Abstract
The cucumber mosaic virus (CMV) 2b viral suppressor of RNA silencing (VSR) inhibits host responses to jasmonic acid (JA), a chemical signal regulating resistance to insects. Previous experiments with a CMV subgroup IA strain and its 2b gene deletion mutant suggested that VSRs might neutralize aphid (Myzus persicae) resistance by inhibiting JA-regulated gene expression. To further investigate this, we examined JA-regulated gene expression and aphid performance in Nicotiana benthamiana infected with Potato virus X, Potato virus Y, Tobacco mosaic virus and a subgroup II CMV strain, as well as in transgenic plants expressing corresponding VSRs (p25, HC-Pro, 126 kDa and 2b). All the viruses or their VSRs inhibited JA-induced gene expression. However, this did not always correlate with enhanced aphid performance. Thus, VSRs are not the sole viral determinants of virus-induced changes in host–aphid interactions and interference with JA-regulated gene expression cannot completely explain enhanced aphid performance on virus-infected plants.
Most plant viruses encode at least one viral suppressor of RNA silencing (VSR) (Voinnet et al. 1999). Among the first VSRs discovered was the 2b VSR encoded by cucumber mosaic virus (CMV) (Brigneti et al., 1998). The 2b VSR inhibits silencing predominantly through sequestration of small dsRNAs (Goto et al., 2007; Chen et al., 2008; González et al., 2010, 2012). Additionally, the 2b VSR can directly interact with and inhibit the ARGONAUTE (AGO) proteins AGO1 and AGO4 (Zhang et al., 2006; González et al., 2010; Harvey et al., 2011). In Arabidopsis thaliana, the 2b VSR encoded by a subgroup IA CMV strain (Fny-CMV) interacted strongly with AGO1 and interfered with microRNA-regulated gene expression and plant development, whereas 2b proteins of subgroup II strains (LS-CMV and Q-CMV) did not interact as strongly with AGO1 or induce such strong developmental defects in the host (Zhang et al., 2006; Lewsey et al., 2007). However, these differences must depend to a certain extent upon the host plant, as it was found that in tomato (Solanum lycopersicum) the LS-CMV 2b VSR inhibited microRNA-mediated regulation of gene expression (Cillo et al., 2009).
The 2b VSR also interferes with signalling mediated by salicylic acid (Ji & Ding, 2001), abscisic acid (Westwood et al., 2013a) and jasmonic acid (JA) (Lewsey et al., 2010). Remarkably, transgenic expression of the 2b VSR in A. thaliana inhibited the normal responses of over 90 % of JA-regulated plant genes to methyljasmonic acid (MeJA) treatment (Lewsey et al., 2010). Among other things, JA orchestrates responses to herbivores including aphids (Ellis et al., 2002; Wasternack, 2007; Rohwer & Erwin, 2008; Bari & Jones, 2009). It was therefore suggested that the 2b protein inhibits resistance to these insects (Lewsey et al., 2010), which are vectors for CMV and many other plant viruses (Palukaitis & García-Arenal, 2003; Westwood & Stevens, 2010). Subsequently, it was shown that in tobacco (Nicotiana tabacum) plants, the 2b VSR inhibits induction of resistance to the aphid Myzus persicae by other viral gene product(s) during infection by Fny-CMV (Ziebell et al., 2011). In contrast, it was reported that VSRs encoded by certain other aphid-transmitted viruses have the ability to enhance JA-mediated signalling: HC-Pro derived from the potyvirus tabacco etch virus and the P6 protein from cauliflower mosaic virus enhanced responses to JA when expressed in transgenic A. thaliana (Endres et al., 2010; Love et al., 2012). However, for the whitefly-transmitted begomovirus tomato yellow leaf curl China virus, the inhibitory effect on JA-regulated gene expression of the βC1 pathogenicity factor encoded by its satellite DNA appears to explain increased whitefly performance on infected plants (Zhang et al., 2012). JA-mediated signalling can also be disrupted by geminiviruses via disruption of jasmonate perception, as in the case of the C2 transactivator protein of tomato yellow leaf curl Sardinia virus (Lozano-Durán et al., 2011).
