Abstract
Objective:
To describe the frequency of mild cognitive impairment (MCI) in Parkinson disease (PD) in a cohort of newly diagnosed incident PD cases and the associations with a panel of biomarkers.
Methods:
Between June 2009 and December 2011, 219 subjects with PD and 99 age-matched controls participated in clinical and neuropsychological assessments as part of a longitudinal observational study. Consenting individuals underwent structural MRI, lumbar puncture, and genotyping for common variants of COMT, MAPT, SNCA, BuChE, EGF, and APOE. PD-MCI was defined with reference to the new Movement Disorder Society criteria.
Results:
The frequency of PD-MCI was 42.5% using level 2 criteria at 1.5 SDs below normative values. Memory impairment was the most common domain affected, with 15.1% impaired at 1.5 SDs. Depression scores were significantly higher in those with PD-MCI than the cognitively normal PD group. A significant correlation was found between visual Pattern Recognition Memory and cerebrospinal β-amyloid 1–42 levels (β standardized coefficient = 0.350; p = 0.008) after controlling for age and education in a linear regression model, with lower β-amyloid 1–42 and 1–40 levels observed in those with PD-MCI. Voxel-based morphometry did not reveal any areas of significant gray matter loss in participants with PD-MCI compared with controls, and no specific genotype was associated with PD-MCI at the 1.5-SD threshold.
Conclusions:
In a large cohort of newly diagnosed PD participants, PD-MCI is common and significantly correlates with lower cerebrospinal β-amyloid 1–42 and 1–40 levels. Future longitudinal studies should enable us to determine those measures predictive of cognitive decline.
Dementia is a frequent and distressing complication of Parkinson disease (PD), with a cumulative incidence approaching 80% in some community studies.1 Clinical features associated with cognitive decline include advanced age and motor subtype, along with specific genetic associations.2–4 Mild cognitive impairment (MCI) in PD has received increased attention in recent years, including formulation of new diagnostic criteria,5 and PD-MCI at baseline assessment has also been associated with increased risk of PD dementia (PDD).4,6,7 Several biomarkers have been proposed to be predictive of cognitive impairment, including a higher burden of Alzheimer disease (AD)-like atrophy on structural MRI8 and lower CSF β-amyloid 1–42 (Aβ42) levels.9 Because of the complexity of the pathophysiologic processes, it is unlikely that a single biomarker will predict PDD, but, taken together, these laboratory, imaging, and clinical risk factors may allow clinicians to predict which patients are most likely to progress to this state.
The Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation–PD (ICICLE-PD) is a twin center longitudinal observational study, the aim of which is to better understand the mechanisms underlying the evolution of PDD from disease onset. Herein, we describe the baseline data from our cohort, focusing on their cognitive profile and the clinical, imaging, and biochemical correlates of PD-MCI. Our hypotheses included that cognitive deficits would be frequent and that they would be associated with lower CSF Aβ42 and elevated tau levels. We also hypothesized that there would be minimal gray matter (GM) atrophy on MRI in those with PD-MCI.
METHODS
Participants.
Between June 2009 and December 2011, patients newly diagnosed with PD from community and outpatient clinics in Newcastle upon Tyne/Gateshead and Cambridgeshire, UK were invited to participate. Written requests for notification of patient details were sought from all general practitioners, neurologists, geriatricians, and PD specialist nurses in each area. PD was diagnosed by a movement disorder specialist according to the UK Brain Bank criteria10 (for details, see e-Methods on the Neurology® Web site at www.neurology.org).
To control for the effects of normal aging and to generate normative values for cognitive tests, unrelated controls of similar age and sex to patients were recruited from community sources. All control subjects underwent clinical and neuropsychological testing, and were given the option of participating in laboratory and MRI studies. None were screened for preexisting cognitive deficits.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Newcastle and North Tyneside Research Ethics Committee and performed according to the Declaration of Helsinki. All subjects provided written informed consent.
Clinical assessment.
Clinical and demographic data were recorded including disease duration, level of education, medication, and family history. Clinical assessments were performed by trained examiners, and included a standardized neurologic examination, the Movement Disorder Society (MDS)-revised Unified Parkinson's Disease Rating Scale11 with Hoehn and Yahr stage,12 and the Geriatric Depression Scale-15 (GDS-15) score.13
Neuropsychological assessment.
