Abstract
Background
HIV is contracted most frequently at birth and during early adulthood. The epidemic may thus impact the demographic structure and the household structure of affected populations.
Methods
This paper reviews earlier evidence of such an impact, uses demographic theory to anticipate its changes over time, and reviews the most recent evidence for indications of these changes.
Results
Modest increases in the male : female ratio are beginning to show within certain age groups only (approximately 15% among 25–34 year olds). Similarly sized increases in the proportion of 15–29 year olds relative to 30–54 year olds are observed in some age pyramids. These ‘youth bulges’ are expected to fade out, whereas an aging effect phases in with the fertility impact of the epidemic. In the longer run, the size of all age groups will be reduced, but relatively less so for middle-aged adults. Proportions of orphans and widows have increased in the most affected countries. Fewer remarriage probabilities for widows were observed. Resulting increases in the proportion of female-headed households should only be temporary, as female mortality is catching up with male mortality. The number of double orphans is beginning to increase, but overall, orphans continue to live predominantly with a family member, most often the grandparents if not with the surviving parent.
Conclusion
To date, the epidemic’s impact on the population and household structure has been limited by demographic (aging) and social (adaptive movements of kin across households) processes that contribute to diffuse the epidemic throughout the entire population and all households.
Keywords: sex ratio, age structure, household structure, widows, orphans
Introduction
Severe HIV epidemics have caused significant mortality increases that primarily affect middle-aged adults [1,2] and children [3]. This differential mortality impact across age categories implies that the age structure of the population and the composition of its households should be significantly altered by a severe HIV epidemic. In turn, the disproportional loss of individuals in their most productive years raises concerns over the welfare of surviving members of affected families and social institutions. These compositional changes can thus be seen as intermediate effects between the immediate mortality impact of the epidemic and its social and economic ‘downstream’ consequences [4].
However, the compositional impacts of the HIV epidemic are perhaps more complex to anticipate than may appear at first sight. First, HIV epidemics also affect reproduction [5], which combined with increased mortality reduces the growth rate of the affected populations. These reduced rates imply further changes in the composition of the population and of households. Second, affected families and societies may react to their loss by bringing in new members or by relocating themselves. This effect is clearer at the level of the household that may receive kin or relatives to cope with the loss, or on the contrary, may dissolve and join existing households. Population movement on a larger scale may also respond to human capital shortages. The compositional changes induced by the HIV epidemic are thus not limited to the ‘first-order’ mortality impact, and the adaptive nature of the household is key to an understanding of the aggregate and dynamic impact of the HIV epidemic. The objective of this paper is to contribute to this understanding, to review the extant empirical record of the epidemic’s impact on population structure and household composition, and to outline the changes to be expected in the near future. The discussion focuses on the countries in eastern and southern Africa, where to date the highest prevalence rates have been estimated among general adult populations.
Population sex ratio
Early evidence and expected changes
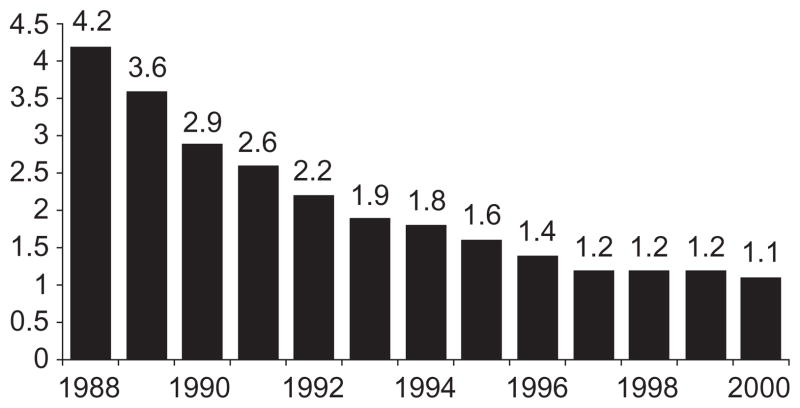
The effect of HIV on the total population sex ratio has received relatively little attention partly because in the most severe epidemics infected females to infected males ratios were observed to be in the range of 1.0 to 1.4 [6,7], values deemed to be too close to the prevailing ratio in the population to alter it significantly. These observed ratios might appear low in an epidemic driven by heterosexual transmission, considering that HIV transmission is approximately two to three times more efficient from male to female than from female to male. Whereas a simplistic equation for the long-term equilibrium value of the sex ratio in the infected population is the square root of the ratio of infectivity (female : male relative to male : female), that is, approximately 1.5, the implicit assumptions underlying that equation are unlikely (non-random sexual mixing patterns, differential age thus survival distribution by sex, etc). More realistically, the difference between men and women in the distribution of the number of sexual partners suggests that the sex ratio may fluctuate substantially over time. A plausible scenario would begin with a high proportion of women at the onset of the epidemic in ‘core groups’, followed by an increasing proportion of men when the epidemic begins to spread to the general population, and finally an increasing proportion of women as infected men in turn infect women in the general population. The steady then softening decline of the sex ratio in the reported numbers of new AIDS cases in Cote d’Ivoire (Fig. 1) [8] is consistent with this scenario to the extent that these numbers are known to be severely under-reported but not necessarily more so for one or the other sex. Also consistent with these dynamics and with a survival time from HIV incidence to death in the order of 10 years [9], female prevalence rates appeared to exceed male prevalence rates in the early 1990s, whereas the HIV impact on mortality continued to be stronger for men than for women [10].
