Abstract
Objective:
To examine the association between alcohol consumption in midlife and subsequent cognitive decline.
Methods:
Data are from 5,054 men and 2,099 women from the Whitehall II cohort study with a mean age of 56 years (range 44–69 years) at first cognitive assessment. Alcohol consumption was assessed 3 times in the 10 years preceding the first cognitive assessment (1997–1999). Cognitive tests were repeated in 2002–2004 and 2007–2009. The cognitive test battery included 4 tests assessing memory and executive function; a global cognitive score summarized performances across these tests. Linear mixed models were used to assess the association between alcohol consumption and cognitive decline, expressed as z scores (mean = 0, SD = 1).
Results:
In men, there were no differences in cognitive decline among alcohol abstainers, quitters, and light or moderate alcohol drinkers (<20 g/d). However, alcohol consumption ≥36 g/d was associated with faster decline in all cognitive domains compared with consumption between 0.1 and 19.9 g/d: mean difference (95% confidence interval) in 10-year decline in the global cognitive score = −0.10 (−0.16, −0.04), executive function = −0.06 (−0.12, 0.00), and memory = −0.16 (−0.26, −0.05). In women, compared with those drinking 0.1 to 9.9 g/d of alcohol, 10-year abstainers showed faster decline in the global cognitive score (−0.21 [−0.37, −0.04]) and executive function (−0.17 [−0.32, −0.01]).
Conclusions:
Excessive alcohol consumption in men (≥36 g/d) was associated with faster cognitive decline compared with light to moderate alcohol consumption.
Alcohol misuse is a leading preventable cause of morbidity and mortality.1 In addition to chronic diseases, alcohol may affect aging outcomes, but this effect remains poorly understood. Light to moderate alcohol consumption is hypothesized to be associated with better cognitive function and lower risk of dementia,2–9 but less is known about the impact of alcohol on cognitive aging trajectories because much of the evidence comes from studies conducted in elderly populations10–17 in which health-related changes in alcohol consumption are likely to influence results.2 Because alcohol consumption declines with age,18 the heavy drinking category is either small12,13,15,17 or not represented at all10,11,14,16 in these studies. Besides notable exceptions,19,20 few studies have examined the impact of alcohol consumption on cognitive aging trajectories before old age. Furthermore, alcohol consumption is often assessed only once, resulting in possible measurement error bias. The objective of the present study was to examine the association of midlife alcohol consumption assessed 3 times over a 10-year period with subsequent cognitive decline using 3 waves of cognitive data.
METHODS
Study population.
The Whitehall II cohort is an ongoing study of British civil servants.21 At study inception (1985–1988), 10,308 participants (67% men, age range 35–55 years) underwent a clinical examination and completed a self-administered questionnaire. Subsequent clinical examinations were undertaken in 1991–1993, 1997–1999, 2002–2004, and 2007–2009.
Standard protocol approvals, registrations, and patient consents.
All participants provided written informed consent, and the University College London ethics committee approved this study.
Ten-year alcohol consumption.
To better characterize long-term alcohol consumption patterns and to reduce measurement error, we calculated mean alcohol consumption over 10 years for each participant using data from 1985–1988, 1991–1993, and 1997–1999 via questions on frequency of alcohol consumption over the previous year and questions on the number of alcoholic drinks (“measures” of spirits, “glasses” of wine, and “pints” of beer) consumed in the last 7 days. Alcoholic drinks were converted to grams of alcohol consumed per week and divided by 7 to yield average daily alcohol consumption in grams/day. Data on the frequency (over the previous year) and quantity (over the previous week) of alcohol consumption were combined to construct a comprehensive measure of alcohol consumption (table e-1 on the Neurology® Web site at www.neurology.org). Participants who reported no alcohol consumption in the previous year at each of the 3 assessments were classified as “alcohol abstainers” while those who reported alcohol consumption in 1985–1988 or 1991–1993 but not in 1997–1999 were categorized as “alcohol cessation in the last 10 years.” Those who reported consuming alcoholic beverages in the previous year but not in the last week at all 3 waves were classified as “occasional drinkers.” The remaining participants were classified into 6 groups on the basis of their average daily alcohol consumption using the 10th/30th/50th/70th/90th percentiles, separately in men and women in the preliminary analysis. These cutoffs were chosen to examine the shape of the association between alcohol and cognition without an a priori assumption. In preliminary analyses (table e-2), those between the 50th and 70th percentile of the distribution were selected as the reference group (12–19.9 g/d in men and 6–9.9 g/d in women). These analyses led us to choose drinkers with alcohol consumption between the 0 and 70th percentile of the distribution as the reference category in the main analyses, corresponding to 0.1 to 19.9 g/d of alcohol in men and 0.1 to 9.9 g/d in women.
