Abstract
Significance: Much evidence shows that electrical stimulation (ES) promotes the wound healing process. The inhibitory effect of ES on bacterial growth has been proposed as a mechanism to explain the useful effects of ES on wound healing. Bacterial burden has been associated with chronic wounds. The extensive use of antibiotics can lead to the spread of multiple drug resistant bacteria. Whether biophysical energies, such as ES, can be used as a treatment modality against pathogenic microorganisms remains an open question.
Recent Advances: The research literature provides evidence for useful effects of ES in terms of inhibition of bacterial growth. The type of ES, its polarity, and the intensity of the current play a major role in establishment of antibacterial effects. Both direct current (DC) and high voltage pulse current are more effective at inhibiting bacterial growth than are other types of ES. The exact mechanism underlying the antibacterial effects of ES is not clear.
Critical Issues: Available evidence indicates that microampere DC (μADC) is better than other ES types for inhibition of bacterial growth. The results of most studies also support the application of cathodal current for bacterial growth inhibition. The current intensity of ES would appear to be tolerable by humans if used clinically for treatment of infected wounds.
Future Directions: The cathodal μADC appears to be more effective for inhibition of microorganism growth. Further research, especially in vivo, is necessary to clarify the inhibitory effects of ES on wound bacterial infections.

Giti Torkaman, PhD
Scope and Significance
Substantial evidence now supports the use of electrical stimulation (ES) for promoting wound healing.1–5 Numerous mechanisms have been suggested to explain the phenomenon of wound healing by ES, including increased angiogenesis due to release of angiogenic factors (vascular endothelial growth factor, fibroblast growth factor-2),6–9 increased circulation10,11 and direct antibacterial effect of ES.12
Over 30 years ago, Rowley was the first to report a bacteriostatic effect of ES.13 Since then, bacteriostatic and bactericidal effects of ES have been extensively documented.12–15 This review focuses on the in vitro and in vivo evidence that supports a role for ES in the inhibition of bacterial growth during wound healing.
Translational Relevance
The research literature provides evidence for useful effects of ES in terms of inhibition of bacterial growth.12–15 The type of ES and its different parameters may be important in establishment of antibacterial effects. ES is suggested to affect bacterial growth via direct and indirect effect but, the exact mechanism underlying this effect is still poorly understood. The examination of ES antibacterial effect based on in vitro and in vivo available evidence can indicate the potential of ES for bacteriostatic and bactericidal effect on microorganism growth that is very important for the wound healing process.
Clinical Relevance
Bacterial invasion is one of the factors that delay the wound healing process.12 Bacteria rupture viable cell membranes and maintain chronic inflammation that prevents wound healing.16 However, the extensive use of antibiotics for the treatment of bacterial infection has led to the spread of multiple drug resistant bacteria. Recent studies have therefore, focused on the potential use of ES as a treatment modality against pathogenic microorganisms.12–15 If the antibacterial effects of ES can be induced in human infected wounds, ES may prove to be a superior antimicrobial agent that would overcome some of the issues currently raised by antibiotic resistance.