Fig. 1. Male : female ratio of AIDS cases, Cote d’Ivoire, 1988–2000.
Source: World Heath Organization [8].
Given the differences in the age profile of incidence for men and women [11], changes should be more visible in some age-specific sex ratios rather than in the more robust overall population ratio. Simulations suggested that increases in the sex ratio should become visible at approximately 35 years of age, about 20 years after the onset of the epidemic [12].
Most recent evidence
A review of intercensal demographic changes in three African countries (Kenya 1979–1999, Malawi 1977–1998, and Zambia 1980–2000) detected increases in the male : female ratio in the age group 25–34 years in two of the three countries [13]. Because these intercensal changes could have been caused by other factors, mainly sex differences in international migration rates, the impact of the HIV epidemic cannot be identified from these census comparisons. Moreover, the observed changes are relatively modest, in the order of 15%. A recent review of mortality trends in several countries found female mortality rates to be impacted more than male rates in the Central African Republic and Uganda, two countries with a relatively early onset of the HIV epidemic [7]. These findings support the expectation that the impact on women will increase relative to the impact on men as the epidemic matures, and the sex ratio may thus be expected to increase further.
Population age structure
Early evidence and expected changes
The initial effects of the epidemic on the age structure are clear. Given the typical survival times from HIV incidence to death and the age profile of incidence [11], mortality will reduce most the number of women aged approximately 30–50 years, men aged approximately 35–60 years, and children of both sexes under the age of 5 years. A reduction in the number of births, resulting from the smaller size and lower fertility rates of their parents’ generation than in the absence of the HIV epidemic, will further reduce the size of the youngest cohorts. Over time, however, these effects will diffuse through the age structure as the cohorts affected in childhood or mid-adulthood age. The long-term effects are perhaps less intuitive: as the epidemic lowers the rates of population change, it is expected to contribute to an aging of the affected populations, which will be counterbalanced at the older ages by the increased mortality of infected adults and the decreased probability of reaching the oldest ages.
Whereas the proportion of middle-aged adults is expected to decline at first, their proportion is expected to increase later relative to younger generations (aging effect) and to older generations that have experienced the impact of HIV for a longer time. This cumulative effect is weak at first for the generations that were in their peak years of sexual activity and partner change when prevalence was still relatively low. The initial effect on the age structure will thus continue as long as the number of deaths keeps growing rapidly, because recent deaths far outnumber earlier deaths in now older cohorts. With 10 years of fast increases in incidence after the epidemic onset and 10 years from adult incidence to death, it could be at least two decades before an age–structural reversal begins. Simulations indeed suggest that the median age of the working age population might reach a minimum 20–25 years after the onset of the epidemic before rising again [12]. In the absence of concomitant fertility or migration changes, demographic theory suggests that, were the epidemic to stabilize at an endemic level that would eventually translate into new stable mortality rates, the age structure would then converge towards a new equilibrium. With interventions being constantly applied, however, the several decades of unchanging incidence and survival rates required by such equilibrium make it an unlikely occurrence. Typically defined as the average number of individuals under 15 years and over 65 years of age per individual aged 15–65 years, population dependency ratios are expected to decline relatively soon, however, because of the shorter survival time of HIV-infected infants and to accelerate when reduced population growth initiates the aging effect. The dependency ratio might also be depressed by the increased mortality of children and older adults, who might be uninfected themselves but might lose to the epidemic the middle-aged adults most likely to care for them in the event of a life-threatening sickness.
As rates of population growth were rather high in the majority of the most affected countries, their current age pyramids exhibit a wide base corresponding to the youngest age groups and a steep gradient as the age pyramid narrows with older ages. For those countries, the expected effect of the epidemic at the youngest ages is to produce age pyramids with a gentler age gradient. The projected effect is more spectacular in the few countries (foremost of which are Botswana, Namibia, and South Africa) where, reduced by recent fertility declines, slow population growth had already resulted in gentle age gradients. In such cases, the projected age pyramid displays a bottleneck as the age pyramid first widens with age before being narrowed by the increase in mortality with age [14]. More spectacular than simple gradient reductions in the age pyramid, the bottleneck effect might be presented as exemplar of the impact of the epidemic in the worst affected countries, whereas the future formation of such a bottleneck depends more on the fertility rates prevailing in the affected population than on its HIV prevalence. Finally, our ability to project the impact of the epidemic on the future age structure is currently limited to adding AIDS-related deaths, with ‘everything else remaining the same’. The magnitude of some projected effects threatens the validity of these underlying assumptions: would couples continue to control their fertility to the same extent in a fast-declining population, for instance, and would dwindling working-age populations leave immigration flows unchanged?
Most recent evidence
The same review of intercensal demographic changes in Kenya, Malawi, and Zambia [13] documented substantial decreases in the dependency ratio, ranging from 8.0% in Malawi to 17.8% in Kenya. Other factors than the HIV epidemic contributed to this change, especially in Kenya, where an important fertility reduction is under way, and only counterfactual projections can estimate how much of the observed change would have occurred even without the epidemic. The study also found evidence of ‘youth bulges’ with the ratio of 15–29 year-olds compared with 30–54 year-olds increasing by approximately 15% in Malawi and Zambia, but not in Kenya, probably because of the stronger compensating aging effect induced by the fertility reduction (Table 1).
Table 1.
