Abstract
An excessive tissue response to prosthetic arterial graft material leads to intimal hyperplasia (IH), the leading cause of late graft failure. Seroma and abnormal capsule formation may also occur after prosthetic material implantation. The matricellular protein Thrombospondin-2 (TSP-2) has shown to be upregulated in response to biomaterial implantation. This study evaluates the uptake and release of small interfering RNA (siRNA) from unmodified and surface functionalized electrospun PET graft materials. ePET graft materials were synthesized using electrospinning technology. Subsets of the ePET materials were then chemically modified to create surface functional groups. Unmodified and surface-modified ePET grafts were dip-coated in siRNAs alone or siRNAs complexed with transfection reagents polyethyleneimine (PEI) or Lipofectamine RNAiMax. Further, control and TSP-2 siRNA-PEI complex treated ePET samples were placed onto a confluent layer of human aortic smooth muscle cells (AoSMCs). Complexation of all siRNAs with PEI led to a significant increase in adsorption to unmodified ePET. TSP-2 siRNA-PEI released from unmodified-ePET silenced TSP-2 in AoSMC. Regardless of the siRNA-PEI complex evaluated, AoSMC migrated into the ePET. siRNA-PEI complexes delivered to AoSMC from dip-coated ePET can result in gene knock-down. This methodology for siRNA delivery may improve the tissue response to vascular and other prosthetics.
Keywords: Polyester, silencing RNA, transfection reagents, human aortic smooth muscle cells, thrombospondin-2
1. INTRODUCTION
Arterial bypass grafts fabricated from poly(ethylene terephthalate) (PET) have been used for over 60 years to repair or replace a diseased segment of artery. PET prosthetic grafts, similar to grafts comprised of expanded polytetrafluoroethylene (ePTFE) or polyurethane, fail due to thrombosis and anastomotic intimal hyperplasia (AIH) at the distal anastomosis.[1, 2] AIH still remains as the leading cause for delayed prosthetic bypass graft failure.[3, 4] Currently, there are no treatments proven to effectively ameliorate the pathologic hyperplasia that occurs at the prosthetic graft-arterial anastomosis.[3, 4] Efforts to study gene expression of AIH using microarray analysis of hyperplastic lesion suggests that the majority of gene expression changes in the neointimal cells occur within the first week of implantation.[5] Moreover, seroma and abnormal capsule formation can occur after prosthetic material implantation, which may compromise the implant.
The expression of one gene in particular, Thrombospondin-2 (TSP-2), increased after one week and was sustained over a 30-day period of graft implantation.[6] TSP-2 is an anti-angiogenic matricellular protein. However, not all its functions are yet known. We have previously shown that TSP-2 regulates smooth muscle cell attachment.[3] Others have shown that TSP-2 inhibition increases vascularity in granulation and capsular tissue that forms in response to implanted materials.[7, 8]
Small interfering RNA (siRNA) is a versatile tool that has the potential to modulate vascular cell gene expression in a temporary manner without the potential adverse effects associated with lentiviral or adenoviral vectors.[9–15] Previous studies have shown the differential susceptibility of vascular cells to siRNA transfection with vascular smooth muscle cells being less susceptible than endothelial cells.[16, 17] Vascular smooth muscle cells of the synthetic phenotype are the predominant cells associated with intimal hyperplasia and therefore a logical target of gene silencing aimed at diminishing the vascular response to implanted materials. Because TSP-2 was found to be consistently upregulated at the anastomosis of biomaterials to a host artery TSP-2 gene was chosen as the gene target in this proof of principal study.
Electrospinning provides a rapidly growing technique for controlled fiber modification as well as new nanocomposite substrates. Several parameters are attributed to the successful formation of a product by electrostatic means.[18] These include: 1) the magnitude of the electric potential in relation to the distance between the emitter and the collector as well as the discharge media, 2) the viscosity of the polymer solution as determined by molecular weight and/or percent solids of the solution and 3) the surface tension at droplet surface as determined by solvent/polymer interaction. This research proposal builds on the rapid development seen in electrospinning over a number of years[19, 20], including those investigating the formation of electrospun tubular structures.[21]
Electrospinning of PET material and siRNA transfection technology were combined to explore the possibility of using ePET to deliver siRNA to aortic smooth muscle cells and achieve effective gene silencing. The goal of this study is to evaluate the uptake, release and bioavailability of siRNA, in native and complexed forms, from unmodified and surface functionalized ePET graft materials. Our hypothesis is siRNA can be incorporated into an electrospun material using a simple dip-coating method, be released from the material and provide targeted knockdown of a specific gene.