Discussion of Findings and Relevant Literature
Parameters of ES for antibacterial effects in research
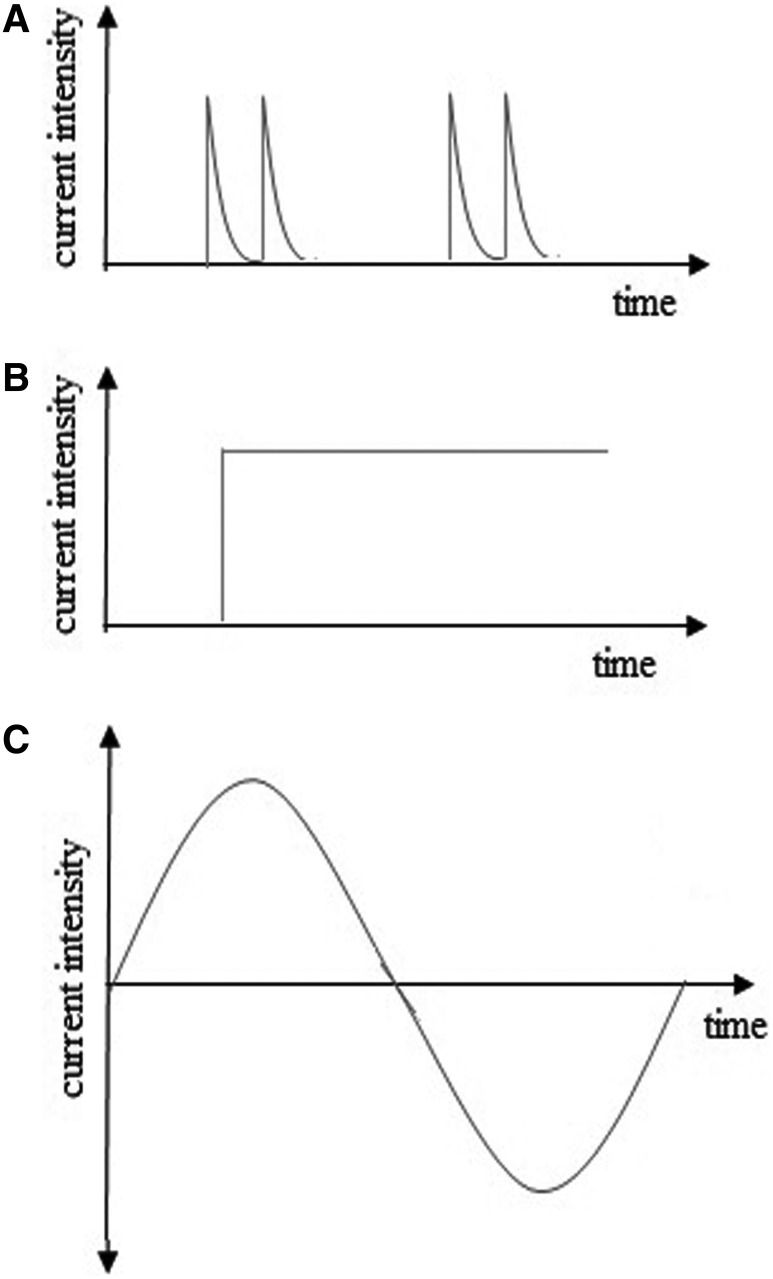
The available research that has examined the capacity for ES to inhibit or destroy pathogens indicates that various parameters of ES (e.g., current type, current density, polarity, etc.) have been employed in the past. Various types of ES have been used in this research, including low-intensity direct current (LIDC), alternating current (AC), and high-voltage pulsed current (HVPC) with diverse parameters (Fig. 1). In an early study, Rowley13 focused on the in vitro antibacterial effects of AC (milliampere level) and cathodal direct current (DC). They reported that the growth rate of Escherichia coli was affected very little or not at all by AC, while a bacteriostatic effect occurred with cathodal DC. Later, Rowley et al.12 demonstrated that cathodal DC had a bacteriostatic effect on the in vivo growth of Pseudomonas aeruginosa when applied to rabbit skin wounds at an amplitude of 1 mA for 72 h.
Figure 1.
Various waveforms of electrical stimulation (ES) are available in research, including (A) high-voltage pulsed current, (B) direct current, and (C) alternating current.