Ratios of 15–29 year olds to 30–54 year olds by sex and urban/rural areas, in Kenya, Malawi, and Zambia.
| Urban
|
Rural
|
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Kenya | ||||
| 1979 | 1.4 | 2.15 | 1.5 | 1.39 |
| 1999 | 1.29 | 2.01 | 1.54 | 1.44 |
| Malawi | ||||
| 1977 | 1.29 | 1.84 | 1.21 | 1.22 |
| 1998 | 1.53 | 2.08 | 1.31 | 1.41 |
| Zambia | ||||
| 1980 | 1.26 | 1.79 | 1.32 | 1.12 |
| 2000 | 1.45 | 1.82 | 1.49 | 1.5 |
Source: Heaton and Stanecki [13].
Even if some impact of the HIV epidemic on the age structure has become visible, the impact to date remains small compared with other demographic factors causing age–structural shifts. Declining fertility in Thailand, for example, brought the dependency ratio down from 92 per 100 in 1970 to 47 per 100 in 2000 [14]. The HIV epidemic probably contributes to declining fertility, and one study estimated that nearly a quarter of recent fertility declines in Zimbabwe were caused by the epidemic [15]. However, the shift to an older age structure and lower dependency ratio is expected to take place over time in high-fertility populations regardless of the HIV epidemic, as they experience their fertility transition.
The impact of the epidemic on the internal age structure of the adult population is perhaps more modest, but also more unusual as it contributes to ‘youth bulges’ that are expected to age into ‘middle-age bulges’. The trend might have negative downstream consequences as the proportion of the most experienced individuals within the working-age population declines, and the early association between incidence and education [16] implies greater losses of human capital. (Eventually, the more educated appear to modify their behavior first and their prevalence declines earlier than the rest of the population [17]). To date, however, there is little evidence of a dramatic shock to the age and sex structure of the overall population and effects are more likely to be found at the level of the affected households.
Household structure
To anticipate and assess the impact of the epidemic on household structure requires first a benchmark description of the extant distribution of household structures in the affected populations. A rich anthropological tradition describes the diversity of African kinship systems [18], and quantitative analyses in countries with available survey data confirm the complexity of family and households to date [19]. (Not to downplay diversity, but in the interests of space, the shorthand ‘benchmark household structure’ will be used in the rest of the paper in lieu of the entire distribution of household structures). Moreover, the institution of marriage was clearly undergoing change before the epidemic in sub-Saharan Africa as in other parts of the world [20,21], further complicating any assessment of the hypothetical benchmark household structure in the absence of the epidemic. This counterfactual scenario being unobservable, holding present households without infected adults as representing it may be the only option available. However, with several households connected by family ties or other relationships, the limits of individual households are not always clearly defined, and one can hardly speak of ‘unaffected’ households when prevalence approaches 20% of adults aged 15 years or more, which in a study in rural Uganda [22] corresponded to 31.3% of the households having at least one HIV-infected resident adult.
In addition to complicating the assessment of a benchmark household structure, the overlap of kinship networks over several households implies that we cannot just focus on mortality increases induced by the epidemic, the ‘first-order’ impact, but must also consider subsequent movements in or out of the household. The adaptive relocation of individuals across households linked by kinship and other networks diffuses the impact of the epidemic across a larger number of households. Moreover, the impact of the epidemic includes observable events, such as death and movement across households, but also ‘non-events’, that is, events that do not take place or take place less frequently than they would have in the absence of the epidemic, such as births and remarriages. Finally, a household directly affected by the epidemic may experience several deaths and adaptations thereto, and its structure will undergo several changes over time. The impact of the epidemic on household structure thus depends not just on the prevalence of the infection and how much mortality increased as a result, but also on the benchmark household structure, on how reproduction, remarriage, and fostering might also change with the epidemic, and on how long the mortality impact of the epidemic has been felt in the population.
Early evidence and expected changes
Chronologically, an infant or child death is likely to be the first adverse event experienced by an affected household [23]. Considering an infected woman surviving 10 years to infection and giving birth to an infected child [9], that birth takes place, on average, more than 5 years before the mother’s death because fertility rates are declining with time since infection [11]. Given the survival probabilities of infants infected at birth, the death of the child is more likely to occur before than after maternal death (but perhaps not before paternal death, as typically the husband is infected before his wife [24]). A child death was unfortunately not a rare event already for parents in most of the areas affected by severe epidemics. Even if the frequency of child deaths increased with the HIV-epidemic [3], one might not expect it to have profound consequences for household structure, and if the population experiences declining trends in child mortality from non-HIV-related causes overall, child mortality may continue to decline [25]. A fairly large demographic literature [26,27] has suggested that the impact on subsequent fertility of changes in infant mortality is typically quite modest. Moreover, in the context of HIV, these effects are likely to be outweighed by other HIV effects on parental fertility [5] and morbidity. In households with an infected mother, AIDS-related infant and child death and reduced fertility might thus combine first to lower the proportion of children in households before any parental death. Everything else being equal, the average number of children in two-parent families should be smaller than in the absence of AIDS, but as was the case in the overall population structure, this effect is difficult to isolate from the effect of declining fertility rates, declining child mortality from non-HIV-related causes and other effects discussed below.