2. MATERIALS AND METHODS
2.1 Confirmation of siRNA Transfection from Solution, Delivery and Target mRNA Knockdown
2.1.1 siRNAs and transfection reagents
siRNAs were obtained from Dharmacon (Lafayette, CO) as follows: 1) non-targeting-non-fluorescent control siRNA (D-001206-13-20), 2) siGLO Red®, a transfection indicator, consisting of non-targeting double stranded siRNA conjugated to the red fluorescent probe DyLight547, 3) 3′ Cholesterol and a 5′ prime Dy547 tagged non-targeting control siRNA (Chol siRNA), 4) TSP-2 siRNA and 5) 3′ Cholesterol TSP-2 siRNA (Chol TSP-2 siRNA). Two transfection reagents were procured: 1) Lipofectamine RNAiMAX® (liposomal transfection reagent; Invitrogen, Carlsbad, CA) and 2) jetPEI™ (a cationic polymer; Polyplus, Strasbourg, France).
2.1.2 siRNA transfection protocol
Primary human aortic smooth muscle cells (AoSMC) (Lonza, Walkersville, MD) were cultured in basal medium (LifeLine, Walkersville, MD) enriched with SMC growth supplements. The cells were maintained at 37°C with 5% CO2. AoSMCs from passages 4–8 were used in the experiments. AoSMC were seeded at a density of 5,000 cells/well in a 96-well plate (Fisher Scientific, Pittsburgh, PA). After 24 hours, AoSMCs were transfected with conditions described in Table 1. For all transfections, siRNA was used in the range of 0.25μg–1.0μg. All transfection reagents were used as recommended by the manufacturer. Complexation of siGLO Red or Chol siRNA with PEI at N/P ratios of 2.5, 5 and 10 were evaluated for delivery efficacy (N/P ratio = ratio of moles of amine groups of PEI to moles of siRNA phosphate groups).
Table 1.
siRNA/transfection reagent combinations evaluated for gene silencing in AoSMC.
| Transfection Reagent | |||
|---|---|---|---|
| siRNA for delivery | Absent | RNAiMAX | PEI (N/P ratios: 2.5, 5 or 10) |
| Unlabeled control siRNA | X | X | |
| siGLO RED | X | X | NP: 2.5, 5 and 10 |
| Chol-siRNA | X | NP: 5 and 10 | |
| siRNA for gene knockdown | |||
| Unlabeled control siRNA | NP: 10 | ||
| TSP-2 siRNA | NP: 10 | ||
| Chol-siRNA | NP: 10 | ||
| Chol TSP-2 siRNA | NP: 10 | ||
2.1.3 Visualization of siRNA delivery
Visualization of AoSMC siRNA delivery was performed using standard fluorescence microscopy.
2.1.4 Quantification of target mRNA knockdown
Standard qRT-PCR was used to quantify and compare level of TSP-2 RNA transcripts. Primers (Table S2) were obtained from Integrated DNA Technologies (Coralville, IA). For quantitative analysis, target gene levels were normalized to B2M levels. Gene expression in cells treated with TSP-2 specific siRNA was measured as fold change over cells treated with control siRNA.