The molecular mechanisms governing vector–plant interactions in virus-infected hosts are poorly understood, and it is likely that there are important host-specific effects at work. Thus, by contrast with its effects in tobacco (Ziebell et al., 2011), Fny-CMV induced a mild resistance to feeding by M. persicae in squash (Cucurbita pepo) plants (Mauck et al., 2010). As CMV is transmitted in a non-persistent manner, in which virus acquisition is favoured by short feeding periods and not by prolonged ingestion, this may aid transmission between squash plants (Mauck et al., 2010). Fny-CMV also induced in squash the increased production of aphid-attracting volatiles (Mauck et al., 2010), as did infection of potato (Solanum tuberosum) by potato leafroll virus, which is persistently transmitted by aphids (Eigenbrode et al., 2002).
It was not entirely clear from these previous studies what roles specific viral gene products play in shaping host–aphid interactions. For example, is it always VSRs that shape changes in host interactions with insects? It was also not clear if only insect-transmitted viruses alter JA-responsive gene expression. Therefore, we compared the effects on JA-responsive gene expression and aphid performance of virus infection and transgenic VSR expression for aphid-transmitted viruses [CMV and the potyvirus potato virus Y (PVY)] and for two viruses that are mechanically transmitted and are not known to have any insect vectors [the tobamovirus tobacco mosaic virus (TMV) and the potexvirus potato virus X (PVX)]. An additional reason for choosing PVY was that previous work had indicated that this virus perturbs JA-mediated signalling (Kovač et al., 2009).
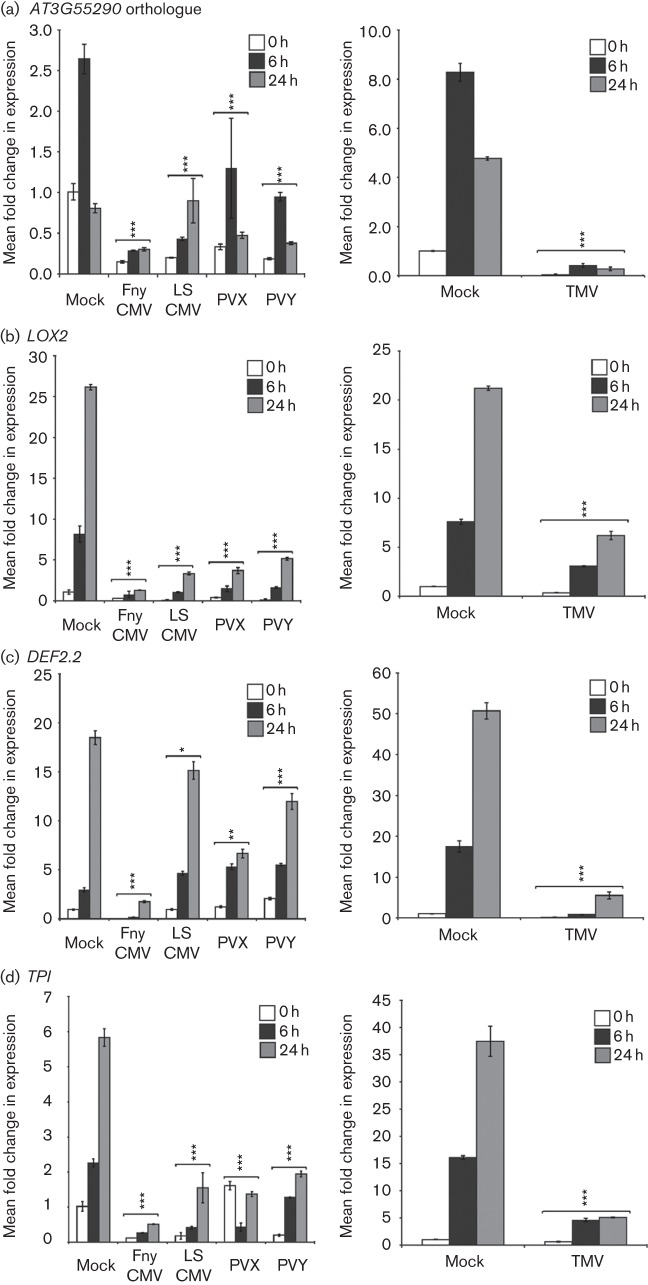
N. benthamiana plants infected with LS-CMV (a subgroup II CMV strain), Fny-CMV, PVY, PVX and TMV were sprayed with 250 µM MeJA and tissue was harvested at 0, 6 and 24 h post-treatment (Fig. 1). MeJA-responsive transcripts were identified from a previously published microarray study, which assessed responses to MeJA in A. thaliana (Lewsey et al., 2010). The TIGR plant transcript assemblies database (http://plantta.jcvi.org/) was used to identify N. benthamiana homologues for selected genes and these were confirmed to be JA-responsive (Fig. 1). Lipoxygenase 2 (LOX2) is an enzyme involved in an early step of JA biosynthesis and its own gene is regulated by JA as part of a positive feedback loop that perpetuates the defence signal (Wasternack, 2007). The transcripts DEFENSIN 2.2 (DEF2.2) and TRYPSIN PROTEIN INHIBITOR (TPI) are key downstream