Global cognitive function was assessed using the Mini-Mental State Examination (MMSE)14 and Montreal Cognitive Assessment (MoCA).15 Participants were assessed “on” dopaminergic medication. Five cognitive domains were assessed. Attention was measured using the Cognitive Drug Research computerized battery.16 Mean response times of simple reaction time, choice reaction time, and digit vigilance were summed to produce a Power of Attention score.16 Digit vigilance accuracy was also evaluated as part of this domain. Memory was assessed with Pattern Recognition Memory (PRM), Spatial Recognition Memory (SRM), and Paired Associates Learning (PAL) from the computerized Cambridge Neuropsychological Test Automated Battery (CANTAB) battery.17 Executive function was determined using the modified (“one touch stockings”) version of the Tower of London task from the CANTAB battery, phonemic fluency (words beginning with “F” in 1 minute),18 and semantic fluency (animals in 90 seconds).19 The pentagon copying item of the MMSE was graded using a modified 0 to 2 rating scale,20 as a measure of visuospatial function. Language domain was assessed using the naming (0–3) and sentence (0–2) subsets of the MoCA test.
PD-MCI was then determined using the recently published MDS criteria.5 Subjects were classified as MCI if they were impaired on 2 tests in one cognitive domain or one impaired test in 2 different domains. Modified level 2 criteria were used, as our neuropsychological battery predated the publication of the PD-MCI criteria; e.g., only one test was specific for the visuospatial domain. Subjects were further classified by domain if impaired in more than one test within that domain. In keeping with previous studies6,21,22 and recent recommendations23 and to reduce the risk of false-positive results caused by multiple testing, level 2 MCI at 1.5 SDs below normative means was used to compare laboratory and imaging data.
MRI acquisition and data preprocessing.
A common MRI protocol was adopted for both sites. Structural images were acquired with a T1-weighted sequence covering the whole brain. Newcastle subjects were scanned using a 3T Philips Intera Achieva scanner with a magnetization-prepared rapid gradient echo sequence (MPRAGE), sagittal acquisition, echo time 4.6 milliseconds, repetition time 9.6 milliseconds, inversion time 1,250 milliseconds, flip angle = 8°, SENSE factor = 2, in-plane field of view 240 × 240 mm with slice thickness 1.2 mm, yielding a voxel size of 1.15 × 1.15 mm. In Cambridge, a 3T Siemens Trio scanner was used with MPRAGE, sagittal acquisition, echo time 2.98 milliseconds, repetition time 2,250 milliseconds, inversion time 900 milliseconds, flip angle = 9°, GRAPPA factor = 2, in-plane field of view 256 × 256 mm with slice thickness 1.0 mm yielding a voxel size of 1.0 × 1.0 mm.
Images were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running in MATLAB 7.14 (MathWorks, Natick, MA). T1-weighted images were segmented into GM, white matter, and CSF using the standard segmentation sequence in SPM. A GM template was created from all subjects using an implementation of a diffeomorphic registration algorithm (DARTEL) included in SPM. GM segments were spatially normalized and warped in DARTEL and transformed to Montreal Neurological Institute space (http://www.mni.mcgill.ca). A separate modulation step was performed to maintain the total amount of GM in each voxel to ensure it was not altered by the normalization procedure. Images were smoothed with an 8-mm, full-width, half-maximum Gaussian kernel. The smoothed, modulated, normalized GM datasets were used for statistical analysis. Total intracranial volume was calculated by summing the total tissue probability of GM, white matter, and CSF.
CSF studies.
Lumbar puncture was performed on a subset of participants between 8 and 10 am after an overnight fast and while withholding PD medications. Samples free of visual contamination by blood were centrifuged (2,000g, 4°C, 10 minutes) within 15 minutes of collection and frozen at −80°C in polypropylene cryovials until analyzed for Aβ42, β-amyloid 1–40 (Aβ40), total tau (T-tau), tau phosphorylated at amino acid 181 (P181-tau), and total α-synuclein (αsyn) (e-Methods).
Genotyping.
DNA was extracted from peripheral blood using standard phenol/chloroform techniques. Genotyping for rs4680 (COMT Val158Met), rs356219 (SNCA), rs1803274 (BuChE), rs9468 (MAPT), rs121434567 (EGF), and rs429358 plus rs7412 (APOE genotype 2-4) was performed using Kompetitive Allele Specific PCR (LGC Genomics, Middlesex, UK).
Statistical analysis.
Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL). Data were examined for normality with visual histograms and the Kolmogorov-Smirnov test. Means were compared using unpaired t tests or analysis of variance for normally distributed data, and Mann-Whitney or Kruskal-Wallis test for non-normally distributed data. Pearson correlation or Spearman rank correlation coefficients were calculated to assess bivariate associations between cognitive, clinical, or biochemical parameters. Linear regression (continuous dependent variables) and logistic regression (ordinal dependent variables) models were used to control for covariates including age and education. Model fit was checked by looking at residuals. Effect sizes were calculated using Glass's Δ. Pearson χ2 tests were used to compare between-group distribution of proportions.
RESULTS
Frequency and profile of MCI.