2.2 Synthesis and Characterization of Unmodified and Surface Functionalized ePET
2.2.1 Electrospinning of ePET materials
Electrospinning of ePET was done by our collaborators using a computer-automated electrospinning apparatus. PET polymer chips were dissolved in hexafluoroisopropanol (HFIP) and mixed for 48 hours on an inversion mixer. After mixing, the PET solution was perfused at a steady rate. As the polymer solution reached the needle port, a voltage of +20kV was applied. The polymer jet was collected onto a 30mm mandrel, resulting in the formation of a flat sheet after removal (ePET). The ePET sheet was then sonicated in absolute ethanol for 30 minutes followed by a 2-minute sonication in distilled water to remove residual solvent. ePET material was then air-dried overnight at room temperature in Kimwipes. All ePET materials (unmodified and surface modified) were sterilized using an Anprolene ethylene oxide sterilizer (25°C, 12 hour cycle, 35% relative humidity, 24 hour degas).
2.2.2 Surface modification of ePET materials
Segments (5cm × 5cm) were cut from the main ePET sheet. One segment was placed into 0.5% (w:v) NaOH at 100°C for 30 minutes followed by a rinse with a copious amount of distilled water. This process creates carboxylic acid groups within the PET polymer chain.[22] Another segment of ePET was placed into 50% ethylenediamine (diluted from concentrate with distilled water) for 6 hours at room temperature followed by an overnight incubation in distilled water and another rinse with distilled water. This process creates amine groups within the PET polymer chain.[23] Formation of functional groups was confirmed via dye uptake studies with methylene blue (carboxylic acid) and acid red 1 (amine).
2.2.3 Evaluation of surface morphology of ePET materials
Scanning Electron Microscopy (SEM) was used to visualize strand morphology. PTFE, utilized in peripheral vascular graft materials, served as reference group.
2.3 AoSMC Attachment to Untreated and Surface Functionalized ePET
2.3.1 Visualization of AoSMC attachment to ePET
AoSMCs (40,000 cells) labeled with fluorescent cytoplasmic dye, Cell Tracker Green (Life Technologies, Grand Island, NY), were seeded onto 5mm × 5mm DyLight Red containing ePET pieces for 3 hours. Standard confocal microscopy was used to visualize cell attachment. PTFE, as the most common peripheral vascular graft material served as reference group.
2.3.2 Quantification of AoSMC attachment to ePET
Segments of untreated and surface modified ePET materials (5mm × 5mm) were placed onto the bottom of 96 well plates. AoSMCs (40,000 cells) were seeded on top of each ePET segment and allowed to attach for 3 hours. ePET pieces were removed, rinsed with PBS and placed in a new well. An Alamar blue assay, previously described by our group [11], was performed to monitor cell attachment and viability. Direct attachment of AoSMC to the tissue culture plate well in absence of ePET was used as control. Again, PTFE served as an experimental control.
2.4 Dip-coating ePET Materials with Different Combinations of siRNA (With and Without PEI)
2.4.1 Visualization of siRNA adsorption to dip-coated ePET
Different siRNA and transfection reagent combinations were made according to Table 2. Segments of untreated ePET and ePET treated with EDA or NaOH (5mm × 5mm) were incubated in the respective siRNA solutions at room temperature for 50 minutes, rinsed with sterile PBS solution (Dulbecco, Radnor, PA), blotted dry and mounted on microscope slides using SlowFade Antifade Kit (Invitrogen) for standard confocal microscopy imaging.
Table 2.
siRNA/transfection solutions tested for dip coating.
| No transfection reagent | RNAiMax | PEI N/P=10 | |
|---|---|---|---|
| Unlabeled control siRNA | X | ||
| siGLO Red | X | X | X |
| Chol-siRNA | X | X | X |
2.4.2 Quantification of siRNA adsorption to dip-coated ePET
Seven 5×5mm ePET samples were placed in a 1.5ml centrifuge tube and submerged in 100μl siRNA (2μg) solution for 45 minutes. Using a NanodropTM UV-spectrophotometer, siRNA concentration was measured in the solution before and after dipping of the ePET pieces. The relative change in siRNA concentration of the solution after dipping was calculated and expressed as a ratio normalized to the starting concentration.
2.4.3 Quantification of AoSMC attachment to dip-coated ePET
ePET segments (5mm × 5mm) were dip-coated as described above with different siRNA and PEI combinations (Table 3). AoSMC (40,000 cells) were seeded on top of each of ePET piece and allowed to attach from 3 hours up to 48 hours. After 3 and 48 hours, ePET pieces were removed, rinsed with PBS and placed in a new well with fresh media. An Alamar blue assay was performed as previously described to monitor cell attachment and viability.[3]
Table 3.
