Abstract
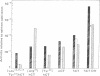
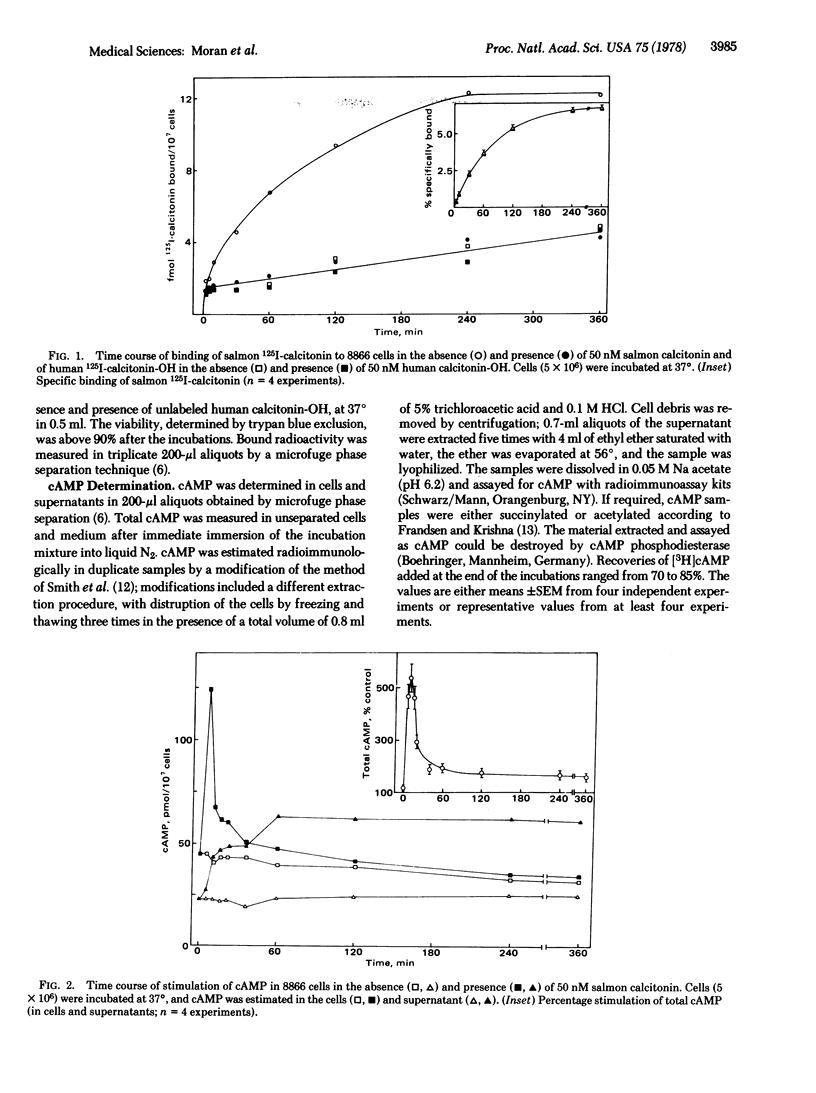
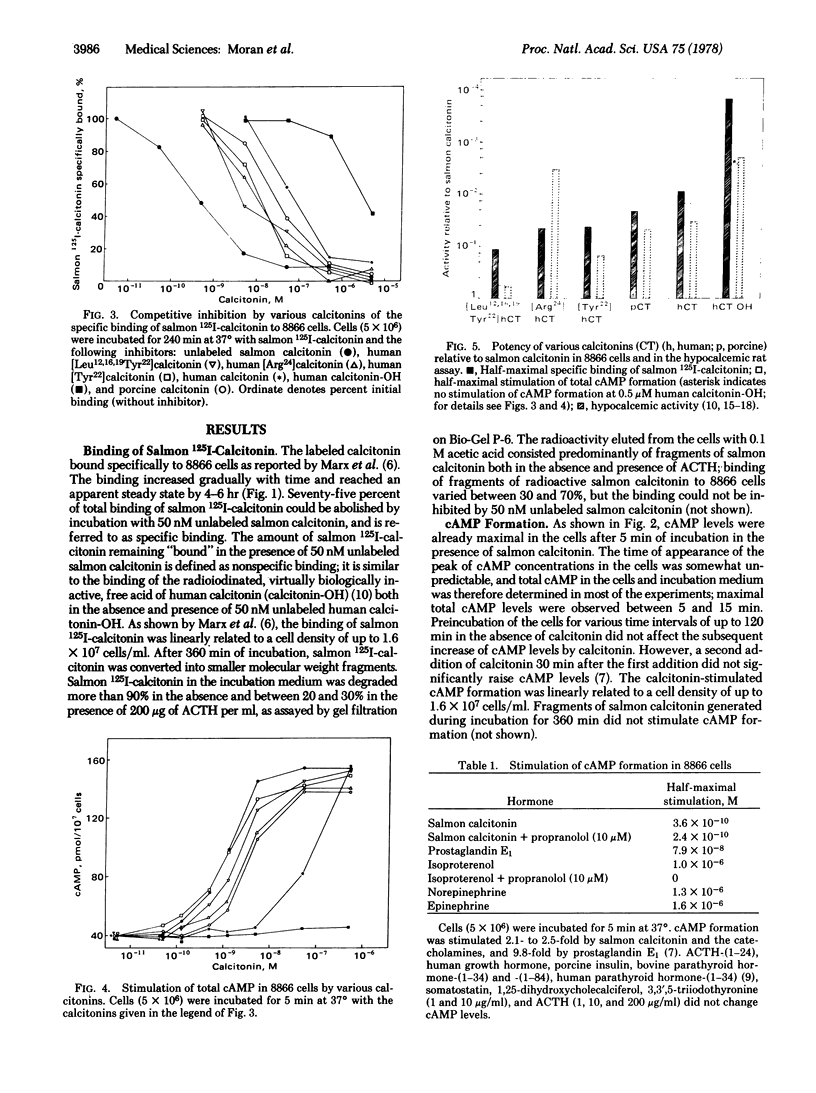
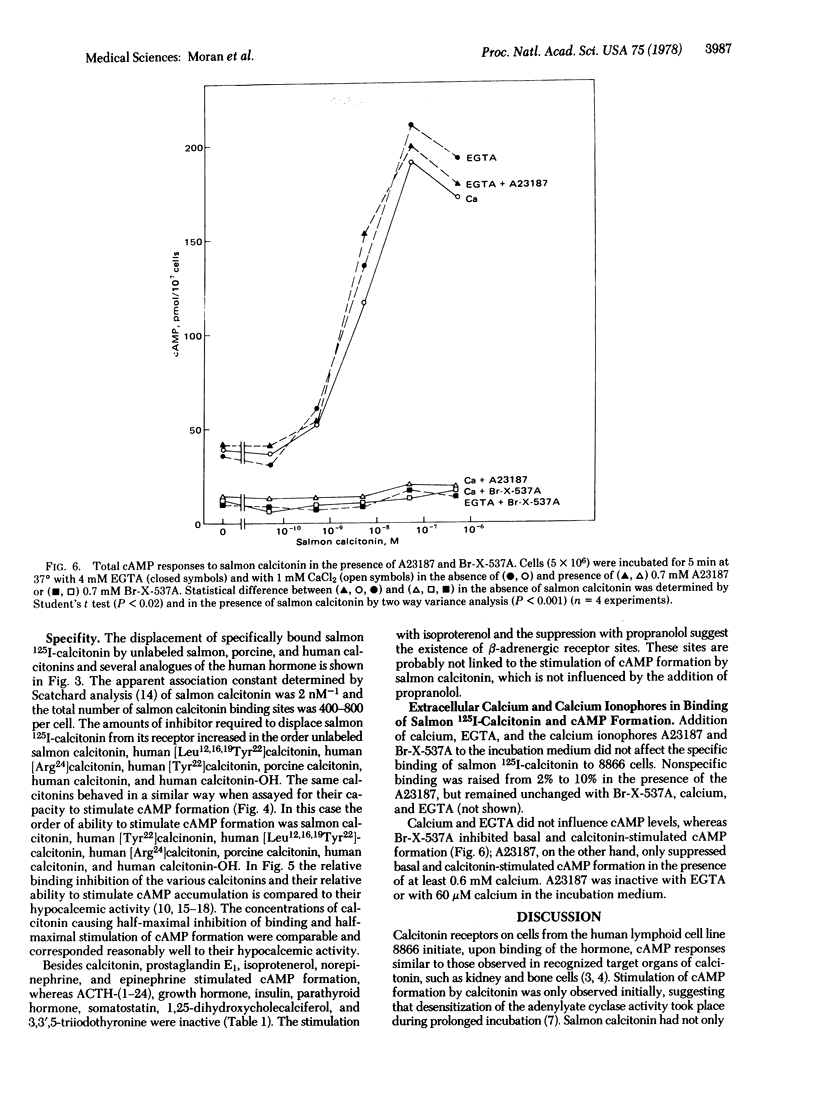
Receptors for calcitonin, as assayed by the specific binding of 125I-labeled salmon calcitonin and stimulation of cyclic AMP formation, were found in 8866 cells derived from a human lymphoid line. The affinity of calcitonin from different species and of various analogues of human calcitonin for the binding sites and their ability to stimulate cyclic AMP formation were closely related to their hypocalcemic activity and presumably reflected biological properties of the hormones. Besides calcitonin, prostaglandin E1 and beta-adrenergic catecholamines stimulated cyclic AMP formation in these cells. The calcium ionophores, A23187 and Br-X-573A, did not influence the specific binding of 125I-labeled salmon calcitonin. A23187, however, suppressed basal and calcitonin-stimulated formation of cyclic AMP in the presence of at least 0.6 mM calcium in the incubation medium. Br-X-537A did not require extracellular calcium to suppress basal and calcitonin-stimulated formation of cyclic AMP, suggesting that the release of calcium from internal stores may regulate adenylyate cyclase activity in 8866 cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardaillou R. Kidney and calcitonin. Nephron. 1975;15(3-5):250–260. doi: 10.1159/000180515. [DOI] [PubMed] [Google Scholar]
- Babcock D. F., First N. L., Lardy H. A. Action of ionophore A23187 at the cellular level. Separation of effects at the plasma and mitochondrial membranes. J Biol Chem. 1976 Jul 10;251(13):3881–3886. [PubMed] [Google Scholar]