Barranco et al.15 applied DC stimulation with stainless steel, platinum, gold, and silver electrodes at amplitudes of 0.4, 4, 40, and 400 μA for 48 h on Staphylococcus aureus in an in vitro model. The authors observed that the silver anode electrode had a bactericidal effect on S. aureus at 0.4 and 4 μA, while other electrodes induced growth inhibition only at 400 μA. Spadaro et al.17 applied DC current with silver, platinum, stainless steel, gold, and copper electrodes on four bacterial species in vitro. They found that, at a high current range (400 μA), electrodes inhibited bacterial growth at both negative and positive poles, while at lower current levels (0.4 and 4 μA), only the silver electrode had a bacteriostatic effect when used as the anode. Bolton et al.18 investigated the effect of DC stimulation on microorganisms in intact human skin. Current was delivered through carbon-filled electrodes for 4 or 24 h with 10, 25, 50, 75, and 100 μA. Bactericidal effects were seen at 4 and 24 h beneath the positive electrode. The authors suggested that the bactericidal effect of the electric current was dependent on the current density and on the acid pH that was generated at the positive electrode. The in vitro study by Karba et al.19 examined the effect of LIDC (0.2–1 mA) on Candida albicans. The authors found that DC for all amplitudes and application times (2, 10, and 18 h) inhibit the C. albicans growth. However, the inhibitory action of DC was dose-dependent and time-dependent with respect to the electric current. In an in vivo study, Wolcott et al.14 used LIDC for treatment of chronic skin ulcer initially colonized with Pseudomonas and proteus organisms. In this study, the cathodal DC (microampere level) was initially applied to the ulcers. The authors observed that the treated ulcers became free of pathogens within a few days. Another in vitro study by Liu et al.20 showed antimicrobial activity of low amperage DC (10 μA) around the cathode when current was applied to S.aureus and Staphylococcus epidermidis for 16 h. Del Pozo et al.21 demonstrated marked reduction of S. epidermidis, S. aureus, and P. aeruginosa biofilm by prolonged exposure to DC stimulation. Current was delivered via stainless steel or graphite electrodes with 20, 200 or 2,000 microamperes for 1–7 days. These authors observed that a higher electrical current intensity resulted in a greater decrease in viable bacteria at all time points studied. Subsequent to these reports, numerous other studies established that application of weak DC (0.1–10 μA) through a silver ion electrode increased the antimicrobial action of silver by increasing the rate of silver release.22–24 Spadaro et al.,17 in an in vitro study, demonstrated that silver, when used as an anode, is extremely bacteriostatic, even at the lowest current, whereas the penetration of the silver ion applied topically is limited. The addition of LIDC to metallic silver increased the penetration depth of silver into the wound site.25
The use of HVPC for inhibition of bacterial growth was initially based on the results of studies using LIDC. Unlike DC, scant research has examined the antibacterial effects of HVPC in vitro and in vivo. Kincaid and Lavoie26 reported that growth of S.aureus, E. coli, and P. aeruginosa (bacterial species that are commonly found in open wounds) was inhibited in vitro at both the anode and cathode after exposure to HVPC for 2 h at 250 V. Similarly, Szuminsky et al.27 evaluated the antibacterial effect of HVPC on four bacterial species (S. aureus, E. coli, Klebsiella, and P. aeruginosa) in an in vitro study. The authors suggested that HVPC at both the positive and negative electrode exerted antimicrobial effects when applied at 500 V for 30 min. Conversely, Guffey and Asmussen28 compared the inhibitory effect of HVPC and DC in an in vitro study and showed that DC stimulation showed antibacterial effects, but HVPC did not, when current was applied for 30 min at <160 V. Daeschlein et al.29 showed that low voltage pulsed current had an antibacterial effect on Gram-positive and Gram-negative bacteria in vitro and they observed this antibacterial effect at both positive and negative polarity.
The efficacy of DC and AC at exerting antibacterial effects has only been investigated in one in vitro study to date, and this showed positive effects of AC. Petrofsky et al.30 exposed three types of bacteria (S. aureus, E. coli, and P. aeruginosa) to either biphasic sine wave, 5 or 20 mA, or DC stimulation, at 100 μA, for 30 min in vitro. The authors reported that DC stimulation had no bacteriostatic effects for any of the bacteria, whereas AC stimulation (both 5 and 20 mA) significantly reduced the growth of P. aeruginosa, but had little effect on S. aureus or or E. coli. Another investigation that compared the antibacterial effects of AC and DC stimulation in vitro showed that AC had no inhibitory effect on growth of P. aeruginosa, whereas both the cathodal and anodal DC stimulation inhibited growth of this organism.31
In a comparable study, Merriman et al.32 evaluated the effects of four types of ES—microampere direct current (μADC), HVPC, low voltage monophasic pulsed current (LVMPC), and low voltage biphasic pulsed current (LVBPC)—on bacterial growth in vitro. They demonstrated an inhibitory effect for μADC and HVPC at both negative and positive polarity whereas LVMPC and LVBPC had no inhibitory effect at any polarity.