Combinations of siRNA/transfection solutions used for dip-coating ePET tested for AoSMC attachment.
| No transfection reagent | PEI N/P=10 | |
|---|---|---|
| ePET | X | X |
| Control siRNA | X | X |
| Chol-siRNA | X | X |
2.4.4 siRNA uptake from siRNA treated ePET: Uptake visualization and target mRNA silencing quantification in AoSMC
siRNA+PEI dip-coated ePET pieces (5mm × 5mm) were placed on top of a confluent layer of AoSMC for 24 hours after which cells were harvested for qRT-PCR to assess level of mRNA knockdown. Visualization of AoSMC siRNA delivery was performed using standard confocal microscopy. Standard qRT-PCR technique was used to measure TSP-2 gene knockdown
2.5 Statistical analysis
Each experiment was performed at least three independent times (n=3). For quantification of AoSMC attachment, siRNA adsorption and qRT-PCR, each treatment in each experiment was done in duplicate. For multivariate analysis, 2-Way ANOVA was used, with a p<0.05 considered statistically significant.
3. RESULTS
3.1 Confirmation of siRNA delivery and target mRNA knockdown
3.1.1 Delivery of siRNA to AoSMCs
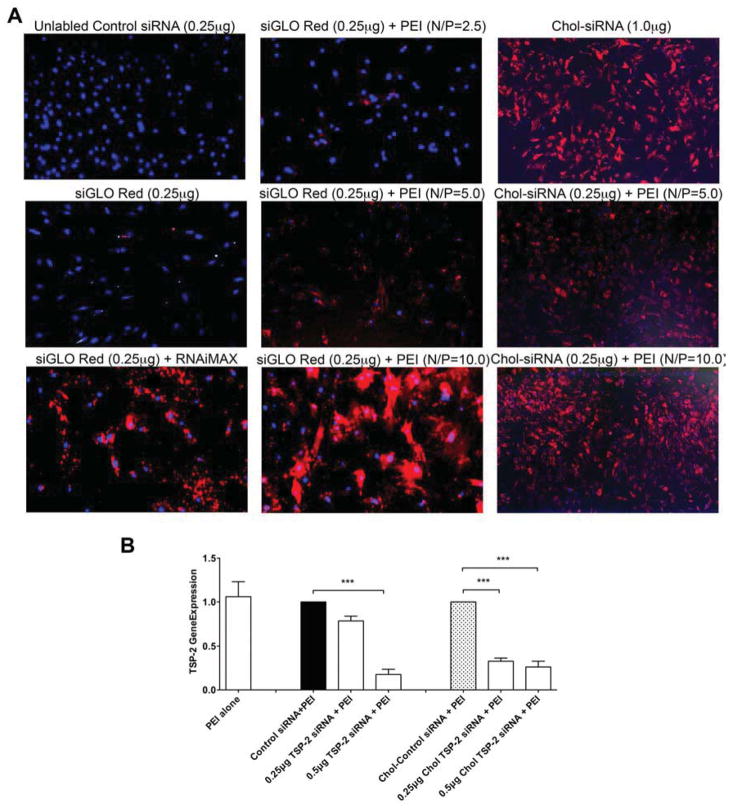
Visualization of AoSMC siRNA transfection was confirmed by fluorescence microscopy and confocal microscopy (Figure 1A). AoSMCs were transfected as described in Table 1. Transfection of AoSMCs with control siRNA did not result in any visible intracellular fluorescence. Transfection of AoSMCs with siGLO Red in the absence of a transfection reagent resulted in only minimal delivery into AoSMCs. Complexing siGLO Red with a commercially available liposomal transfection reagent, RNAiMAX increased siRNA delivery into AoSMCs. Complexing siGLO Red with the cationic polymer PEI led to a N/P ratio dependent increase in delivery into AoSMC. Best transfection results were observed with an N/P ratio = 10. In contrast, even in the absence of a transfection reagent, there was significant delivery of Chol-siRNA into AoSMCs. However, to achieve this level of transfection, a higher concentration of Chol-siRNA (1μg) was required compared to PEI-complexed Chol-siRNA. Chol-siRNA complexed with PEI at N/P ratio = 5 led to modest delivery while N/P ratio = 10 showed significantly higher Chol siRNA delivery. An N/P ratio of 10 was deemed optimal for transfection success and was therefore used in the subsequent experiments.