Households are expected to experience more adult than child deaths on average. A married woman may continue to conceive for an average of 8 years past her infection, and with marital fertility rates currently in the order of 0.3 births per person-year in many of the most affected areas, two to three subsequent births can be expected for each infected married woman. Vertical transmission rates in the order of 0.3–0.4 would yield close to one infected birth per infected married woman, to be considered as an upper bound given the biological and social mechanisms that significantly reduce the fertility of infected women. Therefore, on average, less than one AIDS-related child death per AIDS-related maternal death is expected, and considering paternal death, there should clearly be fewer child deaths than parental deaths. Adult deaths are also likely to have further consequences for household structure. These consequences depend largely on the extant household structure. In extended, multi-generation or multi-family households, the reallocation of work in and out of the house and of childcare responsibilities may take place without a visible change in the structure of the household. An adult loss would be more conspicuous in two-parent households, leaving orphans to live with a single parent, which may prompt another adult from the extended family to move in and help with childcare, or if a surviving spouse is also infected and symptomatic, with caring for the sick spouse, which typically rests mostly with the patient’s family [28,29]. An alternative response to ease the burden would be to send the children to another household within the extended family network [19]. Some of these adaptive changes may be pre-emptive and precede the adult death, and the realization of the spouse’s infection may induce an early separation [30]. Although the timing of these different events may thus vary, the impact on the household is qualitatively similar.
As adult mortality was already relatively high before the epidemic, especially in eastern Africa, families were used to drawing on traditional coping readjustments to face the increased mortality induced by the epidemic (e.g. remarriage of widows or widowers [31–33], child fostering [34–36]). However, the stigma attached to the virus may threaten some traditional adaptive behavior [37], thus increasing the proportion of single-parent households, which appear to have been relatively rare in eastern Africa before the epidemic compared with southern Africa where male out-migration was more intense. This stigma might even lead to the formation of child-headed households [4,38], although earlier evidence showed that most orphans continued to live with the surviving parent [22,35,39]. The residence of AIDS orphans needs to be monitored as the epidemic matures, however, as the likelihood of HIV transmission from one parent to the other, most likely from husband to wife [24], implies that the second parent may also die, leaving ‘double orphans’ behind. Although children who did not reside with any parent traditionally still resided with a relative [19], social scientists appear to be divided on the issue of whether the institution of child fostering within the extended family can meet the expected increase in the number of these orphans [37,38,40,41].
The epidemic’s effects that are hardest to anticipate are those that concern other households, that is, households without infected members. Although the size of some of these families may increase through fostering for example, how will they adapt to this unexpected increase? Will the foster parents reduce their own future fertility to cope with the increased childcare? Another little understood issue is how the family-building behavior of the generation of unmarried young adults might be affected [42].
Most recent evidence
The challenges to validate these expectations empirically are several. First, any cross-section of households will present a mix of households with and without infected members. In two-parent households, the size of the households with an infected mother might be reduced by child death and impaired fertility, whereas the size of other two-parent households might be increased by fostering relatives’ orphans. Further complicating the assessment from cross-sectional data, households with an infected adult are also expected to go through a sequence of events and ‘non-events’. A possible sequence for a two-parent household might be a reduced size two-parent household, a single-parent household, and eventually, household dissolution. Ideally, to assess the impact of the epidemic on household structure would require a good knowledge of benchmark household structure, knowledge of the HIV status of adults in the household, and a longitudinal follow-up of households over time. The last two requirements have been met by a few community-based research projects that were undertaken precisely to study the HIV epidemic after the importance of the epidemic was recognized, and have provided some of the most important and relevant information on the impact of the epidemic on households, such as Rakai and Masaka, rural Uganda, Mwanza, rural Tanzania, or Mutasa, rural Zimbabwe (for a more complete list see Table 1 in Heuveline [11]).
Even in these community-based projects, data on the selected populations before the onset of the epidemic are often lacking, with the notable exception of a study in rural Malawi [43], where researchers were able to return to a population first studied in the 1980s. The study confirmed that HIV-positive widows and widowers were less likely to remarry, but found that HIV infection had no impact on marital break-up. Interestingly, the net effect of HIV on remarriage actually disappears after controlling whether marriage ended in divorce or widowhood, and whether the widow/ widower survived to the end of the follow-up period. It is reasonable to assume that this survival is correlated to the widow/widower’s health at the time of the spouse’s death, and the finding is thus consistent with a situation in which HIV status is actually unknown and inferred by the entourage from visible characteristics indicative of health. This further suggests that the effect of the HIV epidemic on remarriage might be more pervasive than if infection was known, as all widows or widowers displaying these characteristics may be similarly ostracized regardless of their HIV status. In any event, 10 years after HIV infection was first recognized in their husbands, a third of the widows in the study were single heads of their households.
Recent studies have reported that households experiencing an adult AIDS death have difficulty ‘replacing’ those lost adult members in Kenya [44], South Africa [45], and in Tanzania [46]. The Kenyan study actually provided more nuanced evidence on adaptive movements in and out of households struck by an AIDS death, showing that households experiencing the death of an adult other than the head or spouse thereof tended to gain new adult members. When the head of the household or spouse thereof died, on the contrary, the household incurred on average a greater than one-person decline in household size, with older daughters commonly leaving the household after the death of a male head, and younger children more likely to leave after the death of a female head or spouse. These contrasted results suggested that the proportion of single or widowed female-headed households should increase initially. Over time, however, the proportion should peak as infected widows are also expected to die within a few years. In South Africa, the widowhood rate did decline between 1991 and 1996, which seems to be too early to correspond to a catching up of female mortality with male mortality, and an artifact of data differentials cannot be ruled out [47]. However, a community-based study in rural South Africa found that more mothers than fathers died in 2001 [45].