The research literature provides evidence to indicate a useful effect of ES for inhibition of bacterial growth. Review of these results suggests that the type of ES plays a major role in establishment of antibacterial effects. Both DC and HVPC appear to be effective at inhibiting bacterial growth. Although the efficacy of the antibacterial effect of HVPC has been shown, the voltages used in several studies (250 to 500 V) are too high to be tolerated by humans and also may be damaging to the formation of collagen.33 Therefore, μADC would appear to be more effective than other ES types for inhibition of bacterial growth. This also supports the thought that the antibacterial effect may be contributing to the promotion of wound healing that is observed with LIDC.2
When compared with AC and biphasic (charge balanced) current which have no pH effects, DC creates strong pH effects, while HVPC has little to no polar effects, indicating that pH plays a role in establishment of antibacterial effects by ES. Although some studies have indicated antibacterial effects at both the anode and cathode,17,26,27,29,31,32 most research supports the application of cathodal current for bacterial inhibition.12–14,20
The evidence also indicates that different current intensities might affect the antibacterial effects of ES. Overall, the inhibitory effect of ES is proportional to the amplitude and application time of electric current. Thus, higher current intensities create more inhibitory effects than do lower intensities.15,17,19 Collectively, the amplitude of the electric current would appear to be tolerable if used on an infected wound in human tissues. However, further in vivo or clinical wound research is needed to confirm the antibacterial effects observed in in vitro studies.
Antibacterial mechanisms of ES
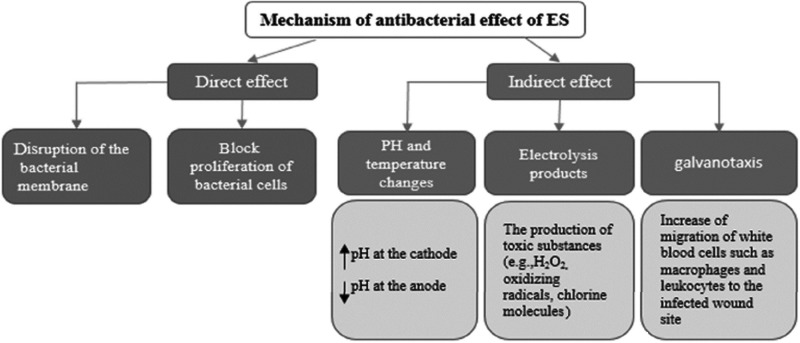
The exact mechanisms by which ES inhibits the growth of microorganisms are unknown and are also controversial. However, two mechanisms are currently proposed to explain the antibacterial effects of ES: a direct effect and an indirect effect (Fig. 2). The direct effect proposes that electric current directly results in bacterial death by disruption of the integrity of the bacterial membrane or electrolysis of molecules on the bacterial cell surface.19,20,24 Evidence has been presented to support a blockage of proliferation of bacterial cells by DC19 but further in vitro and in vivo research is needed to understand the underlying mechanism of these direct antibacterial effects.
Figure 2.
A diagram of direct and indirect effects of ES on microorganism growth. It is suggested that plasma transmembrane potential is related to the cell cycle processes. Low transmembrane potential facilitates cell proliferation, while high potential tends to inhibit it. Transmembrane potential of microbes differs from other cells, such as fibroblasts. Thus, different characteristics of the membrane of microbes might be responsible for inhibition of microbe proliferation by ES.19
Two factors—temperature and pH—were suggested as possible mechanisms to explain the indirect effect of ES. However, according to available evidence, temperature changes are minimal during the application of electric current.27 Thus, temperature changes would not appear to contribute to the antibacterial effects of ES.