Figure 1. Confirmation of siRNA delivery and target mRNA knockdown.
A) Fluorescent microscope images of AoSMC transfected with Control siRNA, siGLO Red or Cholesterol (Chol) siRNA complexed without transfection reagent or with RNAiMax or PEI (N/P ratio of 2.5, 5 or 10) (Mag=10x). B) Thrombospondin-2 (TSP-2) mRNA expression in AoSMC after transfecting with Control siRNA (0.5μg), TSP-2 siRNA (0.25μg or 0.5μg), Chol siRNA (0.25μg) or Chol TSP-2 siRNA (0.25μg or 0.5μg) using PEI.
3.1.2 Target mRNA knockdown in AoSMC
To ascertain that AoSMCs are amenable to TSP-2 siRNA delivery and TSP-2 mRNA knockdown, AoSMCs were transfected with control siRNA, TSP-2 siRNA, Chol-siRNA or Chol-TSP-2 siRNA (Figure 1B). Compared to control siRNA, TSP-2 siRNA (0.5μg) significantly down-regulated TSP-2 mRNA expression (1.0 ±0 vs. 0.18 ± 0.04, 5.6 fold down-regulation). Similarly, compared to Chol-siRNA (control), Chol-TSP-2 siRNA (0.25μg and 0.5μg) significantly down-regulated TSP-2 mRNA expression (1.0 ± 0 vs. 0.33 ± 0.02 and 0.26 ± 0.04, 3.0 and 3.8 fold down-regulation). These data suggest that cholesterol modification of siRNA might significantly increase transfection delivery and mRNA knockdown efficiency.
3.2 Morphology of control PTFE and ePET materials
Using SEM, the ultrastructure of untreated ePET was compared to ePET treated with EDA and with NaOH. PTFE served as a control vascular prosthetic bypass material (Figure 2). As expected, significant differences in the fibrous composition of PTFE and ePET were observed. While PTFE presented with tightly parallel-aligned fiber bundles with little inter-fiber space, ePET consisted of a loosely aligned fiber mesh with variable inter-fiber spaces and a fairly homogenous fiber diameter. EDA treatment of the ePET material led to breakage in some of the fibers, whereas the NaOH treatment did not induce any significant morphologic changes.
Figure 2. Ultrastructure of ePET.

Scanning electron microscopy images of PTFE, untreated ePET and ePET treated with EDA and NaOH, at low and high magnification.
3.3 AoSMC attachment to untreated and treated ePET
3.3.1 Visualization of AoSMC attachment to ePET
Confocal imaging of DyLight 549 containing ePET (Figure 3A) was used to visualize ePET fiber matrix in three dimensions (3D) and to characterize AoSMC ingrowth and morphology. AoSMCs infiltrated the fiber matrix, while maintaining their spindle shaped phenotype. Furthermore, a three dimensional orientation of AoSMCs throughout the matrix was observed. (Figure 3B). Fluorescence microscopy was also performed to examine AoSMC attachment differently treated ePET. PTFE served as reference material. Highest rates of AoSMC attachment were observed in the ePET and ePET treated with EDA materials (Figure 3C).
Figure 3. AoSMC attachment to ePET.
A) Confocal microscopic image of Dy549 (red fluorescence) labeled ePET. B) Confocal microscopic image of AoSMC attachment to Dy549 labeled ePET. (Green = AoSMC Cytoplasm and Blue = Nuclei). C) Fluorescent microscopic images of AoSMC attachment to PTFE, ePET, ePET treated with EDA and ePET treated with NaOH (Mag=10x). D) Quantification of AoSMC attachment to PTFE, ePET, ePET treated with EDA, and ePET treated with NaOH after 3 hours using Alamar Blue assay.