Cognition.
Cognitive testing was introduced to the study in 1997–1999 (age range 44–69 years) and repeated in 2002–2004 (age range 50–74 years) and 2007–2009 (age range 55–80 years). The cognitive test battery included 4 tests.
Short-term verbal memory was assessed with 20 one- or two-syllable words, presented orally at 2-second intervals, and the participants had 2 minutes to recall these words in writing.22
Executive function23 was derived from 3 tests. The timed (10 minutes) Alice Heim 4-I (AH4-I) to test inductive reasoning was composed of a series of 65 verbal and mathematical items of increasing difficulty.24 Two measures of verbal fluency were used: phonemic, assessed via “S” words, and semantic fluency using names of animals.25 One minute was allowed for each test. The mean of the standardized z scores of these 3 tests (mean = 0; SD = 1, using the mean and SD at the first cognitive assessment [1997–1999]) was the measure of executive function.
To provide a summary score of all tests in the cognitive battery, a global cognitive score was created using all 4 tests described above by averaging the z scores of each test. This method has been shown to minimize problems caused by measurement error on the individual tests.26 However, it does not reflect all aspects of cognition because it is limited by the content of the cognitive test battery.
Covariates.
Sociodemographic variables included age, sex, ethnicity (white, south-Asian, black, other), marital status (married/cohabiting vs others), occupational position (high, intermediate, and low representing income and status at work), and education (less than primary school, lower secondary school, higher secondary school, university, and higher university degree).
Health behaviors were assessed by questionnaire in 1985–1988, 1991–1993, and 1997–1999. Smoking history was defined as current smokers, recent ex-smokers (smoking cessation between 1985–1988 and 1997–1999), long-term ex-smokers (smoking cessation before 1985–1988), and never smokers. The frequency of fruit and vegetable consumption was assessed using the question, “How often do you eat fresh fruit or vegetables?” (responses were on an 8-point scale, ranging from “seldom or never” to “2 or more times a day”). The mean frequency of fruit and vegetable consumption over the 3 time points was used in the analyses. The number of hours of moderate and vigorous physical activity at the 3 time points were averaged to represent physical activity between 1985–1988 and 1997–1999.
Health measures were drawn from 1985–1988, 1991–1993, and 1997–1999 and included cumulative history of hypertension, diabetes, cardiovascular disease, and depressive symptoms. Blood pressure was measured twice with the participant sitting after a 5-minute rest using the Hawksley random-zero sphygmomanometer. The average of 2 readings was taken to be the measured blood pressure. History of hypertension was defined as systolic or diastolic blood pressure ≥140 or ≥90 mm Hg, respectively, or use of antihypertensive drugs. Diabetes was defined by fasting glucose ≥7.0 mmol/L or a 2-hour postload glucose ≥11.1 mmol/L, self-reported doctor-diagnosed diabetes, or use of diabetes medication. Coronary heart disease was based on clinically verified events and included myocardial infarction and definite angina.27 Stroke cases were ascertained from participants' general practitioners, information extracted from hospital medical records by study nurses, or data from the National Health Service Hospital Episode Statistics database obtained after linking the participants' unique National Health Service identification numbers to this national database.28 History of cardiovascular disease included history of coronary heart disease or stroke. History of depressive symptoms was defined as scoring ≥4 on the General Health Questionnaire–Depression subscale or use of antidepressant medication.29
Statistical analysis.
Because drinking patterns differ greatly between men and women, analyses were stratified by sex. To allow comparison between cognitive tests, all cognitive scores were standardized using the mean and SD of cognitive scores in 1997–1999. Linear mixed models30 were used to estimate the association between alcohol consumption and 10-year cognitive decline. These models use all available data over the follow-up, handle differences in length of follow-up, and account for the fact that repeated measures on the same individual are correlated. Both the intercept and slope were fitted as random effects, allowing individual differences in cognitive performance at baseline and rate of cognitive decline. The models were adjusted for the covariates, time since baseline and interaction terms between each covariate and time. First, analyses were adjusted for age, sex, ethnicity, education, occupational position, marital status, and health behaviors and then additionally for health measures. We also examined whether age modified the association of alcohol consumption with cognitive decline by introducing interaction terms between time, alcohol categories, and age (continuous variable).
To characterize the effect size of the association between alcohol consumption and cognitive decline, we compared it with the effect of aging using the following formula: (difference in 10 years cognitive change between the group of interest and the reference group)/(mean cognitive change in the study population over 1 year). Finally, among male drinkers, the association with type of alcoholic beverage (beer, wine, or spirits) consumed was examined in a model adjusted for sociodemographic variables and health behaviors and mutually adjusted for categories of consumption of each individual beverage type. These analyses were based only on those who reported consuming alcohol, and for each beverage type the reference group comprised persons not consuming that type of alcohol, although they consumed other types of alcohol. These analyses could not be undertaken in women because there was little heterogeneity in the type of alcohol consumed; 66% of alcohol consumed was wine.