outputs of the JA pathways, encoding proteins that function in anti-insect defence. Also monitored was an N. benthamiana orthologue of the JA-inducible A. thaliana transcript AT3G55290, which encodes a putative protein of no known function in the NAD(P)-binding Rossmann-fold superfamily (Lewsey et al., 2010). PCR primer information is provided in Table S1 (available in the online Supplementary Material). Two housekeeping genes, elongation factor 1α (EF1α) and glyceraldehyde-3-phosphate dehydrogenase, were identified during preliminary work as unresponsive to MeJA treatment and suitable for use as internal PCR standards. Reactions were routinely normalized to expression of EF1α (Table S2 and Spreadsheet S1).
Fig. 1.
Infection by a range of viruses inhibited JA-mediated gene expression. N. benthamiana plants systemically infected with either Fny-CMV, LS-CMV, PVX, PVY or TMV were treated with MeJA at 2 weeks post-inoculation. Aerial tissue from three plants per treatment group was harvested immediately prior to treatment (0 h) and at 6 and 24 h following treatment. RNA was extracted from pooled tissue samples and reverse transcription coupled to quantitative PCR was performed to measure the transcript abundance of the JA-regulated transcripts AT3G55290 (a), lipoxygenase 2 (LOX2) (b), DEFENSIN 2.2 (DEF2.2) (c) and TRYPSIN PROTEINASE INHIBITOR (TPI) (d). Transcript accumulation levels were compared with those in mock-inoculated (Mock) plants. Significant suppression of MeJA-induced gene expression changes at one or both post-treatment time points are indicated (t-test: *P<0.05, **P<0.01, ***P<0.001). Histogram bars represent RNA samples from three plants (technical replicates). Error bars represent sem.
We used reverse transcription coupled to quantitative PCR to assess relative transcript abundance in infected plants following MeJA treatment. These and all other experiments in this study were performed at least three times independently. In all cases, virus infection inhibited induction of all JA-regulated genes tested. In most cases, the basal accumulation of JA-regulated transcripts was depressed in virus-infected plants (Fig. 1). There appeared to be no relationship between inhibition of JA-regulated gene expression by a virus and its transmissibility by aphids. For example, MeJA-induced expression of the N. benthamiana AT3G55290 orthologue, LOX2, DEF2.2 and TPI was inhibited by infection with TMV and PVX, neither of which is transmitted by aphids, as well as by Fny-CMV, LS-CMV and PVY, which are aphid-transmitted viruses (Fig. 1).