- Borle A. B. Regulation of cellular calcium metabolism and calcium transport by calcitonin. J Membr Biol. 1975 Apr 23;21(1-2):125–146. doi: 10.1007/BF01941066. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Studer R. Effects of calcium ionophores on the transport and distribution of calcium in isolated cells and in liver and kidney slices. J Membr Biol. 1978 Jan 12;38(1-2):51–72. doi: 10.1007/BF01875162. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Fairwell T., Ronan R., Sizemore G. W., Arnaud C. D. Human parathyroid hormone: amino-acid sequence of the amino-terminal residues 1-34. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3585–3588. doi: 10.1073/pnas.69.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich F. M., Hunziker W. H., Fischer J. A. Synthetic human calcitonin: analysis of antibodies obtained from various animal species and determination of immunoreactive hormone in human sera. Acta Endocrinol (Copenh) 1975 Nov;80(3):465–486. doi: 10.1530/acta.0.0800465. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci. 1976 Mar 1;18(5):529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- Hait W. N., Weiss B. Characteristics of the cyclic nucleotide phosphodiesterases of normal and leukemic lymphocytes. Biochim Biophys Acta. 1977 Mar 29;497(1):86–100. doi: 10.1016/0304-4165(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Hunt N. H., Ellison M., Underwood J. C., Martin T. J. Calcitonin-responsive adenylate cyclase in a calcitonin-producing human cancer cell line. Br J Cancer. 1977 Jun;35(6):777–784. doi: 10.1038/bjc.1977.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P., Rasmussen H. The effect of A23187 upon calcium metabolism in the human lymphocyte. Biochim Biophys Acta. 1977 Jul 4;468(1):146–156. doi: 10.1016/0005-2736(77)90157-2. [DOI] [PubMed] [Google Scholar]