RESULTS
Of the 10,308 participants at study inception (1985–1988), 7,495 participated in at least 1 of the 3 cognitive assessments. Of these individuals, 7,153 had data on alcohol and other covariates and constituted the analytic sample (figure e-1). The analysis was based on participants similar in age (44.4 vs 44.2 years in 1985–1988, p = 0.10) to those not included in the analysis but comprised more men (70.7% vs 58.6%, p < 0.001) and persons from the higher occupational group (33.3% vs 20.5%, p < 0.001). Among participants included in the analyses, 12.9% contributed 1 wave of cognitive data, 22.4% 2 waves, and 64.7% all 3 waves. Compared with participants with cognitive data at all 3 waves, those with data at 1 and 2 waves had 0.24- and 0.18-SD lower global cognitive score, respectively, in analysis adjusted for age, sex, and occupational position (p < 0.001).
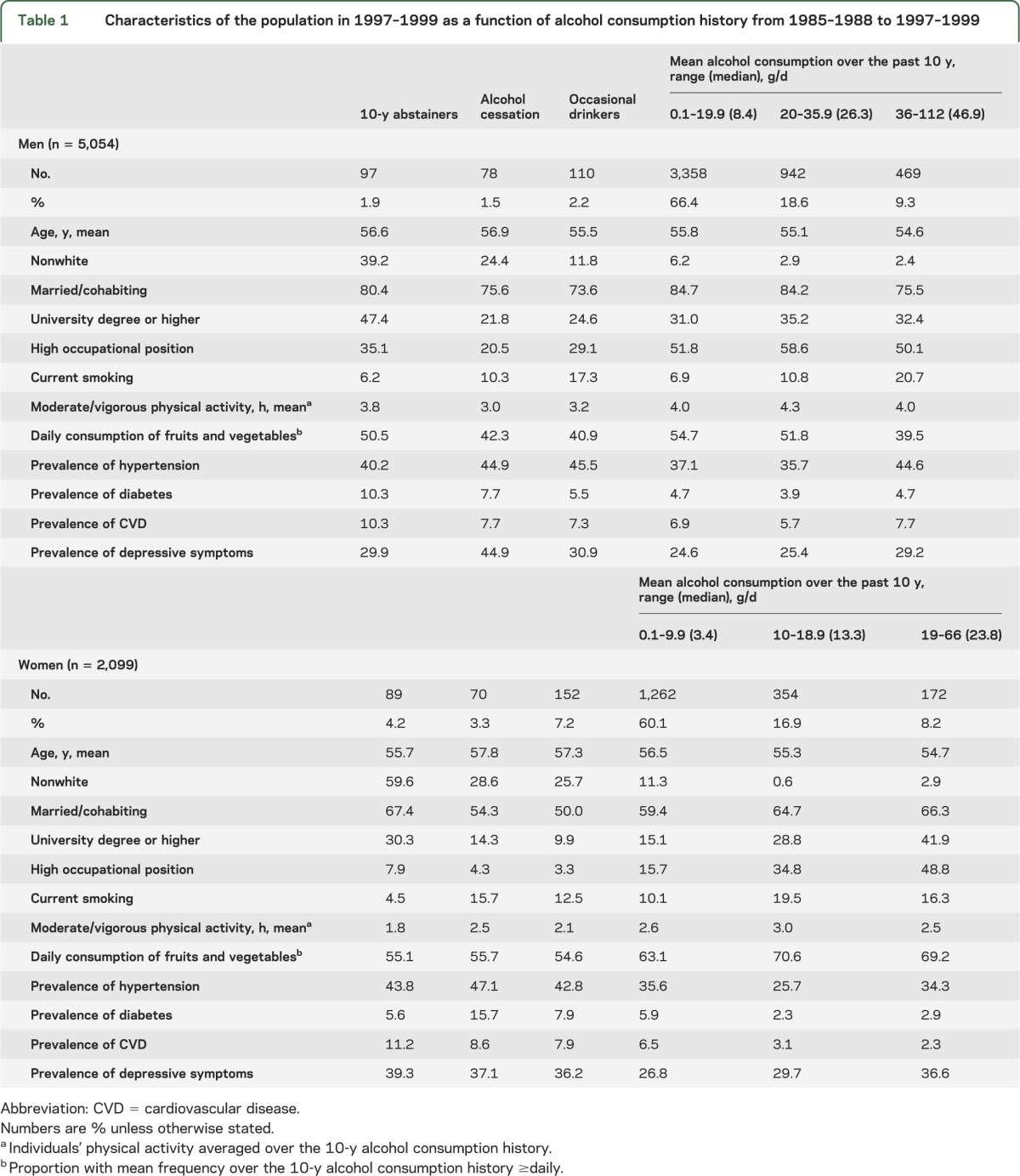
Table 1 shows the characteristics of study participants in 1997–1999 (beginning of cognitive follow-up) as a function of alcohol consumption. The distribution of alcohol consumption differed by sex: women were more likely to be abstainers, quitters, or occasional drinkers (14.7% vs 5.6%), and the quantity of alcohol consumed varied more in men. The frequency of alcohol consumption also differed by the quantity consumed daily (data not shown): in 1985–1988 (in 1997–1999), 95.1% (96.0%) of men drinking ≥36 g/d of alcohol consumed alcohol daily/almost daily compared with 18.7% (38.5%) of those drinking between 0.1 and 19.9 g/d of alcohol. Similarly, 87.4% (92.6%) of women drinking ≥19 g/d of alcohol consumed alcohol daily/almost daily compared with 8.4% (18.8%) of those drinking between 0.1 and 9.9 g/d of alcohol.