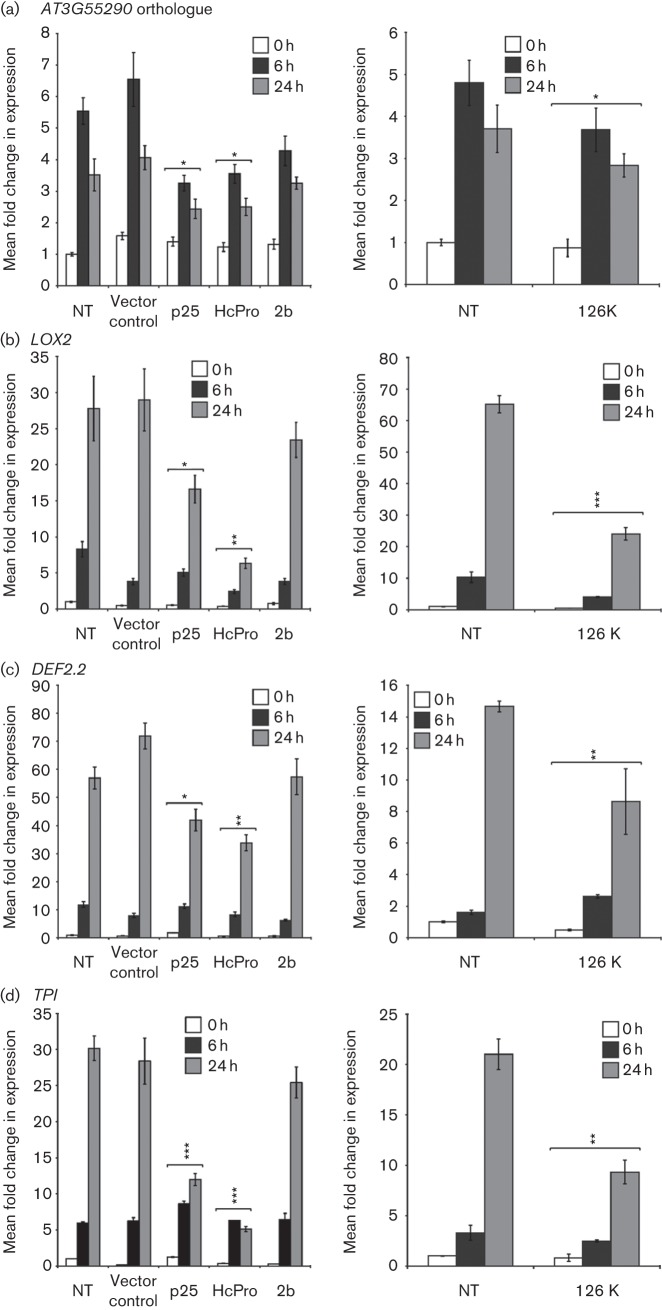
MeJA was applied to transgenic plants expressing VSRs in three plant lines described by Siddiqui et al. (2008): p25 (from PVX) (Chiu et al., 2010), HC-Pro (from PVY) (Anandalakshmi et al., 1998; Brigneti et al., 1998) and 2b (from the subgroup II CMV strain KIN); or the 126 kDa replication protein/VSR (Kurihara et al., 2007; Vogler et al., 2007) encoded by TMV (described by Harries et al., 2008). KIN-CMV is, like LS-CMV, classified as a subgroup II CMV strain (Palukaitis & García-Arenal, 2003) and the amino acid sequences of the 2b proteins of LS-CMV and KIN-CMV are identical. The responses of all four JA-regulated transcripts to MeJA treatment were markedly depressed in plants of lines expressing p25, HC-Pro, and 126 kDa proteins (Fig. 2). However, infection with the corresponding viruses inhibited expression to a greater degree (Fig. 1). It appeared that expression of the 2b protein derived from KIN-CMV did not significantly inhibit accumulation of JA-responsive transcripts following application of MeJA in contrast to the other VSRs tested (Fig. 2). The ability of the PVY-derived HC-Pro to inhibit JA-responsive gene expression in N. benthamiana contrasts with the reported effects of Tobacco etch virus in A. thaliana (Endres et al., 2010).
Fig. 2.
Transgenic expression of VSRs inhibits JA-induced gene expression in N. benthamiana. Non-transgenic (NT) and transgenic N. benthamiana plants expressing p25, HC-Pro or 2b or transformed with an ‘empty’ vector (vector control line) were treated with MeJA. Aerial tissue from three plants per treatment group was harvested immediately prior to treatment (0 h) and at 6 and 24 h following treatment. RNA was extracted from pooled tissue samples and reverse transcription quantitative PCR was performed to measure the transcript abundance of the JA-regulated transcripts for the N. benthamiana orthologue of AT3G55290, LOX2, DEF2.2 and TPI. Significant suppression of MeJA-induced gene expression changes at one or both post-treatment time points is indicated (t-test: *P<0.05, **P<0.01, ***P<0.001). Histogram bars represent RNA samples from three plants (technical replicates). Error bars represent sem.