The investigations of application of DC and HVPC indicate that the pH at the cathode tends to be alkaline18,19,21,26,27,32,34 and, conversely, acidic at the anode (Figs. 3 and 4, respectively).18,19,27,32,34 The pH changes are significantly more marked with DC than with HVPC.32 Due to the waveform of HVPC (it has a low average current), alterations of pH under the electrodes will be very slight.27 Because the pH changes beneath the electrodes are transient, most investigators believe that these changes are also not the primary cause of the antibacterial effects of ES.12,27,34 However, when combined with the direct effect of electric current, they may contribute to inhibition of bacterial growth.
Figure 3.
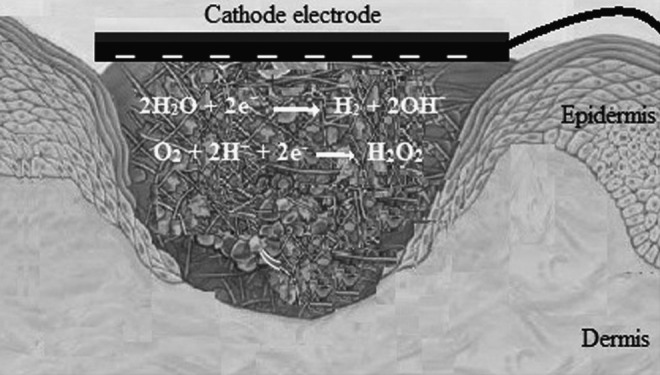
This scheme shows that pH at the cathode (negative pole) tends toward alkaline and H2O2, as an antibacterial substance, produced at the cathode.
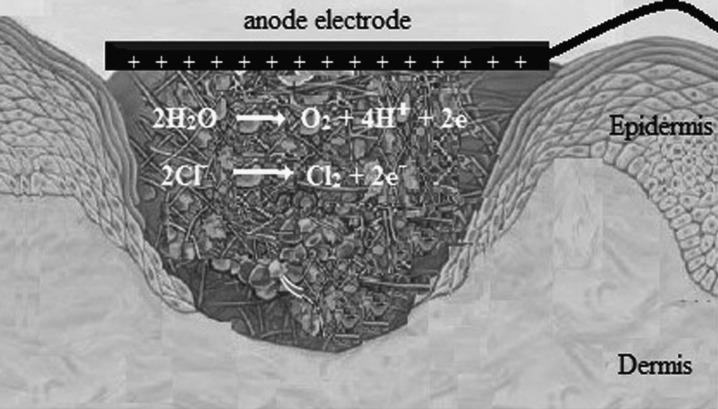
Figure 4.
This scheme shows that pH at the anode (positive pole) tends toward acidic and chlorine molecules, as an antibacterial substance, produced at the anode.
The production of toxic substances (e.g., H2O2, oxidizing radicals, chlorine molecules, etc.) as a result of electrolysis has been suggested as another mechanism to explain the indirect antibacterial effects of ES.18,20,24 Liu et al.20 revealed that a lower level of DC (10 μA) produced the antibacterial substances, H2O2 and chlorine, at the cathode and anode, respectively (Figs. 3 and 4, respectively). It is likely that the other antibacterial substances, such as H2O2, ozone, and hypochlorite are also produced if a higher level of electrical current is applied.20 However, Del Pozo et al.21 did not observe the generation of chlorine or H2O2 when LIDC was applied for 7 days.
Gas formation at the cathode and corrosion and discoloration at the anode were observed for both HVPC and DC.15,26,32 These by products can also affect the growth of microorganisms. However, in the in vivo condition, the antibacterial effects of ES are unlikely to result from electrolysis products. In addition, the human wound environment is much more complex and it may constantly receive a new supply of highly buffered fluids that can remove electrolysis products rapidly from the wound environment.32
One suggested mechanism for indirect antibacterial effects of ES has been that the antibacterial effect of electric current may be the result of galvanotaxis (directional migration of cells to the anode or cathode).35 Research has demonstrated that lymphocytes, neutrophils, and macrophages migrate toward negative polarity,1,36 although some researchers have reported that fibroblast cells and macrophages migrated toward positive polarity.1 Application of ES to a wound can also induce the release of prostaglandins and the other cytokines,37,38 which would attract macrophages to the wound site.28 Thus, the indirect antibacterial effects of ES may be the result of galvanotaxic attraction of white blood cells, such as macrophages and leukocytes to the infected wound rather than a direct response to electrolysis products or pH changes.