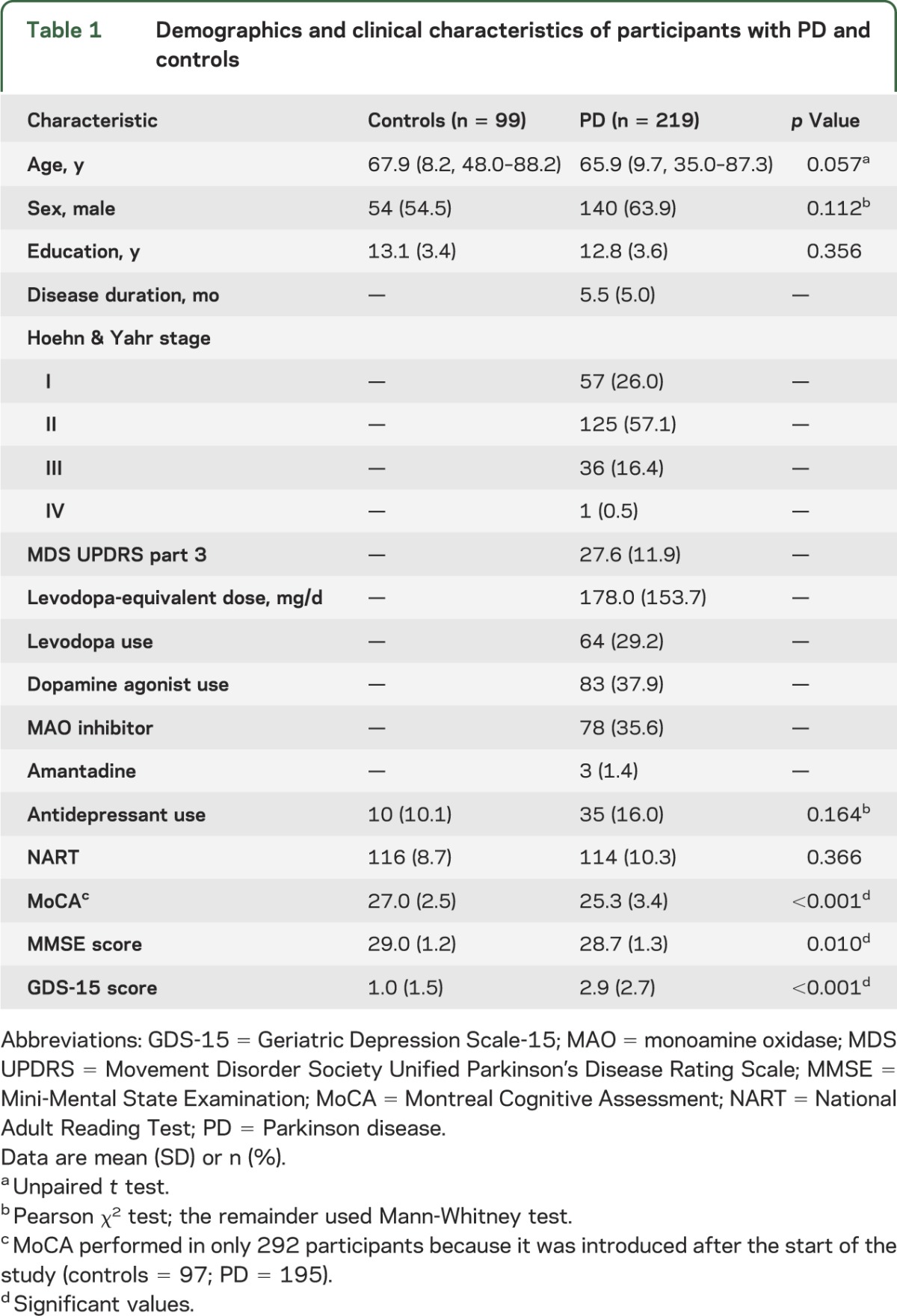
Six hundred eighty-two patients with parkinsonism were approached, and, of these, 226 with idiopathic PD consented. Seven patients were subsequently excluded; therefore, 219 subjects with PD and 99 controls participated (figure 1). Patients who declined (n = 312) were significantly older than those who participated (71.5 vs 65.9 years, p < 0.001, t test). PD participants scored significantly lower on the MoCA and MMSE and higher on the GDS-15 than the controls, but there were no differences in age and years of education (table 1). Control participants tended to be older, and although this did not reach statistical significance, age was included as a covariate in all analyses. Cognitive test scores on all domains, apart from language, were significantly lower in PD than control participants, even after controlling for age (table e-1), with the greatest effect size being seen for tests of attention and executive function.
Figure 1. Flow diagram of participants and assessments.
ICICLE-PD = Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation–Parkinson Disease; VBM = voxel-based morphometry.
Table 1.