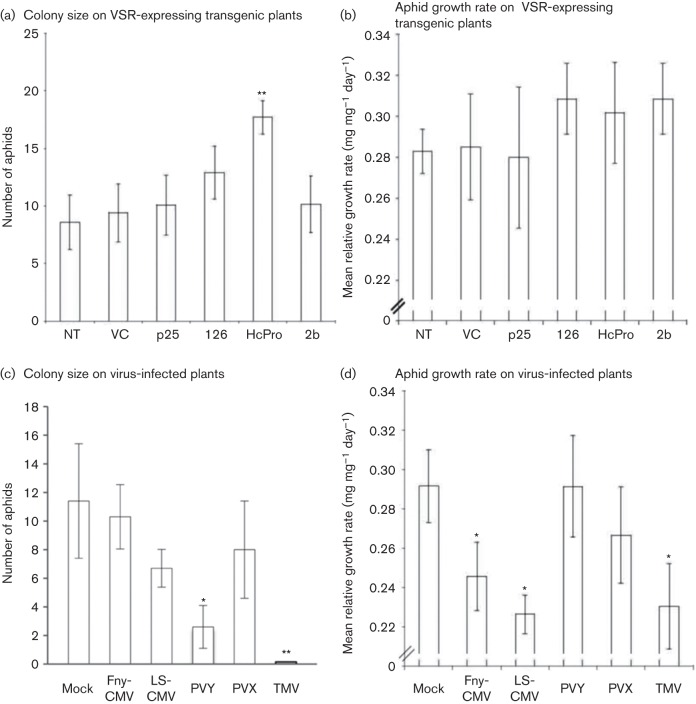
To test whether these viruses or the VSRs they encoded affected aphid performance on plants, we measured aphid growth rates and monitored aphid colony development on virus-infected plants or VSR-expressing transgenic plants (Fig. 3). A colony of M. persicae clone US1L (Devonshire & Sawicki, 1979) was established on N. benthamiana. To determine growth rates, 1-day-old nymphs were individually weighed on a microbalance (MX5; Mettler-Toledo), then placed on test plants and reweighed 5 days later. The mean relative growth rate (MRGR) was calculated as described previously (Leather & Dixon, 1984; Ziebell et al., 2011). Aphid colony growth was measured by counting the progeny of a single nymph 12 days after its placement on a plant.
Fig. 3.
Effects of VSR expression versus virus infection on aphid performance. (a) Aphid colony development was enhanced on N. benthamiana plants expressing HC-Pro derived from PVY. A 1-day-old nymph was placed on a leaf in a clip cage and its progeny counted after 12 days. (b) Mean relative growth rate (MRGR) of aphids was unaltered on transgenic plants expressing VSR proteins derived from PVX (p25), TMV (126 kDa), PVY (HC-Pro) or CMV strain KIN (2b), or on plants transformed with an ‘empty’ vector control (VC) or non-transformed (NT) plants. Nymphs were weighed prior to being placed on the plant and after 5 days. MRGR was calculated according to the method of Leather & Dixon (1984). (c) Colony growth on virus-infected plants was determined as in (a). (d) MRGR for aphids on virus-infected plants. A 1-day-old nymph was weighed and placed contained in a clip cage on a leaf of an infected plant 5 days post-inoculation, reweighed 5 days later and MRGR calculated as in (b). Means of test groups (placed on infected or transgenic plants) and mean values for aphids placed on mock or non-transgenic plants were compared by t-tests: *P<0.05; **P<0.01. Error bars represent sem.