- Loreau N., Lepreux C., Ardaillou R. Calcitonin-sensitive adenylate cyclase in rat renal tubular membranes. Biochem J. 1975 Sep;150(3):305–314. doi: 10.1042/bj1500305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R., Brugger M., Brückner H., Kamber B., Riniker B., Rittel W. Analogues of human calcitonin. V. Influence of basic amino acids in positions 11, 17 and 24 on hypocalcaemic activity in the rat. Acta Endocrinol (Copenh) 1977 May;85(1):102–108. [PubMed] [Google Scholar]
- Maier R., Kamber B., Riniker B., Rittel W. Analogues of human calcitonin. II. Influence of modifications in amino acid positions 1, 8 and 22 on hypocalcemic activity in the rat. Horm Metab Res. 1975 Nov;7(6):511–514. doi: 10.1055/s-0028-1093715. [DOI] [PubMed] [Google Scholar]
- Maier R., Kamber B., Riniker B., Rittel W. Analogues of human calcitonin. IV. Influence of leucine substitutions in positions 12, 16 and 19 on hypocalcaemic activity in the rat. Clin Endocrinol (Oxf) 1976;5 (Suppl):327S–332S. doi: 10.1111/j.1365-2265.1976.tb03841.x. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Aurbach G. D., Gavin J. R., 3rd, Buell D. W. Calcitonin receptors on cultured human lymphocytes. J Biol Chem. 1974 Nov 10;249(21):6812–6816. [PubMed] [Google Scholar]
- Marx S. J., Woodward C. J., Aurbach G. D. Calcitonin receptors of kidney and bone. Science. 1972 Dec 1;178(4064):999–1001. doi: 10.1126/science.178.4064.999. [DOI] [PubMed] [Google Scholar]
- Murad F., Brewer H. B., Jr, Vaughan M. Effect of thyrocalcitonin on adenosine 3':5'-cyclic phosphate formation by rat kidney and bone. Proc Natl Acad Sci U S A. 1970 Feb;65(2):446–453. doi: 10.1073/pnas.65.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J., Dyball R. E. New calcium-mobilising agent. Nature. 