Demographics and clinical characteristics of participants with PD and controls
Among the 5 cognitive domains, memory impairment was the most common domain affected in PD participants at 1.5 SDs below normative values (15.1%). This was followed by visuospatial dysfunction in 13.2% of participants, attention/working memory impairment in 12.3%, and executive dysfunction in 11% at 1.5 SDs (figure 2). Only 5.0% of participants were impaired in the language domain at 1 SD below the control mean. PD-MCI criteria were met by 65.8% of PD participants at 1 SD below normative values, 42.5% at 1.5 SDs, and 22.4% at 2 SDs. Participants who met level 2 criteria were older, completed fewer years of education, had greater motor disability, and scored higher on the GDS than those who were cognitively normal (table 2).
Figure 2. Graphical representation of cognitive subtypes of MCI impaired according to single cognitive domain.

(A) Participants with Parkinson disease (PD). (B) Control group. MCI = mild cognitive impairment.
Table 2.
Cognitive profiles of patients with PD according to MCI criteria

Voxel-based morphometric intergroup comparisons.
One hundred forty-eight participants with PD consented to MRI, and scans were performed on 50 controls. Analysis was performed in SPM8 using the general linear model, with covariates of age, scanner location, and total intracranial volume. Using a voxelwise threshold of p = 0.001 (uncorrected), there were no clusters of significantly reduced GM in the patients with PD-MCI (1.5 SDs) compared with those who were cognitively normal or controls. In the PD-MCI group who were 2 SDs below control means, there was a small (1,376 voxels) cluster of reduced GM in the left superior temporal gyrus (Montreal Neurological Institute coordinates −51, −16, +9) (p = 0.012 familywise error corrected).
CSF and MCI.
Sixty-seven participants with PD consented to lumbar puncture. No samples were discarded because of blood contamination. There were significant correlations between age and T-tau and P181-tau (r = 0.412 and r = 0.466, respectively, p < 0.001, Spearman ρ), age and Aβ40 (r = 0.315, p = 0.009, Pearson coefficient), and age and αsyn (r = 0.278, p = 0.023, Spearman ρ). General characteristics and CSF values are shown in table 3.
Table 3.
CSF profile according to cognitive status
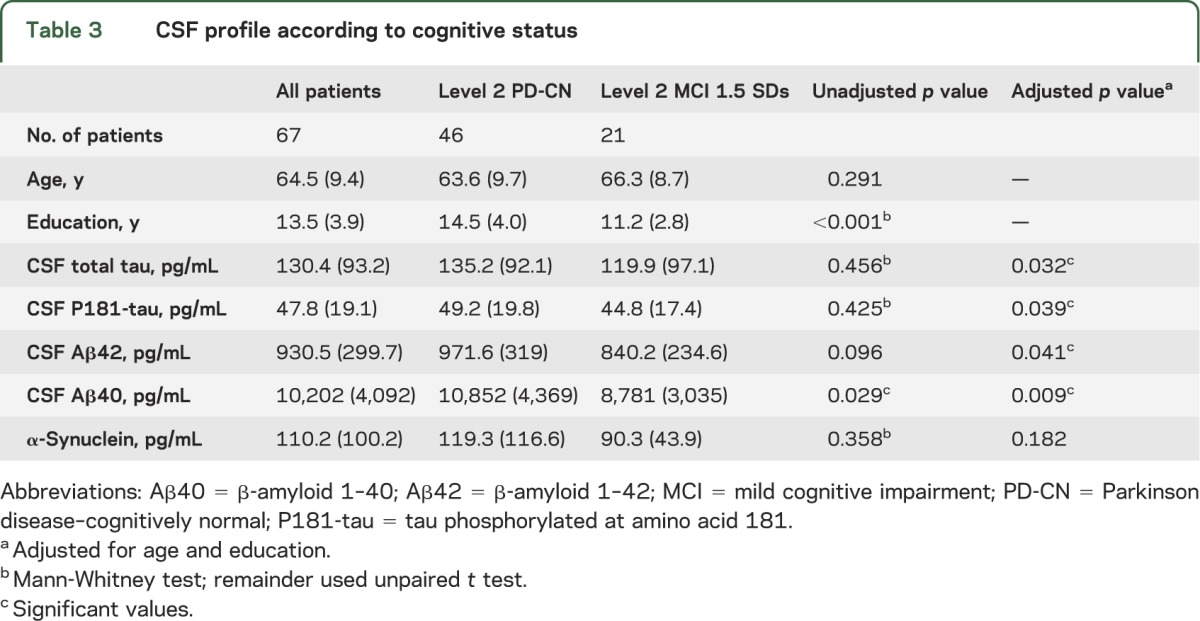
A significant correlation was found between performance on visual PRM and Aβ42, which remained significant after controlling for age and education in a linear regression model (β standardized coefficient = 0.350, p = 0.008). MoCA score and Aβ42 were significantly correlated after controlling for the above variables (β standardized coefficient = 0.282, p = 0.041). Other neuropsychological tests were not correlated with Aβ42 levels. When participants were dichotomized into those who met the criteria for level 2 MCI at 1.5 SDs (table 3), there were significant differences in mean Aβ42 and Aβ40 levels after controlling for age and education in a logistic regression model (B = −2.28 × 10−3, p = 0.041; B = −3.10 × 10−4, p = 0.009, respectively). CSF T-tau and P181-tau showed no significant difference initially, but after controlling for the aforementioned variables, were significantly lower in those with level 2 MCI (B = −0.009, p = 0.032; B = −0.042, p = 0.039, respectively). Combinations of biomarkers (Aβ42/α-syn; Aβ40/α-syn; T-tau/α-syn; P181-tau/α-syn; Aβ42/T-tau) were also evaluated against cognitive scores, but there was no significant difference in those with level 2 MCI at 1.5 SDs and these ratios did not correlate with performance on neuropsychological tests.