Table 1.
Characteristics of the population in 1997–1999 as a function of alcohol consumption history from 1985–1988 to 1997–1999
Mean 10-year cognitive decline in men was −0.42 of the baseline SD (95% confidence interval: −0.44, −0.40) for the global cognitive score, −0.39 (−0.41, −0.37) for executive function, and 0.28 (−0.31, −0.25) for memory. The corresponding numbers for women were −0.39 (−0.42, −0.37) for the global cognitive score, −0.38 (−0.40, −0.35) for executive function, and −0.25 (−0.30, −0.20) for memory.
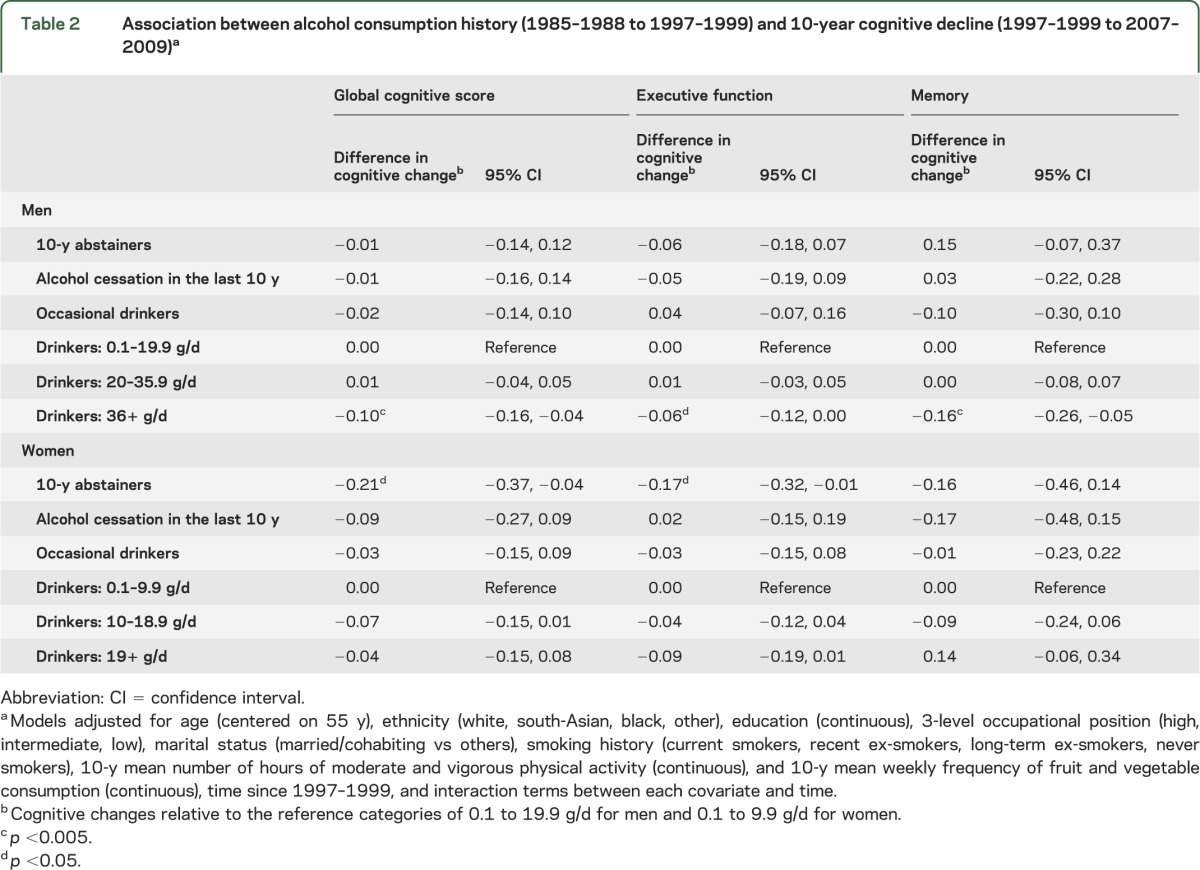
In preliminary analyses, we examined the association between detailed categories of alcohol consumption and 10-year cognitive decline (table e-2). Because no differences in cognitive decline were observed among male drinkers consuming up to 19.9 g/d of alcohol (70th percentile), participants drinking 0.1 to 19.9 g/d were combined and constituted the reference category in the main analyses (table 2). Similarly, in women, alcohol consumption between 0.1 and 9.9 g/d was the reference category. In men, those consuming ≥36 g/d showed faster declines on all cognitive measures compared with those consuming 0.1 to 19.9 g/d (difference [95% confidence interval] in 10-year decline in the global cognitive score was −0.10 [−0.16, −0.04]). The effect size is comparable to 2.4 extra years of cognitive decline in the global cognitive score (2.4 = −0.10/−0.042, 10-year change in the global cognitive score being −0.42 SD in the total male population), 