1975 May 29;255(5507):414–415. doi: 10.1038/255414a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Lardy H. A. Ionophore A23187: the effect of H+ concentration on complex formation with divalent and monovalent cations and the demonstration of K+ transport in mitochondria mediated by A23187. Biochemistry. 1976 Mar 9;15(5):935–943. doi: 10.1021/bi00650a001. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Rittel W., Maier R., Brugger M., Kamber B., Riniker B., Sieber P. Struktur-Wirkungsbeziehungen beim menschlichen Calcitonin. III. Die biologische Aktivität verkürzter oder an den Kettenenden modifizierter, synthetischer Analoger. Experientia. 1976 Feb 15;32(2):246–248. doi: 10.1007/BF01937791. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Johnston C. C., Jr Hormonal responsiveness of adenylate cyclase activity from separate bone cells. Endocrinology. 1974 Jul;95(1):130–139. doi: 10.1210/endo-95-1-130. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer M. L., Levitzki A. The interaction of catecholamines, Ca2+ and adenylate cyclase in the intact turkey erythrocyte. Arch Biochem Biophys. 1975 Mar;167(1):371–376. doi: 10.1016/0003-9861(75)90473-7. [DOI] [PubMed] [Google Scholar]
- Stolc V. Mechanism of regulation of adenylate cyclase activity in human polymorphonuclear leukocytes by calcium, guanosyl nucleotides, and positive effectors. J Biol Chem. 1977 Mar 25;252(6):1901–1907. [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Gillan D. J. Inhibition by thyrocalcitonin (calcitonin) of the cyclic AMP-mediated stimulation of thymocyte proliferation by epinephrine. Horm Metab Res. 1971 Sep;3(5):348–351. doi: 10.1055/s-0028-1094121. [DOI] [PubMed] [Google Scholar]