Genotyping.
Two hundred four participants with PD and 95 controls consented to genotyping. At level 2 MCI 1.5 SDs below normative values, there were no differences in the proportions of genotypes within that group compared with participants with PD who were cognitively normal.
DISCUSSION
We have shown that in a large cohort of participants newly diagnosed with PD, cognitive impairment is common, with 42.5% meeting level 2 MDS PD-MCI criteria at 1.5 SDs below normative means. The clinical profile of these subjects differed from those with normal cognition, being significantly older, with greater motor impairment and depression. Applying the new PD-MCI criteria to an incident PD cohort, we have used a multimodal approach to profile such cases. We have shown that Aβ42 and Aβ40 levels are significantly lower in those with PD-MCI, and therefore may serve as a useful biomarker of cognitive decline if shown to be predictive during planned longitudinal assessment.
Previous studies of patients with early or untreated PD have shown frequencies of PD-MCI between 14.8% and 36%.24–26 Two factors may explain the higher prevalence of PD-MCI in our study: first, adoption of the new MDS PD-MCI criteria, which may be less conservative than other definitions of MCI, and second, the recruitment of a largely community-based cohort of patients (as opposed to secondary or tertiary care). In addition, by using 2 computerized test systems, we may have increased the precision of measuring cognition. Similar to other studies, however, we found that memory impairment was frequently affected.21,25 Prominent frontal/executive deficits have been found in other work in PD-MCI,22,26 and there is evidence that these deficits may affect other neuropsychological domains. For example, executive deficits may explain a significant proportion of the variance in memory impairments.27
Although the precise pathophysiologic substrates underlying cognitive impairment in PD remain unclear and may vary among subjects, there is increasing evidence for a synergistic role between αsyn and Aβ accumulation.28 Tau may also contribute, because it has been shown that polymorphisms in the MAPT gene increase the risk of PDD.4 Furthermore, cholinergic dysfunction is likely to influence cognitive decline, with evidence from autopsy studies of a greater cholinergic deficit in subjects with dementia with Lewy bodies (DLB) than in AD.29 Determining the factors that influence the progression of cognitive decline in PD, and the individuals at risk of ultimately developing PDD, is critical for targeted intervention and drug discovery studies.
In keeping with our hypotheses, and in line with previous studies, CSF Aβ42 and Aβ40 levels were significantly lower in those with impaired cognition.30–32 Aβ42 correlated with both global cognition and PRM, the latter being sensitive to deficits of temporal lobe function. Although the pathophysiology of PD-MCI is poorly understood, it is most likely the result of a complex interaction among neurotransmitter dysfunction, synaptic pathology, protein aggregation, and neuronal damage. This reduction in Aβ42 in those with PD-MCI, even at this early stage of disease, suggests that these patients may have alterations in Aβ metabolism and a higher burden of temporal lobe Alzheimer-type pathology, a finding strengthened by the correlation with PRM on neuropsychological testing.
Contrary to our hypotheses, we found a trend toward decreased T-tau and P181-tau in subjects with PD-MCI, consistent with some31 but not all30,33 previous studies, and in contrast to findings in AD. Indeed, there is evidence that tau levels are higher in AD and DLB than PDD.34 Our findings are consistent with an autopsy study in PD-MCI that demonstrated little neurofibrillary tangle deposition and indicates that the findings are not just attributable to coexistent AD.35 αsyn did not correlate with cognitive scores in this study. Previous work has demonstrated reduced αsyn in PD, but to date there has been little investigation of αsyn and cognition, although one small study in DLB found that lower αsyn levels correlated with global cognition and verbal fluency.36 It should be noted, however, that only total (not oligomeric) αsyn was measured in our study; the latter may represent a more toxic species in PD.37
Genotyping did not add to the prediction of PD-MCI defined with a 1.5-SD threshold. Previous studies have demonstrated that certain genotypes predict cognitive decline during longitudinal assessment,4,38 but were not associated with differences at baseline.38 It is possible therefore that genetic influences will become manifest over time, with baseline genotype predicting the trajectory of cognitive decline.