1.5 extra years for executive function, and 5.7 extra years for memory. In women, compared with those consuming 0.1 to 9.9 g/d, the 10-year abstainers experienced faster decline in the global cognitive score and executive function corresponding to approximately 5.0 extra years of cognitive decline. Women reporting alcohol consumption ≥19 g/d also showed faster decline in executive function corresponding to 2.4 extra years of decline in this cognitive domain; however, the association did not reach statistical significance (p = 0.09). After further adjustment for health measures, the associations were slightly attenuated (table e-3). No age differences were found in the association between alcohol consumption and cognitive decline in men (p values range = 0.15–0.32) or women (p values range = 0.35–0.91).
Table 2.
Association between alcohol consumption history (1985–1988 to 1997–1999) and 10-year cognitive decline (1997–1999 to 2007–2009)a
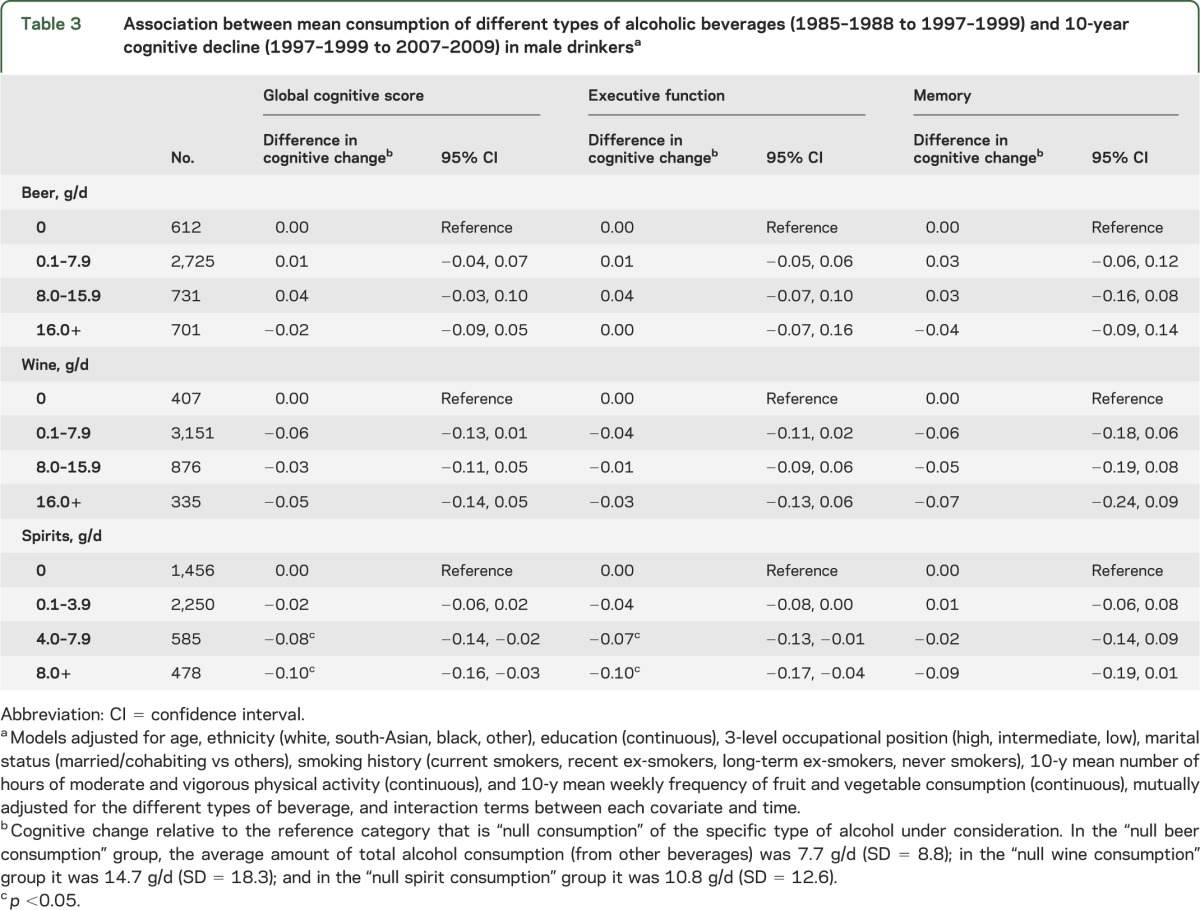
We examined the association between type of alcohol consumed and cognitive decline in male drinkers (table 3). Higher consumption of spirits was found to be associated with faster cognitive decline. No clear association was found in relation to beer or wine. In models adjusted for each type of alcohol, the association of total alcohol consumption with cognitive decline remained evident suggesting that one type of alcohol did not drive the association between total alcohol consumed and cognitive decline (data not shown).