Take-Home Messages.
Basic science advances
• In vitro studies have demonstrated antibacterial effects of ES.
• The type of ES plays a major role in establishment of antibacterial effects. Both DC and HVPC are more effective in inhibition of bacterial growth than are other ES types.
• Although an antibacterial effect of ES is shown at both the anode and cathode, most of the evidence supports the application of a cathodal current.
• The bacterial inhibitory action of ES is proportional to the amplitude and application time of the electric current.
• The electric current directly results in bacterial death by disruption of the integrity of the bacterial membrane or by electrolysis of molecules on the cell surface.
• Changes in pH were suggested as a possible mechanism for the indirect effects of ES (especially DC) on inhibition of bacterial growth.
• The production of toxic substances (e.g., H2O2, oxidizing radicals, chlorine molecules) and galvanotaxic effects of ES might be other mechanisms for indirect antibacterial effects of ES.
Clinical science advances
• ES has a potential for bacteriostatic and bactericidal effects on in vivo microorganism growth.
• Cathodal microampere DC and HVPC might provide a treatment modality for promoting the healing of chronic wounds with bacterial burdens.
• Further experimental studies, especially in vivo, are necessary to clarify the direct and indirect inhibitory effects of ES on wound bacterial infections.
In general, the direct bacterial inhibitory effects of ES appear to be more important than indirect effects, although both contribute to the overall antibacterial response. However, further studies, especially in vivo, must be done to elucidate the exact mechanism that gives rise to the observed antibacterial effects of ES.
Conclusion
The available evidence indicates that ES inhibits the growth of microorganisms. Cathodal μADC appears to be more effective than other types of ES. The exact mechanisms that result in antibacterial effects of ES are unknown, but direct effects of ES on the bacteria appear to be more important than indirect effects. The number of studies on this topic is limited and further research, especially in vivo, is necessary to clarify the mechanisms underlying the antibacterial effects of ES in infected wounds.
Abbreviations and Acronyms
- AC
alternating current
- DC
direct current
- ES
electrical stimulation
- HVPC
high-voltage pulsed current
- LIDC
low intensity direct current
- LVBPC
low-voltage biphasic pulsed current
- LVMPC
low-voltage monophasic pulsed current
- μADC
microampere direct current
Acknowledgments and Funding Sources
The authors gratefully acknowledge Prof. Luther Kloth (Marquette University, Milwaukee, WI) for his beneficial suggestions and comments on this study. The author has not received funding for this study.
Author Disclosure and Ghostwriting
The authors have no competing interests. No ghostwriters were used to write this article.
About the Authors
Mohammad Reza Asadi, MSc, is a PhD candidate in the Department of Physical Therapy at Tarbiat Modares University under supervision of Dr. Giti Torkaman. His current research interest is the effect of electrical stimulation on the expression of VEGF and its receptors in diabetic foot ulcer. Dr. Giti Torkaman, PhD, is a professor in the Department of Physical Therapy at Tarbiat Modares University. Dr. Torkaman's research program focuses on the use of physical modalities such as electrical stimulation and laser radiation to accelerate the healing process.