In the voxel-based morphometric analyses, limited GM loss in the left superior temporal gyrus was seen in only the most severely impaired MCI category. Atrophy in PD-MCI has been observed by others but these studies have generally included patients with longer disease duration.39 The absence of significant GM loss is consistent with previous work in early PD that adopted voxel-based morphometry protocols40 and also with the presumed topographic spread of PD, suggesting that at least some of the early cognitive deficits observed may be the result of functional neurotransmitter imbalance rather than structural GM loss.
The principal strengths of this hypothesis-driven prospective study are its multimodal design and recruitment of a large cohort with early disease. Through the longer-term follow-up of cases over time, we will determine whether those currently classified as PD-MCI at baseline are at increased risk of PDD, and the subtypes that ultimately predict cognitive decline to this state. Previous cross-sectional studies of MCI have been limited by small sample sizes and disease heterogeneity, along with limited follow-up and absence of a matched control group.
Limitations of our study include the fact that not all of our participants were treatment-naive. Furthermore, a significant proportion of patients declined to participate and these individuals tended to be older. Nonetheless, our multisource recruitment strategy and inclusion of community, geriatric, and neurology clinics as sources of referral make the results relevant to understand the nature of PD in the community. Regarding cognitive measures, attention, memory, and executive function were well covered, but testing of visuospatial function and language was more limited, which may explain the low frequencies of impairment observed in these domains. However, the inability to copy pentagons has been shown to be a significant predictor of dementia,4 and there is evidence that language function is less likely to be affected in early cognitive dysfunction in PD.22 In addition, the memory tasks utilized in this study were largely dependent on visual cues and thus executive, visuospatial, and/or attention dysfunction may have had an impact on memory performance, which may alter the interpretation of domain impairment. The inclusion of additional tests such as a word list or story recall task would be beneficial in future studies. Lastly, our study did not include CSF examination in controls.
This study has shown that MCI is more common than previously reported in patients with newly diagnosed PD. In addition, we have shown that this is correlated with lower Aβ42 and Aβ40 levels, and in particular Aβ42 correlated with memory function. The longitudinal assessment of this cohort will now allow us to determine which clinical, laboratory, and imaging measures best predict those who will ultimately develop PDD.
Supplementary Material
GLOSSARY
- Aβ40
β-amyloid 1–40
- Aβ42
β-amyloid 1–42
- AD
Alzheimer disease
- αsyn
α-synuclein
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- DARTEL
diffeomorphic registration algorithm
- DLB
dementia with Lewy bodies
- GDS-15
Geriatric Depression Scale-15
- GM
gray matter
- ICICLE-PD
Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation–Parkinson Disease
- MCI
mild cognitive impairment
- MDS
Movement Disorder Society
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- MPRAGE
magnetization-prepared rapid gradient echo sequence
- PD
Parkinson disease
- PDD
Parkinson disease dementia
- P181-tau
tau phosphorylated at amino acid 181
- PRM
Pattern Recognition Memory
- T-tau
total tau
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Yarnall, Dr. Breen, and Dr. Duncan were involved with coordination of the study. They were also involved with participant recruitment, clinical assessment, data collection, and statistical analysis, and they drafted the manuscript. Dr. Khoo was involved with the study design and coordination of the study. He was also involved with participant recruitment, clinical assessment, data collection, and manuscript revision. Dr. Coleman was involved with statistical analysis and reviewed the manuscript. Dr. Firbank was involved with MRI data analysis and reviewed the manuscript. Dr. Nombela was involved with data acquisition and reviewed the manuscript. Dr. Winder-Rhodes was involved with data acquisition and reviewed the manuscript. Dr. Evans was involved in data acquisition and reviewed the manuscript. Dr. Rowe was involved with data acquisition and reviewed the manuscript. Prof. Mollenhauer is a principal investigator. She analyzed the CSF samples and reviewed and approved the final manuscript. Dr. Kruse analyzed the CSF samples and reviewed and approved the final manuscript. Dr. Hudson analyzed the DNA samples and reviewed and approved the final manuscript. Prof. Chinnery is a principal investigator. He analyzed the DNA samples and reviewed and approved the final manuscript. Prof. O’Brien is a principal investigator and coapplicant for the funding grant. He was involved in the study supervision and reviewed and approved the final manuscript. Prof. Robbins is a principal investigator, was involved in the study design, and reviewed the manuscript. Prof. Wesnes is a principal investigator, was involved in the study design and statistical analysis, and reviewed the manuscript. Prof. Brooks is a principal investigator and coapplicant for the main funding grant. He was also involved with the study design and reviewed the manuscript. Prof. Barker is a principal investigator and coapplicant for the main funding grant. He was involved with the study design and reviewed the manuscript. Prof. Burn is the chief investigator and main applicant for the funding grant. He was involved with the study design, supervised the study, and reviewed the manuscript.