3.3.2 Quantification of AoSMC attachment to ePET
AoSMC attachment was also quantified by Alamar blue assay three hours after seeding AoSMCs onto different materials (Figure 3D). Compared to the tissue culture well and PTFE, ePET showed significantly higher attachment of AoSMCs (0.28 ± 0.04 and 0.36 ± 0.02 vs. 0.59 ± 0.05, units in OD). No significant differences were observed between ePET, ePET (NaOH) and ePET (EDA), although there appeared to be a trend for AoSMCs to attach less to ePET reacted with NaOH than to ePET or ePET reacted with EDA. These results suggested that ePET provided a better surface for cell attachment and viability profile compared to cells grown on tissue culture plates or PTFE. ePET treatment with NaOH and EDA did not change cell attachment ability of ePET.
3.4 Dip-coating ePET in different combinations of siRNA (with and without PEI)
3.4.1 Visualization of siRNA adsorption to dip-coated ePET
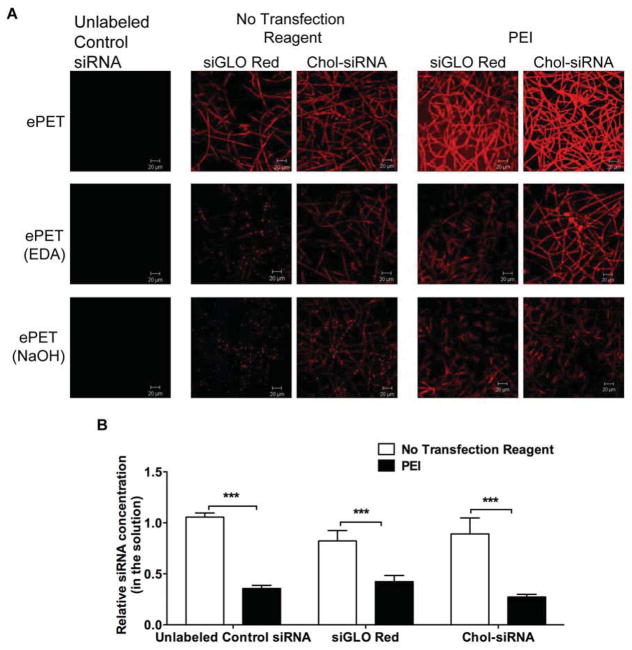
Confocal microscopy was used to visualize siRNA adsorption to untreated and treated ePET (Figure 4A). ePET (untreated and surface functionalized with EDA or NaOH) dip-coated in solution of Control siRNA showed no fluorescence. Dipping ePET in solution of siGLO Red or Chol siRNA in the absence of a transfection reagent led only to modest adsorption. Dipping ePET in PEI complexed siGLO Red or Chol-siRNA solutions significantly increased adsorption to untreated ePET but not to EDA or NaOH treated ePET. Based on these findings, the subsequent experiments were pursued with ePET coated with PEI complexed siRNA (N/P ratio of 10).
Figure 4. Dip Coating ePET with Different siRNA Solutions.
A) Confocal microscopic images showing adsorption of Control siRNA, siGLO Red or Chol siRNA complexed without or with PEI (N/P ratio of 10) to dip-coated ePET, ePET treated with EDA or ePET treated with NaOH. B) Quantification of adsorption of Control siRNA, siGLO Red or Chol siRNA complexed without or with PEI (N/P ratio of 10) to dip-coated ePET. C) Quantification of attachment of AoSMC to uncoated ePET or ePET dip-coated in Control siRNA, siGLO Red or Chol siRNA complexed with PEI after 3 and 48 hours.