References
- 1.Kloth LC: Electrical stimulation for wound healing: a review of evidence for in vitro studies, animal experiment, and clinical trial. Int J Low Extrem Wounds 2005; 4:23. [DOI] [PubMed] [Google Scholar]
- 2.Balakatounis KC. and Angoules AG: Low-intensity electrical stimulation in wound healing: review of the efficacy of externally applied currents resembling the current of injury. Eplasty 2008; 8:e28. [PMC free article] [PubMed] [Google Scholar]
- 3.Houghton PE, Kincaid CB, Lovell M, Campbell KE, Keast DH, Woodbury MG, and Harris KA: Effect of electrical stimulation on chronic leg ulcer size and appearance. Phys Ther 2003; 83:17. [PubMed] [Google Scholar]
- 4.Houghton PE, Campbell KE, Fraser CH, Harris C, Keast DH, Potter PJ, Hayes KC, and Woodbury MG: Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch Phys Med Rehabil 2010; 91:669. [DOI] [PubMed] [Google Scholar]
- 5.Petrofsky JS, Lawson D, Berk L, and Suh H: Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation for 30 min three times per week. J Diabetes 2010; 2:4. [DOI] [PubMed] [Google Scholar]
- 6.Bai H, McCaig CD, Forrester JV, and Zhao M: DC electrical fields induce distinct preangiogenic responses in microvascular and macrovascular cells. Arterioscler Thromb Vasc Biol 2004; 24:1234. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Bai H, Wang E, Forrester JV, and McCaig CD: Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci 2004; 117:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asadi MR, Torkaman G, and Hedayati M: Effect of sensory and motor electrical stimulation in vascular endothelial growth factor expression of muscle and skin in full-thickness wound. J Rehabil Res Dev 2011; 48:195. [DOI] [PubMed] [Google Scholar]
- 9.Asadi MR, Torkaman G, and Hedayati M: The role of sensory and motor intensity of electrical stimulation on FGF-2 expression, inflammation, vascularization, and mechanical strength of full-thickness wounds. J Rehabil Res Dev 2013; 50:489. [DOI] [PubMed] [Google Scholar]
- 10.Kloth LC: How to use electrical stimulation for wound healing. Nursing 2002; 32:17. [DOI] [PubMed] [Google Scholar]
- 11.Petrofsky JS, Schwab E, Lo T, Cuneo M, George J, Kim J, and Al-Malty A: Effect of electrical stimulation on skin blood flow in controls and in and around stage III and IV wounds in hairy and non hairy skin. Med Sci Monit 2005; 11:309. [PubMed] [Google Scholar]
- 12.Rowley BA, McKenna JM, Chase GR, and Wolcott LE: The influence of electrical current on an infecting microorganism in wounds. Ann NY Acad Sci 1974; 238:543. [DOI] [PubMed] [Google Scholar]
- 13.Rowley BA: Electrical current effects on E. coli growth rates. Proc Soc Exp Biol Med 1972; 139:929. [DOI] [PubMed] [Google Scholar]
- 14.Wolcott L, Wheeler P, Hardwicke H, and Rowley BA: Accelerated healing of skin ulcers by electrotherapy: preliminary clinical results. South Med J 1969; 62:795. [DOI] [PubMed] [Google Scholar]
- 15.Barranco S, Spadero J, and Berger T: In vitro effect of weak direct current on Staphylococcus aureus. Clin Orthop 1974; 100:250. [PubMed] [Google Scholar]
- 16.Neubauer T, Bayer GS, and Wagner M: Open fractures and infection. Acta Chir Orthop Traumotol Cech 2006; 73:301. [PubMed] [Google Scholar]
- 17.Spadaro JA, Berger TJ, Barranco SD, Chapin SE, and Becker RO: Antibacterial effects of silver electrodes with weak direct current. Antimicrob Agents Chemother 1974; 6:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolton L, Foleno B, Means B, and Petrucelli S: Direct current bactericidal effect on intact skin. Antimicrob Agents Chemother 1980; 18:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karba R, Gubina M, and Vodovnik L: Growth Inhibition in Candida albicans due to low intensity constant direct current. J Bioelectricity 1991; 10:1 [Google Scholar]