Aphid colony growth was not affected to any significant degree on 2b-transgenic, 126 kDa-transgenic or p25-transgenic plants but was significantly enhanced on plants expressing HC-Pro (Fig. 3a). We also measured MRGR for aphids placed on these transgenic plants but found no significant differences (Fig. 3b). The positive effect of HC-Pro expression on aphid colony growth was not reflected in colony growth data from PVY-infected plants; infection with PVY markedly inhibited aphid reproduction (Fig. 3c). Infection of host plants with Fny-CMV, LS-CMV and PVX did not significantly inhibit aphid colony growth (Fig. 3c), although the growth rates for aphids placed on plants infected with Fny-CMV and LS-CMV were depressed (Fig. 3d). Aphid growth rates were not inhibited on PVY-infected plants (Fig. 3d). Aphid growth and colony production were both markedly decreased on TMV-infected plants (Fig. 3c, d), but this was most likely due to the initiation of systemic necrosis, which is a feature of TMV infection in N. benthamiana.
We have shown that infection by TMV, PVX, PVY and CMV inhibits induction of JA-dependent genes in N. benthamiana plants. However, the effect was seen not only during infection by aphid-transmitted viruses (CMV and PVY), but also during infection by mechanically transmitted viruses (TMV and PVX). Therefore, there is no simple correlation between the ability of a virus or its corresponding VSR to interfere with JA-induced host gene expression with the mode of transmission of that virus. The aphid-transmitted viruses PVY and CMV did affect aphid performance, with effects on colony size and growth rate, respectively. This may indicate some similarities with the model proposed by Mauck et al. (2010) for CMV-infected squash in which host quality was diminished by the virus to favour migration of aphids, leading to increased CMV transmission. It is notable, however, that expression of HC-Pro in transgenic plants increased aphid colony growth, whereas PVY infection inhibited it. These conflicting observations lead to two conclusions. First, as transgenic HC-Pro expression and PVY infection both inhibited JA-regulated gene expression but only HC-Pro expression increased aphid performance, virus-induced changes in host–aphid interactions cannot be explained purely by inhibition of JA-mediated gene expression. Secondly, the results indicate, as do recent experiments with Fny-CMV in A. thaliana (Westwood et al., 2013b), that virus-induced changes in host–aphid interactions are not solely regulated by VSRs but must be conditioned by the direct or indirect interactions of more than one viral gene product with the host and, perhaps, with each other.
Acknowledgements
We gratefully acknowledge Kirsi Lehto and Rick Nelson for providing transgenic plant lines, Neil Boonham for providing PVY, Glen Powell, Mark Stevens, Zhiyou Du, Simon ‘Niels’ Groen, Heiko Zeibell and Wing-Sham Lee for useful discussions, Adrienne Pate for technical support and David Pate for kindly lending us a microbalance for this work. This work was supported by grants from the Leverhulme Trust (F/09741/F, RPG-2012-667), UK Biotechnology and Biological Sciences Research Council (BB/D014376/1, BB/J011762/1) and the Cambridge University Isaac Newton Trust (12.07/I).
References
- Anandalakshmi R., Pruss G. J., Ge X., Marathe R., Mallory A. C., Smith T. H., Vance V. B. (1998). A viral suppressor of gene silencing in plants. Proc Natl Acad Sci U S A 95, 13079–13084 10.1073/pnas.95.22.13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R., Jones J. D. (2009). Role of plant hormones in plant defence responses. Plant Mol Biol 69, 473–488 10.1007/s11103-008-9435-0 [DOI] [PubMed] [Google Scholar]