3.4.2 Quantification of siRNA adsorption to ePET
Based on the confocal microscopy results, siRNA adsorption to ePET dip-coated with Control siRNA, siGLO Red and Chol siRNA was quantified. siRNA concentration was quantified via measurement of siRNA in the incubation solution pre and post dipping using UV spectrophotometer (Nanodrop) and a ratio of before/after concentration was calculated (Figure 4B). A ratio of ‘1’ suggests that there was no change in siRNA concentration in the solution, indicating siRNA did not get adsorbed onto the material. A ratio of less than 1 suggests that the siRNA concentration in the solution after dipping is lower than in the beginning of the dipping indicating higher adsorption of the siRNA to ePET. Control siRNA did not adsorb to ePET in the absence of PEI (1.05±0.02), while siGLO Red and Chol siRNA in the absence of PEI only modestly adsorbed (0.82 ± 0.05 and 0.89 ± 0.09, respectively). Complexing all siRNAs to PEI led to significant adsorption to ePET (Control siRNA = 0.36 ± 0.02; siGLO Red = 0.42 ± 0.03; Chol-siRNA = 0.27 ± 0.01). These results confirm that complexation of unmodified siRNA, siGLO Red and Chol siRNA with PEI (N/P ratio = 10) significantly enhanced adsorption onto the ePET material.
3.4.3 Quantification of AoSMC attachment to dip-coated ePET
AoSMC attachment assay suggested that siRNA-PEI coating of ePET did not interfere with AoSMC attachment at three hours nor did it lead to cell loss after 48 hours of cell seeding (Figure 4C).
3.4.4 siRNA transfection and target mRNA knockdown in AoSMC using dip-coated ePET
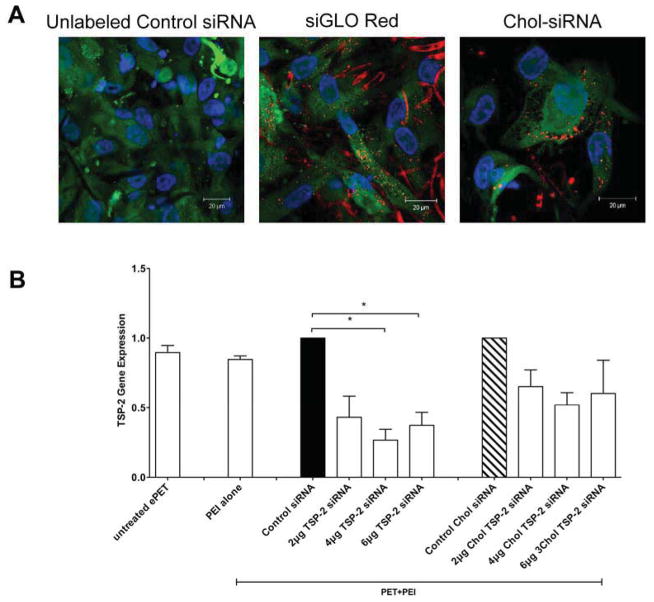
Confocal microscopy illustrated that AoSMC attached and grew into siRNA+PEI dip-coated ePET (Figure 5A). Control siRNA coated ePET exhibited no red fluorescence in AoSMC. AoSMCs exposed to siGLO-Red (no PEI) dip-coated ePET only showed minimal red fluorescence (data not shown). In contrast, exposure of AoSMCs to siGLO Red+PEI or Chol-siRNA+PEI dip-coated ePET resulted in significant siRNA uptake.
Figure 5. siRNA Delivery and Target Gene Knock-Down in AoSMC.
A) Confocal microscopic images of AoSMC transfected with ePET dip-coated in control siRNA, siGLO Red or Chol siRNA complexed with PEI. 40000 AoSMCs were placed on top of dip-coated ePET for 48 hours for transfection or dip-coated ePET was placed on top of confluent layer of AoSMC for 24 hours for transfection. B) Thrombospondin-2 (TSP-2) mRNA expression in AoSMC after transfection with ePET dip-coated in Control siRNA (6μg), TSP-2 siRNA (2, 4 or 6μg), Chol siRNA (6μg) or Chol TSP-2 siRNA (2, 4 or 6μg) complexed with PEI.