Table 3.
Association between mean consumption of different types of alcoholic beverages (1985–1988 to 1997–1999) and 10-year cognitive decline (1997–1999 to 2007–2009) in male drinkersa
DISCUSSION
Our study indicates that men who consumed ≥36 g/d of alcohol experienced faster 10-year decline in all cognitive domains, with an effect size comparable to 1.5 to 5.7 extra years of cognitive decline. An important limitation of previous studies of the association between alcohol consumption and cognitive function is their cross-sectional nature, whereby reverse causation is a major concern leading to difficulties in interpretation of the results.2,6–8,31 In addition, cross-sectional analyses are susceptible to cohort effects and residual confounding, for example by socioeconomic factors. A study using mendelian randomization in a Chinese male cohort (N = 4,707) showed no evidence of higher cognitive scores in moderate drinkers,32 suggesting that the hypothesized protective effect of moderate alcohol consumption might be attributable to confounding by unmeasured factors or reverse causation. However, that study was underpowered to detect small effects of moderate alcohol consumption. Our study, with the focus on cognitive decline, adds to this literature as it is unclear whether alcohol consumption influences cognitive aging trajectories.
Much of the evidence on alcohol consumption and cognitive decline derives from studies based on elderly populations,10–17 where individuals need to have survived long enough to participate in the study and may have reduced their alcohol consumption because of health concerns.2,31 Some studies suggest moderate alcohol consumption to be associated with slower cognitive decline10,11,13,15–17 compared with abstinence whereas others found no association between alcohol consumption and cognitive decline.12,14 In many studies, the effect of heavy drinking was difficult to assess because of the small number of heavy drinkers.10–17 Our study based on middle-aged participants suggests that heavy drinking is associated with faster decline in all cognitive domains in men. In women, there was only weak evidence that heavy drinking was associated with a faster decline in executive function, but abstinence from alcohol was associated with faster decline in the global cognitive score and executive function. However, the number of abstainers was small, and this category is likely to represent a group of selected individuals whose characteristics differ from other participants; for instance, a considerably higher proportion of these women were nonwhite (59.6%) compared with other women (10.5%). Although analyses were adjusted for a range of covariates, residual confounding may be an issue and estimates for this category ought to be interpreted with caution.
The effect of type of alcohol on cognitive outcomes has been studied in relation to dementia; persons drinking wine have been found to be at lower risk of dementia,4,5,9 although results are not consistent.6,8 In the present study, we found a dose-response association between consumption of spirits and cognitive decline in men whereas no clear association was found with other alcoholic beverages. Nevertheless, the association with total alcohol consumption was not driven by a single type of alcohol. Our results on the type of alcohol are not comparable to those on total alcohol because the effects observed for total alcohol consumption are at ≥36 g/d and the number of participants drinking this quantity of a given type of alcohol is small in our study.