- Brigneti G., Voinnet O., Li W. X., Ji L. H., Ding S. W., Baulcombe D. C. (1998). Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J 17, 6739–6746 10.1093/emboj/17.22.6739 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen H. Y., Yang J., Lin C., Yuan Y. A. (2008). Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b. EMBO Rep 9, 754–760 10.1038/embor.2008.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu M.-H., Chen I. H., Baulcombe D. C., Tsai C.-H. (2010). The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol Plant Pathol 11, 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo F., Mascia T., Pasciuto M. M., Gallitelli D. (2009). Differential effects of mild and severe Cucumber mosaic virus strains in the perturbation of microRNA-regulated gene expression in tomato map to the 3′ sequence of RNA 2. Mol Plant Microbe Interact 22, 1239–1249 10.1094/MPMI-22-10-1239 [DOI] [PubMed] [Google Scholar]
- Devonshire A. L., Sawicki R. M. (1979). Insecticide-resistant Myzus persicae as an example of evolution by gene duplication. Nature 280, 140–141 10.1038/280140a0 [DOI] [Google Scholar]
- Eigenbrode S. D., Ding H., Shiel P., Berger P. H. (2002). Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc Biol Sci 269, 455–460 10.1098/rspb.2001.1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Karafyllidis I., Turner J. G. (2002). Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol Plant Microbe Interact 15, 1025–1030 10.1094/MPMI.2002.15.10.1025 [DOI] [PubMed] [Google Scholar]
- Endres M. W., Gregory B. D., Gao Z., Foreman A. W., Mlotshwa S., Ge X., Pruss G. J., Ecker J. R., Bowman L. H., Vance V. (2010). Two plant viral suppressors of silencing require the ethylene-inducible host transcription factor RAV2 to block RNA silencing. PLoS Pathog 6, e1000729 10.1371/journal.ppat.1000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González I., Martínez L., Rakitina D. V., Lewsey M. G., Atencio F. A., Llave C., Kalinina N. O., Carr J. P., Palukaitis P., Canto T. (2010). Cucumber mosaic virus 2b protein subcellular targets and interactions: their significance to RNA silencing suppressor activity. Mol Plant Microbe Interact 23, 294–303 10.1094/MPMI-23-3-0294 [DOI] [PubMed] [Google Scholar]
- González I., Rakitina D., Semashko M., Taliansky M., Praveen S., Palukaitis P., Carr J. P., Kalinina N., Canto T. (2012). RNA binding is more critical to the suppression of silencing function of Cucumber mosaic virus 2b protein than nuclear localization. RNA 18, 771–782 10.1261/rna.031260.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K., Kobori T., Kosaka Y., Natsuaki T., Masuta C. (2007). Characterization of silencing suppressor 2b of cucumber mosaic virus based on examination of its small RNA-binding abilities. Plant Cell Physiol 48, 1050–1060 10.1093/pcp/pcm074 [DOI] [PubMed] [Google Scholar]
- Harries P. A., Palanichelvam K., Bhat S., Nelson R. S. (2008). Tobacco mosaic virus 126-kDa protein increases the susceptibility of Nicotiana tabacum to other viruses and its dosage affects virus-induced gene silencing. Mol Plant Microbe Interact 21, 1539–1548 10.1094/MPMI-21-12-1539 [DOI] [PubMed] [Google Scholar]
- Harvey J. J. W., Lewsey M. G., Patel K., Westwood J., Heimstädt S., Carr J. P., Baulcombe D. C. (2011). An antiviral defense role of AGO2 in plants. PLoS ONE 6, e14639 10.1371/journal.pone.0014639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L. H., Ding S. W. (2001). The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid-mediated virus resistance. Mol Plant Microbe Interact 14, 715–724 10.1094/MPMI.2001.14.6.715 [DOI] [PubMed] [Google Scholar]
- Kovač M., Müller A., Milovanovič Jarh D., Milavec M., Düchting P., Ravnikar M. (2009). Multiple hormone analysis indicates involvement of jasmonate signalling in the early defence of potato to potato virus YNTN. Biol Plant 53, 195–199 10.1007/s10535-009-0034-y [DOI] [Google Scholar]
- Kurihara Y., Inaba N., Kutsuna N., Takeda A., Tagami Y., Watanabe Y. (2007). Binding of tobamovirus replication protein with small RNA duplexes. J Gen Virol 88, 2347–2352 10.1099/vir.0.82994-0 [DOI] [PubMed] [Google Scholar]
- Leather S. R., Dixon A. F. G. (1984). Aphid growth and reproductive rates. Entomol Exp Appl 35, 137–140 10.1111/j.1570-7458.1984.tb03373.x [DOI] [Google Scholar]
- Lewsey M., Robertson F. C., Canto T., Palukaitis P., Carr J. P. (2007). Selective targeting of miRNA-regulated plant development by a viral counter-silencing protein. Plant J 50, 240–252 10.1111/j.1365-313X.2007.03042.x [DOI] [PubMed] [Google Scholar]