STUDY FUNDING
The authors acknowledge the study funders, Parkinson's UK, Lockhart Parkinson's Disease Research Fund, and Michael J. Fox Foundation (MJFF). The research was supported by the NIHR Newcastle Biomedical Research Unit based at Newcastle upon Tyne Hospitals NHS Foundation Trust/Newcastle University, and an NIHR Biomedical Research Centre award to the University of Cambridge/Addenbrooke's Hospital. Newcastle University and the University of Cambridge acknowledge the support of the NIHR, through the Dementias and Neurodegenerative Diseases Research Network. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
DISCLOSURE
A. Yarnall is supported by grants from the Lockhart Parkinson's Disease Research Fund and MJFF. She has received honoraria from Teva-Lundbeck and sponsorship from Teva-Lundbeck, UCB, GlaxoSmithKline, Genus, and AbbVie for attending conferences. D. Breen is supported by a grant from Big Lottery Fund/Parkinson's UK, has received a Raymond and Beverly Sackler Studentship, and received honoraria from UCB. G. Duncan is supported by a grant from the Lockhart Parkinson's Disease Fund and has received sponsorship from UCB and Abbott for attending conferences. T. Khoo has received honoraria and educational grants from Teva-Lundbeck and Hospira and sponsorship from GSK and UCB for attending conferences. S. Coleman, M. Firbank, and C. Nombela report no disclosures. S. Winder-Rhodes is supported by a Merck Sharp and Dohme Studentship. J. Evans has received honoraria from UCB. J. Rowe is supported by the Wellcome Trust (088324), Medical Research Council, NIHR, Parkinson's UK, Evelyn Trust, and James S. McDonnell Foundation. B. Mollenhauer has received honoraria from Teva Pharmaceuticals, Orion Corporation, and GSK. She is an associate editor for the Journal of Alzheimer's Disease. She holds or has pending patents re: method of differentially diagnosing dementias; novel ELISA-based quantification of α-synuclein proteins in CSF and peripheral blood products using 384-well plates; and microRNA expression profiling of CSF. She serves as a consultant for Bayer Schering Pharma AG and receives research support from Teva Pharmaceutical Industries Ltd., Desitin Pharmaceuticals, GmbH, Boehringer Ingelheim, GE Healthcare, MJFF, the American Parkinson's Disease Association, and the Stifterverband für die Deutsche Wissenschaft (Dr. Werner Jackstädt-Stipend). N. Kruse reports no disclosures. G. Hudson is supported by a Parkinson's UK Senior Fellowship. P. Chinnery is a Wellcome Trust Senior Fellow in Clinical Science and a UK NIHR Senior Investigator. He receives additional support from the Wellcome Trust Centre for Mitochondrial Research, the Medical Research Council Centre for Translational Muscle Disease research, the Association Française contre les Myopathies, and EU FP7 TIRCON, and the NIHR Newcastle Biomedical Research Centre. J. O'Brien has acted as consultant for GE Healthcare and TauRx, and has been on the advisory board for Lilly, Novartis, and Nutricia. He has received grants from Lilly and received honoraria from GE Healthcare and Novartis. T. Robbins consults for Cambridge Cognition and receives royalties for CANTAB. He also consults for Lilly, GSK, Merck, Sharp & Dohme, Shire Pharmaceuticals, Lundbeck, and Teva, and has been in receipt of recent research grants from Lilly, GSK, and Lundbeck. K. Wesnes has for the last 3 years been an employee of Bracket, a division of United BioSource Corporation, which provides services to the clinical trial industry for numerous pharmaceutical companies. D. Brooks is a consultant for GE Healthcare, Shire Pharmaceuticals, and Cytox plc. He has received grants from the EU FP7 programme, MJFF, Medical Research Council, Parkinson's UK, Alzheimer Research UK, Lundbeck Foundation, Danish Council for Independent Research, and GE Healthcare. R. Barker has received grants from NIHR, Wellcome Trust, Cure-PD, Parkinson's UK, Rosetrees Trust, EU-FP7 programme, Evelyn Trust, and MJFF. He has received honoraria from Teva-Lundbeck and GSK in the past 2 years and is co–editor-in-chief for the Archives of Clinical Neuroscience and Rehabilitation and Journal of Neurology and associate editor for the Journal of Parkinson's Disease. D. Burn has received grants from NIHR, Wellcome Trust, GSK Ltd., Parkinson's UK, and MJFF. He has received honoraria from Teva-Lundbeck and UCB in the past 2 years and acted as consultant for GSK and Archimedes. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844 [DOI] [PubMed] [Google Scholar]
- 2.Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uc EY, McDermott MP, Marder KS, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology 2009;73:1469–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain 2009;132:2958–2969 [DOI] [PubMed] [Google Scholar]