Most recent cross-national trends exhibited an increasing prevalence of orphans in high HIV-prevalence countries and a decrease in low-prevalence countries [48]. The most recent data continue to show most orphans living with their parents or with a relative, foremost of which are the grandparents, and the proportion of child-headed households remains very small at the national level [48]. To date, the HIV epidemic appears to have affected the institution of child fostering less than that of remarriage. However, the situation might worsen as most orphans currently still have a parent left to take care for them, but they can be expected to lose this parent in the coming years. In rural Malawi, 73% of the households dissolved entirely after the death of the second parent [43]. Moreover, the most common substitute carers, the grandparents, are currently from a generation that was largely unaffected by the HIV epidemic, which may not be the case in the future.
To gain insight into the near future, we may focus on countries in southern Africa, where more children were raised by a single parent before the epidemic (a study in rural South Africa [45] found 54% of all children not living with their father in 2000), and thus the effect of the epidemic might be felt sooner. Indeed, already a quarter of children do not live with either parent in Botswana, Namibia, and South Africa [48]. A one-year follow-up of households in rural South Africa found higher frequencies of children leaving the household or even households dissolving entirely than in previous studies, but the number of child-headed families remained extremely small [45]. Similar results were recently reported from another study in rural Uganda [49], where the epidemic is relatively mature and the proportion of orphans living with either biological parent has now dropped to 35%. Most of them continue to live with a family member, most often a grandparent. Nationwide, however, Uganda is the only country among those for which we have trend data, where the proportion of grandparents as caregivers decreased [48]. A sign that the HIV epidemic might be affecting the institution of child fostering can be found in rural Malawi, where although grandparents continue to be the most frequent alternative to parents, aunts and uncles are much more frequent foster parents for the children of HIV-positive parents (37%) than for the children of HIV-negative parents (20%) [43]. Evidence to date therefore fits the hypothesis of the ‘increase in the incidence of orphanhood, widowhood and incomplete coresidence units’ that was anticipated in computer simulations more than a decade ago [50]. The maturation of the epidemic might bring about more profound changes, but the evidence to date is too sketchy to reach such a conclusion yet.
Discussion
To date, the HIV epidemic contributed to modest increases in the sex ratio within certain age groups, and within the adult population, in the proportion of individuals under the age of 30 years. The most important change in the age structures of the population in eastern and southern Africa, however, is generated by the underlying fertility declines; a trend to which the AIDS epidemic certainly contributes, but without being the dominant factor of change. This contribution is not alarming as it ushers in lower dependency ratios in a region where they have traditionally been quite high. A more negative effect, albeit somewhat more subtle, is the relative loss of the most experienced (older) and the most educated (selection effect) members of the workforce.
The effects are felt more pointedly by the households of HIV-positive individuals, although kinship networks that connect several households contribute to diffuse the effect throughout the population. The clearest evidence to date are increasing numbers of single or widowed female-headed households, and increasing numbers of children not living with their biological parents. Female-headed households were not rare in the populations of southern Africa, where male migration rates were high, nor were foster children in most parts of sub-Saharan Africa. Increases in single-parent households in the United States and Europe have generated a fair amount of concern, building on a large body of research that shows the negative consequences for children of growing up with a single parent, generally their mother [51]. Similar results were found in developing countries, Indonesia and Mexico for example [52], but a study of primary school completion in South Africa found a higher completion rate among children in female-headed households unless their own mother was away [53].
In any event, the increase in female-headed households might only be a transitory effect of the epidemic, as its impact on female mortality is soon expected to exceed its impact on male mortality. On the contrary, the increase in the number of children who cannot live with their biological parents is expected to continue. The evidence to date continues to show these children most often living with a relative of their parents rather than forming their own, child-headed households. Among these relatives, evidence continues to point at grandparents as the most frequent recourse, but there are timid signs that aunts and uncles might be called upon more often in the near future. Independently of the HIV epidemic, the health of fostered children has been shown to be worse than that of other children [54]. Although some psychological effects of orphanhood were documented in Uganda [55], no difference in reported health and anthropometric measurements was found in the rural Malawi study described above [43]. A number of studies had found very little or no differences in school enrollment [36,39,56–58], but a couple of recent studies in urban and rural Uganda [49,59] reported small effects in the expected direction in enrollment and in the likelihood to be at the appropriate grade level for age [60]. One study in rural Zimbabwe [61] focused on school completion rather than on mere attendance. The authors reported differences in the expected direction for maternal orphans, but found girls whose fathers had passed away were more likely to complete primary school. If these results are so mixed, it might be partly because the institution of child fosterage represents a variety of situations. The wellbeing of a foster child might be different if the decision to foster is taken jointly by the sending and receiving families in the interests of the child, rather than being imposed by some external constraints [62,63]. One international study of school enrollment in sub-Saharan Africa also found that outcomes for orphans worsened the lesser the degree of relatedness of the orphan to the household head [64]. One may fear that as the epidemic evolves, AIDS orphans will increasingly be pushed into more reluctant and distant relatives’ households.