- Lewsey M. G., Murphy A. M., Maclean D., Dalchau N., Westwood J. H., Macaulay K., Bennett M. H., Moulin M., Hanke D. E. & other authors (2010). Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Mol Plant Microbe Interact 23, 835–845 10.1094/MPMI-23-7-0835 [DOI] [PubMed] [Google Scholar]
- Love A. J., Geri C., Laird J., Carr C., Yun B.-W., Loake G. J., Tada Y., Sadanandom A., Milner J. J. (2012). Cauliflower mosaic virus protein P6 inhibits signaling responses to salicylic acid and regulates innate immunity. PLoS ONE 7, e47535 10.1371/journal.pone.0047535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Durán R., Rosas-Díaz T., Gusmaroli G., Luna A. P., Taconnat L., Deng X. W., Bejarano E. R. (2011). Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. Plant Cell 23, 1014–1032 10.1105/tpc.110.080267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck K. E., De Moraes C. M., Mescher M. C. (2010). Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc Natl Acad Sci U S A 107, 3600–3605 10.1073/pnas.0907191107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palukaitis P., García-Arenal F. (2003). Cucumoviruses. Adv Virus Res 62, 241–323 10.1016/S0065-3527(03)62005-1 [DOI] [PubMed] [Google Scholar]
- Rohwer C. L., Erwin J. E. (2008). Horticultural applications of jasmonates: a review. J Hortic Sci Biotechnol 83, 283–304 [Google Scholar]
- Siddiqui S. A., Sarmiento C., Truve E., Lehto H., Lehto K. (2008). Phenotypes and functional effects caused by various viral RNA silencing suppressors in transgenic Nicotiana benthamiana and N. tabacum. Mol Plant Microbe Interact 21, 178–187 10.1094/MPMI-21-2-0178 [DOI] [PubMed] [Google Scholar]
- Vogler H., Akbergenov R., Shivaprasad P. V., Dang V., Fasler M., Kwon M. O., Zhanybekova S., Hohn T., Heinlein M. (2007). Modification of small RNAs associated with suppression of RNA silencing by tobamovirus replicase protein. J Virol 81, 10379–10388 10.1128/JVI.00727-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O., Pinto Y. M., Baulcombe D. C. (1999). Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc Natl Acad Sci U S A 96, 14147–14152 10.1073/pnas.96.24.14147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C. (2007). Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100, 681–697 10.1093/aob/mcm079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood J. H., Stevens M. (2010). Resistance to aphid vectors of virus disease. Adv Virus Res 76, 179–210 10.1016/S0065-3527(10)76005-X [DOI] [PubMed] [Google Scholar]
- Westwood J. H., McCann L., Naish M., Dixon H., Murphy A. M., Stancombe M. A., Bennett M. H., Powell G., Webb A. A. R., Carr J. P. (2013a). A viral RNA silencing suppressor interferes with abscisic acid-mediated signalling and induces drought tolerance in Arabidopsis thaliana. Mol Plant Pathol 14, 158–170 10.1111/j.1364-3703.2012.00840.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood J. H., Groen S. C., Du Z., Murphy A. M., Anggoro D. T., Tungadi T., Luang-In V., Lewsey M. G., Rossiter J. T. & other authors (2013b). A trio of viral proteins tunes aphid-plant interactions in Arabidopsis thaliana. PLoS ONE 8, e83066. 10.1371/journal.pone.0083066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yuan Y. R., Pei Y., Lin S. S., Tuschl T., Patel D. J., Chua N. H. (2006). Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev 20, 3255–3268 10.1101/gad.1495506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Luan J. B., Qi J. F., Huang C. J., Li M., Zhou X. P., Liu S. S. (2012). Begomovirus-whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol Ecol 21, 1294–1304 10.1111/j.1365-294X.2012.05457.x [DOI] [PubMed] [Google Scholar]
- Ziebell H., Murphy A. M., Groen S. C., Tungadi T., Westwood J. H., Lewsey M. G., Moulin M., Kleczkowski A., Smith A. G. & other authors (2011). Cucumber mosaic virus and its 2b RNA silencing suppressor modify plant-aphid interactions in tobacco. Sci Rep 1, 187 10.1038/srep00187 [DOI] [PMC free article] [PubMed] [Google Scholar]