- 20.Liu WK, Brown MR, and Elliott TS: Mechanisms of the bactericidal activity of low amperage electric current (DC). J Antimicrob Chemother 1997; 39:687. [DOI] [PubMed] [Google Scholar]
- 21.Del Pozo JL, Rouse MS, Mandrekar JN, Fernandez Sampedro M, Steckelberg JM, and Patel R: Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidisbiofilms. Antimicrob Agents Chemother 2009; 53:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu CS, McManus AT, Pruitt BA, Jr., and Mason AD, Jr.: Therapeutic effects of silver nylon dressings with weak direct current on Pseudomonas aeruginosa-infected burn wounds. J Trauma 1988; 28:1488. [DOI] [PubMed] [Google Scholar]
- 23.Ong PC, Laatsch LJ, and Kloth LC: Antibacterial effects of a silver electrode carrying microamperage direct current in vitro. J Clin Electrophysiol 1994; 6:14 [Google Scholar]
- 24.Pareilleux A. and Sicard N: Lethal effects of electric current on escherichia coli. Appl Microbiol 1970; 19:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falcone AE. and Spadaro JA: Inhibitory effects of electrically activated silver material on cutaneous wound bacteria. Plast Reconstr Surg 1986; 77:455. [DOI] [PubMed] [Google Scholar]
- 26.Kincaid C. and Lavoie K: Inhibition of bacterial growth in vitro following stimulation with high voltage, monophasic pulsed current. Phys Ther 1989; 69:651. [DOI] [PubMed] [Google Scholar]
- 27.Szuminsky N, Albers A, Unger P, and Eddy JG: Effect of narrow, pulsed high voltages on bacterial viability. Phys Ther 1994; 74:660. [DOI] [PubMed] [Google Scholar]
- 28.Guffey JS. and Asmussen MD: In vitro bactericidal effects of high voltage pulsed current versus direct current against Staphylococcus aureus. J Clin Electrophysiol 1989; 1:5 [Google Scholar]
- 29.Daeschlein G, Assadian O, Kloth LC, Meinl C, Ney F, and Kramer A: Antibacterial activity of positive and negative polarity low-voltage pulsed current (LVPC) on six typical Gram-positive and Gram-negative bacterial pathogens of chronic wounds. Wound Repair Regen 2007; 15:399. [DOI] [PubMed] [Google Scholar]
- 30.Petrofsky J, Laymon M, Chung W, Collins K, and Yang TN: Effect of electrical stimulation on bacterial growth. J Orthop Neurolsurg 2008; 31:43 [Google Scholar]
- 31.Maadi H, Haghi M, Delshad R, Kangarloo H, Mohammadnezhady MA, and Hemmatyar GR: Effect of alternating and direct currents on Pseudomonas aeruginosagrowth in vitro. Afr J Biotechnol 2010; 9:6373 [Google Scholar]
- 32.Merriman HL, Hegyi CA, Albright-Overton CR, Carlos J, Putnam RW, and Mulcare JA: A comparison of four electrical stimulation types on Staphylococcus aureus growth in vitro. J Rehabil Res Dev 2004; 41:139. [DOI] [PubMed] [Google Scholar]
- 33.Bourguignon GJ. and Bourguignon LYW: Electrical stimulation of protein and DNA synthesis wounds. J Am Acad Dermatol 1991; 25:401880252 [Google Scholar]
- 34.Newton R. and Karselis T: Skin pH following high voltage pulsed galvanic stimulation. Phys Ther 1983; 63:1593. [DOI] [PubMed] [Google Scholar]
- 35.Kloth LC: Electrical stimulation in tissue repair. In: Wound Healing: Alternatives in Management, edited by McCulloch JM, Kloth LC, and Feedar JA. Philadelphia: F.A. Davis Co, 1995, p. 275 [Google Scholar]
- 36.Talebi G, Torkaman G, Firoozabadi M, and Shariat S: Effect of anodal and cathodal micro-amperage direct current on the skin wound healing: a biomechanical and histological study. J Biomech 2007; 40:S665 [Google Scholar]
- 37.Hofbauer R, Moser D, Kaye AD, Knapp S, Gmeiner B, Kapiotis S, Wagner O, and Frass M: Prostaglandin E(1) is able to increase migration of leukocytes through endothelial cell monolayers. Microvasc Res 2000; 59:354. [DOI] [PubMed] [Google Scholar]
- 38.McLoughlin TJ, Mylona E, Hornberger TA, Esser KA, and Pizza FX: Inflammatory cells in rat skeletal muscle are elevated after electrically stimulated contractions. J Appl Physiol 2003; 94:876. [DOI] [PubMed] [Google Scholar]