The mechanisms underlying the association between alcohol consumption and cognition are complex. The main hypothesis focuses on cerebro- and cardiovascular pathways,33 involving effects that play out over an extended period of time. Light to moderate alcohol consumption is associated with better vascular outcomes,34 while both abstinence and heavy alcohol consumption are associated with higher risk of vascular disease,35 which, in turn, may increase the risk of cognitive impairment.36 Furthermore, heavy alcohol consumption has detrimental short- and long-term effects on the brain,7,37 including direct neurotoxic effect,7 proinflammatory effects,7,38 and indirect impact via cerebrovascular disease35 and vitamin deficiency.39
Our study has limitations. First, because alcohol consumption was self-reported, some participants may have underestimated their consumption.40 Second, we were unable to distinguish regular heavy drinking from binge drinking because we did not have data on the number of drinks consumed in a single occasion. However, most of the participants classified in the high alcohol consumption category consumed alcohol daily or almost daily suggesting that binge drinking was not frequent in this population and is therefore unlikely to be the sole explanation for the associations we observed. Third, in women, heavy alcohol consumption was half that in men and the social pattern was different. For example, female heavy drinkers were more likely to have a higher occupational position, while this was not true in men. Thus, the interpretation of sex differences in our study is not straightforward and further research is needed to test whether the effect of alcohol on cognition differs by sex. Fourth, the cognitive test battery was not exhaustive, particularly in relation to assessment of long-term memory. Furthermore, it is possible that more specific tests of executive function would have yielded stronger associations with alcohol consumption. Fifth, missing data were higher in those with lower cognitive scores, suggesting that our results might, if anything, be underestimates of the true associations. Finally, although the sample covered a wide socioeconomic range, with salaries ranging from £4,995 to £150,000/year (US $7,824–$234,954), data are from white-collar civil servants, not fully representative of the general population, particularly the unemployed or blue-collar workers.
This study suggests that men consuming 36 g/d or more of alcohol in midlife were more likely to experience faster 10-year cognitive decline in all cognitive domains; in women, there was weaker evidence of this effect occurring at ≥19 g/d, but only for executive function. Our findings are in agreement with previous studies showing that moderate alcohol consumption is probably not deleterious for cognitive outcomes, but they also show that heavy alcohol consumption in midlife is likely to be harmful for cognitive aging, at least in men.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. The Whitehall II study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants, and data entry staff, who make the study possible.
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
S.S. wrote the first draft of the manuscript and performed the statistical analysis. All authors contributed to interpretation of results and revisions of the manuscript.
STUDY FUNDING
The Whitehall II study is supported by the British Medical Research Council (K013351), British Heart Foundation; National Heart, Lung, and Blood Institute (R01HL036310); and US NIH National Institute on Aging (R01AG013196; R01AG034454).
DISCLOSURE
S. Sabia reports no disclosures. A. Elbaz has received funding from the French Agence nationale de la recherche (ANR, 2009–2012). A. Britton is currently funded by the European Research Council (309337). S. Bell was supported by a UK Economic and Social Research Council PhD studentship and is currently funded by the European Research Council (309337). A. Dugravot reports no disclosures. M. Shipley is supported by the British Heart Foundation. M. Kivimaki is supported by the Academy of Finland, the UK Medical Research Council (K013351), the US NIH (R01HL036310, R01AG034454), and by a professorial fellowship from the Economic and Social Research Council. A. Singh-Manoux is supported by the National Institute on Aging, NIH (R01AG013196, R01AG034454). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373:2223–2233 [DOI] [PubMed] [Google Scholar]
- 2.Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry 2009;17:542–555 [DOI] [PubMed] [Google Scholar]
- 3.Elias PK, Elias MF, D'Agostino RB, Silbershatz H, Wolf PA. Alcohol consumption and cognitive performance in the Framingham Heart Study. Am J Epidemiol 1999;150:580–589 [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc 2004;52:540–546 [DOI] [PubMed] [Google Scholar]
- 5.Mehlig K, Skoog I, Guo X, et al. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in Goteborg. Am J Epidemiol 2008;167:684–691 [DOI] [PubMed] [Google Scholar]
- 6.Neafsey EJ, Collins MA. Moderate alcohol consumption and cognitive risk. Neuropsychiatr Dis Treat 2011;7:465–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panza F, Frisardi V, Seripa D, et al. Alcohol consumption in mild cognitive impairment and dementia: harmful or neuroprotective? Int J Geriatr Psychiatry 2012;27:1218–1238 [DOI] [PubMed] [Google Scholar]
