatori F., Di Donato S., Bocci G., Orlandini C., Lastella M., Federici F., Iannopollo M., Falcone A., Ricci S., Del Tacca M., and Danesi R. (2008). 5-fluorouracil pharmacokinetics predicts disease-free survival in patients administered adjuvant chemotherapy for colorectal cancer. Clin Cancer Res 14,2749–2755 [DOI] [PubMed] [Google Scholar]
- Duranton B., Holl V., Schneider Y., Carnesecchi S., Gosse F., Raul F., and Seiler N. (2003). Polyamine metabolism in primary human colon adenocarcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Amino Acids 24,63–72 [DOI] [PubMed] [Google Scholar]
- Failli A., Legitimo A., Orsini G., Castagna M., Spisni R., Miccoli P., and Consolini R. (2013). Antiproliferative effects of 5-fluorouracil and oxaliplatin in colon cancer cell lines: comparison of three different cytotoxicity assays. J Biol Regul Homeost Agents 27,275–284 [PubMed] [Google Scholar]
- Flood A., Mai V., Pfeiffer R., Kahle L., Remaley A.T., Lanza E., and Schatzkin A. (2007). Elevated serum concentrations of insulin and glucose increase risk of recurrent colorectal adenomas. Gastroenterology 133,1423–1429 [DOI] [PubMed] [Google Scholar]
- Fraile R.J., Baker L.H., Buroker T.R., Horwitz J., and Vaitkevicius V.K. (1980). Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res 40,2223–2228 [PubMed] [Google Scholar]
- Fritz V., and Fajas L. (2010). Metabolism and proliferation share common regulatory pathways in cancer cells. Oncogene 29,4369–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs J.J., Mangu P.B., Anderson H., Balaban E.P., Dignam J.J., Hryniuk W.M., Morrison V.A., Pini T.M., Runowicz C.D., Rosner G.L., Shayne M., Sparreboom A., Sucheston L.E., and Lyman G.H. (2012). Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 30,1553–1561 [DOI] [PubMed] [Google Scholar]
- Guo X., Goessl E., Jin G., Collie-Duguid E.S., Cassidy J., Wang W., and O'Brien V. (2008). Cell cycle perturbation and acquired 5-fluorouracil chemoresistance. Anticancer Res 28,9–14 [PubMed] [Google Scholar]
- Jover R., Nguyen T.P., Perez-Carbonell L., Zapater P., Paya A., Alenda C., Rojas E., Cubiella J., Balaguer F., Morillas J.D., Clofent J., Bujanda L., Rene J.M., Bessa X., Xicola R.M., Nicolas-Perez D., Castells A., Andreu M., Llor X., Boland C.R., and Goel A. (2011). 5-Fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology 140,1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullumstro E., Kollind M., Lydersen S., and Edna T.H. (2009). Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol 48,361–367 [DOI] [PubMed] [Google Scholar]
- Kline C.L., Sheikh H.S., Scicchitano A., Gingrich R., Beachler C., Finnberg N.K., Liao J., Sivik J., and El-Deiry W.S. (2011). Preliminary observations indicate variable patterns of plasma 5-fluorouracil (5-FU) levels during dose optimization of infusional 5-FU in colorectal cancer patients. Cancer Biol Ther 12,557–568 [DOI] [PubMed] [Google Scholar]
- Li L.H., Dong H., Zhao F., Tang J., Chen X., Ding J., Men H.T., Luo W.X., Du Y., Ge J., Tan B.X., Cao D., and Liu J.Y. (2013). The upregulation of dihydropyrimidine dehydrogenase in liver is involved in acquired resistance to 5-fluorouracil. Eur J Cancer 49,1752–1760 [DOI] [PubMed] [Google Scholar]
- Longley D.B., Harkin D.P., and Johnston P.G. (2003). 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3,330–338 [DOI] [PubMed] [Google Scholar]
- Lu P., Weaver V.M., and Werb Z. (2012). The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196,395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur K., Vetter C., Hinz A., Tomas N., Henrich H., Niggemann B., and Zanker K.S. (2011). Diabetogenic glucose and insulin concentrations modulate transcriptome and protein levels involved in tumour cell migration, adhesion and proliferation. Br J Cancer 104,345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt J.A., and Mayer R.J. (2005). Systemic therapy for colorectal cancer. N Engl J Med 352,476–487 [DOI] [PubMed] [Google Scholar]
- Meyerhardt J.A., Sato K., Niedzwiecki D., Ye C., Saltz L.B., Mayer R.J., Mowat R.B., Whittom R., Hantel A., Benson A., Wigler D.S., Venook A., and Fuchs C.S. (2012). Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst 104,1702–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Hose C., Langley J., Cronise P., Vaigro-Wolff A., et al. (1991). Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83,757–766 [DOI] [PubMed] [Google Scholar]