- 5.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord 2006;21:1343–1349 [DOI] [PubMed] [Google Scholar]
- 7.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 2013;70:580–586 [DOI] [PubMed] [Google Scholar]
- 8.Weintraub D, Dietz N, Duda JE, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain 2012;135:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology 2010;75:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170 [DOI] [PubMed] [Google Scholar]
- 12.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 13.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1983;17:37–49 [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 15.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 16.Wesnes KA, McKeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo-controlled international study using the Cognitive Drug Research computerised assessment system. Dement Geriatr Cogn Disord 2002;13:183–192 [DOI] [PubMed] [Google Scholar]
- 17.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994;5:266–281 [DOI] [PubMed] [Google Scholar]
- 18.Benton AL. Differential behavioural effects of frontal lobe disease. Neuropsychologia 1968;6:53–60 [Google Scholar]
- 19.Goodglass H. The Assessment of Aphasia and Related Disorders. Philadelphia: Lea & Febiger; 1972 [Google Scholar]
- 20.Ala TA, Hughes LF, Kyrouac GA, Ghobrial MW, Elble RJ. Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2001;70:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010;75:1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord 2007;22:1272–1277 [DOI] [PubMed] [Google Scholar]
- 23.Dalrymple-Alford JC, Livingston L, MacAskill MR, et al. Characterizing mild cognitive impairment in Parkinson's disease. Mov Disord 2011;26:629–636 [DOI] [PubMed] [Google Scholar]
- 24.Poletti M, Frosini D, Pagni C, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:601–606 [DOI] [PubMed] [Google Scholar]
- 25.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G; Norwegian ParkWest Study Group. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009;72:1121–1126 [DOI] [PubMed] [Google Scholar]
- 26.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK: the CamPaIGN study. Brain 2004;127:550–560 [DOI] [PubMed] [Google Scholar]
- 27.Bronnick K, Alves G, Aarsland D, Tysnes OB, Larsen JP. Verbal memory in drug-naive, newly diagnosed Parkinson's disease: the retrieval deficit hypothesis revisited. Neuropsychology 2011;25:114–124 [DOI] [PubMed] [Google Scholar]
- 28.Lashley T, Holton JL, Gray E, et al. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson's disease patients. Acta Neuropathol 2008;115:417–425 [DOI] [PubMed] [Google Scholar]
- 29.Tiraboschi P, Hansen LA, Alford M, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology 2000;54:407–411 [DOI] [PubMed] [Google Scholar]
- 30.Compta Y, Marti MJ, Ibarretxe-Bilbao N, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord 2009;24:2203–2210 [DOI] [PubMed] [Google Scholar]
- 31.Montine TJ, Shi M, Quinn JF, et al. CSF Abeta (42) and tau in Parkinson's disease with cognitive impairment. Mov Disord 2010;25:2682–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollenhauer B, Trenkwalder C, von Ahsen N, et al. Beta-amyloid 1-42 and tau-protein in cerebrospinal fluid of patients with Parkinson's disease dementia. Dement Geriatr Cogn Disord 2006;22:200–208 [DOI] [PubMed] [Google Scholar]
- 33.Alves G, Bronnick K, Aarsland D, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry 2010;81:1080–1086 [DOI] [PubMed] [Google Scholar]
- 34.Parnetti L, Tiraboschi P, Lanari A, et al. Cerebrospinal fluid biomarkers in Parkinson's disease with dementia and dementia with Lewy bodies. Biol Psychiatry 2008;64:850–855 [DOI] [PubMed] [Google Scholar]
- 35.Adler CH, Caviness JN, Sabbagh MN, et al. Heterogeneous neuropathological findings in Parkinson's disease with mild cognitive impairment. Acta Neuropathol 2010;120:827–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reesink FE, Lemstra AW, van Dijk KD, et al. CSF alpha-synuclein does not discriminate dementia with Lewy bodies from Alzheimer's disease. J Alzheimers Dis 2010;22:87–95 [DOI] [PubMed] [Google Scholar]
- 37.El-Agnaf OM, Walsh DM, Allsop D. Soluble oligomers for the diagnosis of neurodegenerative diseases. Lancet Neurol 2003;2:461–462 [DOI] [PubMed] [Google Scholar]
- 38.Morley JF, Xie SX, Hurtig HI, et al. Genetic influences on cognitive decline in Parkinson's disease. Mov Disord 2012;27:512–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melzer TR, Watts R, MacAskill MR, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:188–194 [DOI] [PubMed] [Google Scholar]
- 40.Dalaker TO, Zivadinov R, Larsen JP, et al. Gray matter correlations of cognition in incident Parkinson's disease. Mov Disord 2010;25:629–633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