Overall, some limited evidence on the negative impact of the epidemic has begun to appear, but without invalidating the earlier conclusion that to date the family systems of the most affected populations have been quite resilient and are able to shelter their members from the worst anticipated effects [65]. Of course, this is no guarantee that the expected further changes in household structure will have no dramatic effect in the future. Even with the epidemic in its third decade, it might be too early to find evidence of the strongest effects. With the numbers of cases that typically grow rapidly throughout the first decade and the duration from incidence to death in the order of 10 years, it may take nearly 20 years after the onset of the epidemic for any household level impact to be detectable, 10 years after the epidemic has peaked for the full scale of the epidemic’s impact on households to appear. We need to continue to monitor households in the affected populations to find out whether their resilient family systems continue to withstand the pressure as the epidemic matures and its impact increases. We also need research to determine at what costs this might be achieved; that is, what families have to sacrifice in order to cope with the worst effects of the epidemic.
References
- 1.Porter K, Zaba B. The empirical evidence for the impact of HIV on adult mortality in the developing world – data from serological studies. AIDS. 2004;18 (Suppl 2):S9–S17. doi: 10.1097/00002030-200406002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Blacker J. The impact of AIDS on adult mortality: evidence from national and regional statistics. AIDS. 2004;18 (Suppl 2):S19–S26. doi: 10.1097/00002030-200406002-00003. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Brahmbhatt H, Ghys P. Child mortality and HIV infection in sub-Saharan Africa: a review. AIDS. 2004;18 (Suppl 2):S27–S34. doi: 10.1097/00002030-200406002-00004. [DOI] [PubMed] [Google Scholar]
- 4.Barnett T, Blaikie P. AIDS in Africa: the present and future impact. London: Bellhaven Press; 1992. [Google Scholar]
- 5.Lewis JJC, Ronsmans C, Ezeh A, Gregson S. The population impact of HIV on fertility in Africa. AIDS. 2004;18 (Suppl 2):S35–S43. doi: 10.1097/00002030-200406002-00005. [DOI] [PubMed] [Google Scholar]
- 6.Berkley S, Naamara W, Okware S, Downing R, Konde-Lule J, Wawer M, et al. AIDS and HIV infection in Uganda: are more women infected than men? AIDS. 1990;4:1237–1242. doi: 10.1097/00002030-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gregson S, Garnett GP. Contrasting gender differentials in HIV-1 prevalence and associated mortality increase in eastern and southern Africa: artifact of data or natural course of epidemics? AIDS. 2000;14 (Suppl 3):S85–S99. [PubMed] [Google Scholar]
- 8.World Heath Organization. HIV/AIDS Epidemiological Surveillance for the WHO African Region 2002. WHO Regional Office for Africa; Brazzaville and Harare: 2003. [Google Scholar]
- 9.Morgan D, Mahe C, Mayanja B, Okongo JM, Lubega R, Whit-worth JAG. HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries? AIDS. 2002;16:597–603. doi: 10.1097/00002030-200203080-00011. [DOI] [PubMed] [Google Scholar]
- 10.Timaeus IM. Impact of the HIV epidemic on mortality in sub-Saharan Africa: evidence from national surveys and censuses. AIDS. 1998;12 (Suppl 1):S15–S27. [PubMed] [Google Scholar]
- 11.Heuveline P. HIV and population dynamics: a general model and maximum-likelihood standards for east Africa. Demography. 2003;40:217–245. doi: 10.1353/dem.2003.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregson S, Garnett GP, Anderson RM. Assessing the potential impact of the HIV-1 epidemic on orphanhood and the demographic structure of populations in sub-Saharan Africa. Popul Stud. 1994;48:435–458. [Google Scholar]
- 13.Heaton LM, Stanecki KA. A descriptive analysis of dependency ratios and other demographic indicators in populations affected by HIV/AIDS. Paper presented at the scientific meeting ‘Empirical evidence for the demographic and socioeconomic impact of AIDS’; Durban, South Africa. 26–28 March 2003. [Google Scholar]
- 14.United Nations. World population prospects: the 2002 revision. New York: United Nations; 2003. [Google Scholar]
- 15.Terceira N, Gregson S, Zaba B, Mason PR. The contribution of HIV to fertility decline in rural Zimbabwe, 1985–2000. Popul Stud. 2003;57:149–164. doi: 10.1080/0032472032000097074. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves JR, Glynn JR. Educational attainment and HIV-1 infection in developing countries: a systematic review. Trop Med Int Health. 2002;7:489–498. doi: 10.1046/j.1365-3156.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 17.Fylkesnes K, Musonda RM, Sichone M, Ndhlovud Z, Tembob F, Monzee M. Declining HIV prevalence and risk behaviours in Zambia: evidence for, surveillance and population-based surveys. AIDS. 2001;15:907–916. doi: 10.1097/00002030-200105040-00011. [DOI] [PubMed] [Google Scholar]
- 18.Radcliffe-Brown AR, Forde D, editors. African systems of kinship and marriage. London, New York: Oxford University Press; 1950. [Google Scholar]
- 19.McDaniel A, Zulu E. Mothers, fathers and children: regional patterns in child–parent residence in sub-Saharan Africa. African Popul Stud. 1996;11:1–28. [Google Scholar]
- 20.Parkin DJ, Nyamwaya D. Transformations of African marriage. Manchester: Manchester University Press; 1987. [Google Scholar]
- 21.Weisner TS, Bradley C, Kilbride PL, editors. African families and the crisis of social change. Westport, CT: Bergin and Garvey; 1997. [Google Scholar]
- 22.Nalugoda F, Wawer MJ, Konde-Lule JK, Menon R, Gray RH, Serwadda D, et al. HIV infection in rural households, Rakai district, Uganda. Health Trans Rev. 1997;7 (Suppl 2):127–140. [Google Scholar]
- 23.Ng’weshemi J, Urassa M, Isingo R, Mwaluko G, Ngalula J, Boerma T, et al. HIV impact on mother and child mortality in rural Tanzania. J Acquired Immune Defic Syndr. 2003;33:393–404. doi: 10.1097/00126334-200307010-00015. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter LM, Kamali A, Ruberantwari A, Malamba SS, Whit-worth JAG. Rates of HIV-1 transmission within marriage in rural Uganda in relation to the HIV sero-status of the partners. AIDS. 1999;13:1083–1089. doi: 10.1097/00002030-199906180-00012. [DOI] [PubMed] [Google Scholar]
- 25.Zaba B, Marston M, Floyd S. The effect of HIV on child mortality trends in sub-Saharan Africa. [Accessed May 2004];United Nations Population Bulletin 2004. http://www.un.org/esa/population/publications/adultmort/Zaba.pdf.