- Noh G.Y., Hwang D.Y., Choi Y.H., and Lee Y.Y. (2010). Effect of diabetes mellitus on outcomes of colorectal cancer. J Korean Soc Coloproctol 26,424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey V., Chaube B., and Bhat M.K. (2011). Hyperglycemia regulates MDR-1, drug accumulation and ROS levels causing increased toxicity of carboplatin and 5-fluorouracil in MCF-7 cells. J Cell Biochem 112,2942–2952 [DOI] [PubMed] [Google Scholar]
- Peters G.J., Lankelma J., Kok R.M., Noordhuis P., van Groeningen C.J., van der Wilt C.L., Meyer S., and Pinedo H.M. (1993). Prolonged retention of high concentrations of 5-fluorouracil in human and murine tumors as compared with plasma. Cancer Chemother Pharmacol 31,269–276 [DOI] [PubMed] [Google Scholar]
- Pollak M. (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8,915–928 [DOI] [PubMed] [Google Scholar]
- Sadoff L. (1998). Overwhelming 5-fluorouracil toxicity in patients whose diabetes is poorly controlled. Am J Clin Oncol 21,605–607 [DOI] [PubMed] [Google Scholar]
- Saif M.W., Choma A., Salamone S.J., and Chu E. (2009). Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst 101,1543–1552 [DOI] [PubMed] [Google Scholar]
- Saigusa S., Toiyama Y., Tanaka K., Okugawa Y., Fujikawa H., Matsushita K., Uchida K., Inoue Y., and Kusunoki M. (2012). Prognostic significance of glucose transporter-1 (GLUT1) gene expression in rectal cancer after preoperative chemoradiotherapy. Surg Today 42,460–469 [DOI] [PubMed] [Google Scholar]
- Sargent D.J., Marsoni S., Monges G., Thibodeau S.N., Labianca R., Hamilton S.R., French A.J., Kabat B., Foster N.R., Torri V., Ribic C., Grothey A., Moore M., Zaniboni A., Seitz J.F., Sinicrope F., and Gallinger S. (2010). Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 28,3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert P., Baker L.H., Reed M.L., and Vaitkevicius V.K. (1975). Comparison of continuously infused 5-fluorouracil with bolus injection in treatment of patients with colorectal adenocarcinoma. Cancer 36,123–128 [DOI] [PubMed] [Google Scholar]
- Stein K.B., Snyder C.F., Barone B.B., Yeh H.C., Peairs K.S., Derr R.L., Wolff A.C., and Brancati F.L. (2010). Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Dig Dis Sci 55,1839–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Nozaki M., Onda M., Tanaka N., and Kato S. (2001). Variations in 5-fluorouracil concentrations of colorectal tissues as compared with dihydropyrimidine dehydrogenase (DPD) enzyme activities and DPD messenger RNA levels. Clin Cancer Res 7,2783–2787 [PubMed] [Google Scholar]
- Tomas N.M., Masur K., Piecha J.C., Niggemann B., and Zanker K.S. (2012). Akt and phospholipase Cγ are involved in the regulation of growth and migration of MDA-MB-468 breast cancer and SW480 colon cancer cells when cultured with diabetogenic levels of glucose and insulin. BMC Res Notes 5, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. (1956). On the origin of cancer cells. Science 123,309–314 [DOI] [PubMed] [Google Scholar]
- Wlodkowic D., Telford W., Skommer J., and Darzynkiewicz Z. (2011). Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol 103,55–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpin B.M., Meyerhardt J.A., Chan A.T., Ng K., Chan J.A., Wu K., Pollak M.N., Giovannucci E.L., and Fuchs C.S. (2009). Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol 27,176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.M., Azzariti A., Tommasi S., Lacalamita R., Colucci G., Johnston P.G., Church S.W., and Paradiso A. (2002). Combination of 5-fluorouracil and irinotecan on modulation of thymidylate synthase and topoisomerase I expression and cell cycle regulation in human colon cancer LoVo cells: clinical relevance. Clin Colorectal Cancer 2,182–188 [DOI] [PubMed] [Google Scholar]
- Yamagishi S., Shimada H., Ishikawa T., Fujii S., Tanaka K., Masui H., Yamaguchi S., Ichikawa Y., Togo S., and Ike H. (2005). Expression of dihydropyrimidine dehydrogenase, thymidylate synthase, p53 and p21 in metastatic liver tumor from colorectal cancer after 5-fluorouracil-based chemotherapy. Anticancer Res 25,1237–1242 [PubMed] [Google Scholar]
- Yoshikawa R., Kusunoki M., Yanagi H., Noda M., Furuyama J.I., Yamamura T., and Hashimoto-Tamaoki T. (2001). Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res 61,1029–1037 [PubMed] [Google Scholar]
- Zhou J., Zhou Y., Yin B., Hao W., Zhao L., Ju W., and Bai C. (2010). 5-Fluorouracil and oxaliplatin modify the expression profiles of microRNAs in human colon cancer cells in vitro. Oncol Rep 23,121–128 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.