- 8.Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing 2008;37:505–512 [DOI] [PubMed] [Google Scholar]
- 9.Truelsen T, Thudium D, Gronbaek M. Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology 2002;59:1313–1319 [DOI] [PubMed] [Google Scholar]
- 10.Espeland MA, Gu L, Masaki KH, et al. Association between reported alcohol intake and cognition: results from the Women's Health Initiative Memory Study. Am J Epidemiol 2005;161:228–238 [DOI] [PubMed] [Google Scholar]
- 11.Ganguli M, Vander BJ, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology 2005;65:1210–1217 [DOI] [PubMed] [Google Scholar]
- 12.Lobo E, Dufouil C, Marcos G, et al. Is there an association between low-to-moderate alcohol consumption and risk of cognitive decline? Am J Epidemiol 2010;172:708–716 [DOI] [PubMed] [Google Scholar]
- 13.Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med 2005;352:245–253 [DOI] [PubMed] [Google Scholar]
- 14.Stott DJ, Falconer A, Kerr GD, et al. Does low to moderate alcohol intake protect against cognitive decline in older people? J Am Geriatr Soc 2008;56:2217–2224 [DOI] [PubMed] [Google Scholar]
- 15.Wright CB, Elkind MS, Luo X, Paik MC, Sacco RL. Reported alcohol consumption and cognitive decline: the Northern Manhattan Study. Neuroepidemiology 2006;27:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes DE, Cauley JA, Lui LY, et al. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc 2007;55:259–264 [DOI] [PubMed] [Google Scholar]
- 17.Bond GE, Burr RL, McCurry SM, Rice MM, Borenstein AR, Larson EB. Alcohol and cognitive performance: a longitudinal study of older Japanese Americans. The Kame Project. Int Psychogeriatr 2005;17:653–668 [DOI] [PubMed] [Google Scholar]
- 18.Lifestyle Statistics HaSCIC. Statistics on alcohol: England, 2013. Available at: http://www.hscic.gov.uk/article/2021/Website-Search?productid=11719&q=alcohol+statistics&sort=Relevance&size=10&page=1&area=both#top. Accessed September 3, 2013 [Google Scholar]
- 19.Richards M, Hardy R, Wadsworth ME. Alcohol consumption and midlife cognitive change in the British 1946 birth cohort study. Alcohol Alcohol 2005;40:112–117 [DOI] [PubMed] [Google Scholar]
- 20.Zanjani F, Downer BG, Kruger TM, Willis SL, Schaie KW. Alcohol effects on cognitive change in middle-aged and older adults. Aging Ment Health 2013;17:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251–256 [DOI] [PubMed] [Google Scholar]
- 22.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott R. Executive functions and their disorders. Br Med Bull 2003;65:49–59 [DOI] [PubMed] [Google Scholar]
- 24.Heim AW. AH 4 Group Test of General Intelligence. Windsor, UK: NFER-Nelson Publishing Company Ltd.; 1970 [Google Scholar]
- 25.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologica 1967;5:135–140 [Google Scholar]
- 26.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology 2010;75:1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrie JE, Langenberg C, Shipley MJ, Marmot MG. Birth weight, components of height and coronary heart disease: evidence from the Whitehall II study. Int J Epidemiol 2006;35:1532–1542 [DOI] [PubMed] [Google Scholar]
- 28.Britton A, Milne B, Butler T, et al. Validating self-reported strokes in a longitudinal UK cohort study (Whitehall II): extracting information from hospital medical records versus the Hospital Episode Statistics database. BMC Med Res Methodol 2012;12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson R, Deary IJ, Shipley B. A hierarchy of distress: Mokken scaling of the GHQ-30. Psychol Med 2008;38:575–579 [DOI] [PubMed] [Google Scholar]
- 30.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–974 [PubMed] [Google Scholar]
- 31.Anstey KJ. Alcohol exposure and cognitive development: an example of why we need a contextualized, dynamic life course approach to cognitive ageing—a mini-review. Gerontology 2008;54:283–291 [DOI] [PubMed] [Google Scholar]
- 32.Au Yeung SL, Jiang CQ, Cheng KK, et al. Evaluation of moderate alcohol use and cognitive function among men using a Mendelian randomization design in the Guangzhou Biobank Cohort Study. Am J Epidemiol 2012;175:1021–1028 [DOI] [PubMed] [Google Scholar]
- 33.Collins MA, Neafsey EJ, Mukamal KJ, et al. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res 2009;33:206–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA 2003;289:579–588 [DOI] [PubMed] [Google Scholar]
- 36.Ivan CS, Seshadri S, Beiser A, et al. Dementia after stroke: the Framingham Study. Stroke 2004;35:1264–1268 [DOI] [PubMed] [Google Scholar]
- 37.Zhu W, Volkow ND, Ma Y, Fowler JS, Wang GJ. Relationship between ethanol-induced changes in brain regional metabolism and its motor, behavioural and cognitive effects. Alcohol Alcohol 2004;39:53–58 [DOI] [PubMed] [Google Scholar]
- 38.Imhof A, Woodward M, Doering A, et al. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille). Eur Heart J 2004;25:2092–2100 [DOI] [PubMed] [Google Scholar]
- 39.Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol 2011;7:284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilsnack SC, Wilsnack RW. International gender and alcohol research: recent findings and future directions. Alcohol Res Health 2002;26:245–250 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