- 26.Preston SH, editor. The effects of infant and child mortality on fertility. New York: Academic Press; 1978. [Google Scholar]
- 27.Montgomery MR, Cohen B, editors. From death to birth: mortality decine and reproductive change. New York: Academic Press; 1978. [PubMed] [Google Scholar]
- 28.Ankrah EM. AIDS and the social side of health. Soc Sci Med. 1991;32:967–980. doi: 10.1016/0277-9536(91)90155-6. [DOI] [PubMed] [Google Scholar]
- 29.Ntozi JPM. AIDS morbidity and the role of the family in patient care in Uganda. Health Trans Rev. 1997;7 (Suppl 1):1–22. [PubMed] [Google Scholar]
- 30.Caldwell JC, Caldwell P, Quiggin P. The social context of AIDS in sub-Saharan Africa. Popul Dev Rev. 1989;15:185–234. [Google Scholar]
- 31.Seeley J. The extended family and support for people with AIDS in south-west Uganda: a safety net with hole. AIDS Care. 1993;5:117–122. doi: 10.1080/09540129308258589. [DOI] [PubMed] [Google Scholar]
- 32.Grinstead OA, Gregorich SE, Choi KH, Coates T. Voluntary HIV-1 Counselling and Testing Efficacy Group: positive and negative life events after counseling and testing: the Voluntary HIV-1 Counseling and Testing Efficacy Study. AIDS. 2001;15:1045–1052. doi: 10.1097/00002030-200105250-00013. [DOI] [PubMed] [Google Scholar]
- 33.Mukiza-Gapere J, Ntozi JPM. Impact of AIDS on the family and mortality in Uganda. Health Trans Rev. 1995;5 (Suppl 2):191–200. [PubMed] [Google Scholar]
- 34.Ntozi JPM. Widowhood, remarriage and migration dution the HIV/AIDS epidemic in Uganda. Health Trans Rev. 1997;7 (Suppl 1):125–144. [PubMed] [Google Scholar]
- 35.Isiugo-Abanihe U. Child fostering in West Africa. Popul Dev Rev. 1985;11:53–74. [Google Scholar]
- 36.Ntozi JPM. Effect of AIDS on children: the problem of orphans in Uganda. Health Trans Rev. 1997;7 (Suppl 1):23–40. [PubMed] [Google Scholar]
- 37.Ryder RW, Kamenga M, Nkusu M, Batter V, Heyward WL. AIDS orphans in Kinshasa, Zaire: incidence and socioeconomic consequences. AIDS. 1994;8:673–679. doi: 10.1097/00002030-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Foster G, Makufa C, Drew R, Kralovec E. Factors leading to the establishment of child-headed households: the case of Zimbabwe. Health Trans Rev. 1997;7 (Suppl 2):155–168. [Google Scholar]
- 39.Urassa M, Boerma JT, Ng’weshemi JZL, Isingo R, Schapink D, Kumugola Y. Orphanhood, child fostering and the AIDS epidemic in rural Tanzania. Health Trans Rev. 1997;7 (Suppl 2):141–153. [Google Scholar]
- 40.Hunter SS. Orphans as a window on the AIDS epidemic in sub-Saharan Africa. Soc Sci Med. 1990;31:681–690. doi: 10.1016/0277-9536(90)90250-v. [DOI] [PubMed] [Google Scholar]
- 41.Ankrah EM. The impact of HIV/AIDS on the family and other significant relationship: the African clan revisited. AIDS Care. 1993;5:5–22. doi: 10.1080/09540129308258580. [DOI] [PubMed] [Google Scholar]
- 42.Mukiza-Gapere J, Ntozi JPM. Impact of AIDS on marriage patterns, customs and practices in Uganda. Health Trans Rev. 1995;5 (Suppl 2):201–208. [PubMed] [Google Scholar]
- 43.Floyd S, Crampin AC, Glynn JR, Madise N, Nyondo A, Khondowe MM, et al. The impact of HIV on household structure in rural Malawi. Paper presented at the scientific meeting ‘Empirical evidence for the demographic and socioeconomic impact of AIDS’; Durban, South Africa. 26–28 March 2003. [Google Scholar]
- 44.Yamano T, Jayne TS. Measuring the impacts of prime-age adult death on rural households in Kenya. Paper presented at the scientific meeting ‘Empirical evidence for the demographic and socioeconomic impact of AIDS’; Durban, South Africa. 26–28 March 2003. [Google Scholar]
- 45.Hosegood V, Timaeus I. The impact of adult deaths on household structure and rural South Africa. Paper presented at the scientific meeting ‘Empirical evidence for the demographic and socioeconomic impact of AIDS’; Durban, South Africa. 26–28 March 2003. [Google Scholar]
- 46.Urassa M, Boerma JT, Isingo R, Ngalula J, Ng’weshemi J, Mwaluko G, Zaba B. The impact of HIV/AIDS on mortality and household mobility in rural Tanzania. AIDS. 2001;14:2017–2023. doi: 10.1097/00002030-200110190-00015. [DOI] [PubMed] [Google Scholar]
- 47.Merli MG, Palloni A, Sharma M, Cottrell EB. Changes in household composition and living arrangements in South Africa in the 1990s. Paper presented at the workshop ‘Population and Health in Africa’; Bellagio, Italy. 3–13 December 2003. [Google Scholar]
- 48.Monasch R, Boerma JT. The situation of orphans in a region affected by HIV/AIDS: a review of population-based household surveys from 40 countries in sub-Saharan Africa. AIDS. 2004;18 (Suppl 2):S55–S66. doi: 10.1097/00002030-200406002-00007. [DOI] [PubMed] [Google Scholar]
- 49.Busingye J, Pickering J, Ruberantwari A, Whitworth J. Orphans in the HIV/AIDS era: a study in rural Uganda. Paper presented at the scientific meeting ‘Empirical evidence for the demographic and socioeconomic impact of AIDS’; Durban, South Africa. 26–28 March 2003. [Google Scholar]
- 50.Palloni A, Lee YJ. Some aspects of the social context of HIV and its effects on women, children, families. Popul Bull UN. 1992;33:64–87. [PubMed] [Google Scholar]
- 51.Heuveline P, Timberlake JM, Furstenberg FF., Jr Shifting child-rearing to single mothers. results from 17 Western countries. Popul Dev Rev. 2003;29:47–71. doi: 10.1111/j.1728-4457.2003.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gertler P, Levine D, Martinez S, Bertozzi S. The presence and presents of parents: do parents matter for more than their money?. Paper presented at the scientific meeting ‘Empirical evidence for the demographic and socioeconomic impact of AIDS’; Durban, South Africa. 26–28 March 2003. [Google Scholar]
- 53.Townsend N, Madhavan S, Tollman S, Garenne M, Kahn K. Children’s residence patterns and educational attainment in rural South Africa. Popul Stud. 2002;56:215–225. [Google Scholar]
- 54.Bledsoe CH, Ewbank DC, Isiugo-Abanihe UC. The effect of child fostering on feeding practices and access to health services in rural Sierra Leone. Soc Sci Med. 1988;27:627–636. doi: 10.1016/0277-9536(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 55.Sengendo J, Nambi J. The psychological effect of orphanhood: a study of orphans in Rakai district. Health Trans Rev. 1997;7 (Suppl 1):105–124. [PubMed] [Google Scholar]
- 56.Foster G, Shakespeare R, Chinemana F, Jackson H, Gregson S, Marange C, Mashumba S. Orphan prevalence and extended family care in a peri-urban community in Zimbabwe. AIDS Care. 1995;7:3–17. doi: 10.1080/09540129550126911. [DOI] [PubMed] [Google Scholar]
- 57.Kamali A, Seeley JA, Nunn AJ, Kengeya-Kayondo JF, Ruberantwari A, Mulder DW. The orphan problem: experience of a sub-Saharan Africa rural population in the AIDS epidemic. AIDS Care. 1996;8:509–515. doi: 10.1080/09540129650125470. [DOI] [PubMed] [Google Scholar]
- 58.Lloyd CB, Blanc AK. Children’s schooling in sub-Saharan Africa: the role of fathers; mothers and others. Popul Dev Rev. 1996;22:265–298. [Google Scholar]
- 59.Muller O, Sen G, Nsubuga A. HIV/AIDS, orphans and access to school education in Kampala, Uganda. AIDS. 1999;13:146–147. [PubMed] [Google Scholar]
- 60.Bicego G, Rutstein S, Johnson K. Dimensions of the emerging orphan crisis in sub-Saharan Africa. Soc Sci Med. 2003;56:1235–1247. doi: 10.1016/s0277-9536(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 61.Nyamukapa C, Gregson S, Wambe M. Extended family childcare arrangements and orphan education in eastern Zimbabwe. Paper presented at the scientific meeting ‘Empirical evidence for the demographic and socioeconomic impact of AIDS’; Durban, South Africa. 26–28 March 2003. [Google Scholar]
- 62.Bledsoe C, Ewbank D, Isiugo-Abanihe U. The effect of child fostering on feeding practices and access to health services in rural Sierra Leone. Soc Sci Med. 1988;27:627–636. doi: 10.1016/0277-9536(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 63.Madhavan S. Fosterage patterns in the age of AIDS: continuity and change. Soc Sci Med. 2004;58:1443–1454. doi: 10.1016/S0277-9536(03)00341-1. [DOI] [PubMed] [Google Scholar]
- 64.Case A, Paxson C, Ableidinger J. Orphans in Africa: Parental Death, Poverty, and School Enrollment. Demography. 2004;41 doi: 10.1353/dem.2004.0019. in press. [DOI] [PubMed] [Google Scholar]
- 65.Caldwell JC. The impact of the African AIDS epidemic. Health Trans Rev. 1997;7 (Suppl 2):169–188. [Google Scholar]