3.4.5 Target mRNA knockdown in AoSMC
As in the confocal microscopy imaging studies, ePET dip-coated with different siRNA combinations of siRNA+PEI were placed over a confluent layer of AoSMCs. Exposure of AoSMCs to TSP-2 siRNA+PEI dip-coated ePET led to a significant decrease of TSP-2 mRNA expression compared to control siRNA+PEI dip-coated ePET (1.0 ± 0 vs. 0.43 ± 0.15, 0.26 ± 0.07 and 0.37 ± 0.09, fold expression). Although exposure of AoSMCs to TSP-2 Chol siRNA+PEI dip-coated ePET led to a reduction in TSP-2 gene expression, it did not reach statistical significance (1.0 ± 0 vs. 0.65 ± 0.12, 0.52 ± 0.08 and 0.60 ± 0.23, fold expression) (Figure 5B). These results suggest that transfection via siRNA+PEI dip-coated ePET is a feasible approach for gene silencing.
4. DISCUSSION
Thus far, no efficacious approach to the modification of the vascular response to implanted biomaterials has been identified. Electrospun PET offers a material that has an extracellular matrix (ECM)-like structure and expanded surface area. This provides the opportunity to incorporate therapeutic agents such as siRNA into the ePET material in order to locally deliver bioactive agents.
siRNA transfection of AoSMC in vitro can be achieved using various liposomal transfection reagents such as RNAiMax. [13, 16] However, given the in vivo liability of liposomal formulations, the cationic polymer-based transfection reagent PEI was also tested. Polyethyleneimine (PEI), which has been used as a siRNA transfection reagent [24], stabilizes siRNA complexes in vivo while exhibiting minimal local and systemic toxicity.[25–27] The present study revealed that PEI complexation of siRNA leads to significant gene knockdown in vitro. The data further showed that AoSMCs quite readily attached to ePET. In fact, three-dimensional AoSMC infiltration was noted throughout the ePET fabric. No significant changes in cell attachment were noted between ePET and its surface functionalized derivatives.
PEI complexation of siRNA resulted in superior siRNA adsorption to ePET as compared to RNAiMax, a commonly used liposomal transfection reagent. Confocal microscopy imaging findings were confirmed by analyzing siRNA concentrations in coating solutions before and after dipping of ePET segments. Unlabeled siRNA was only minimally adsorbed to ePET while siGLO Red and Chol-siRNA in the absence of a transfection reagent were modestly adsorbed to ePET, which may be explained by the dye-like properties of the DyLight549 group. As seen in the confocal images, complexation of PEI and siGLO-Red or Chol-siRNA significantly enhanced siRNA adsorption, which could be attributed to Van der Waal forces between the PEI and PET. Interestingly, EDA and NaOH treatment of ePET did not significantly change the siRNA coating results.
AoSMC attachment and viability were not adversely affected by the presence of the PEI-siRNA coating. Confocal imaging confirmed siRNA uptake into infiltrating AoSMCs both from PEI-siRNA and PEI-Chol-siRNA coated ePET. However, only in the case of PEI-siRNA coated ePET did the uptake result in significant gene silencing. Our previous studies demonstrated that a threshold of intracellular siRNA has to be exceeded to achieve significant gene silencing.[13] Also, some of the visualized PEI-Chol-siRNA may have been trapped within the cell membrane and did not enter the cytosol. Thus, this entrapped PEI-Chol-siRNA may not have contributed to the intracellular siRNA pool. While the purpose of cholesterol is to aid in the cellular uptake of siRNA, the interaction of PEI may have led to complexes that upon cell entry did not sufficiently release the siRNA into the cytoplasm.
In summary, ePET effectively adsorbs PEI-siRNA using a simple dip-coating technique. Additionally, this coating does not impair AoSMC attachment or viability and results in significant gene silencing in the infiltrating cells. While PET has been used for decades in various vascular prosthetic devices, the polymer has also been used as non-absorbable suture material, in prosthetic meshes for hernia repair and for orthopedic surgery.[4, 28–41] Amongst other complications, seroma and fabric contraction have been documented after implantation of PET products.[34, 37, 38, 42] Thus, PEI-mediated siRNA coating of PET may be used to address these aspects of wound healing and thereby improve biocompatibility and longevity of medical devices such as hernia meshes and others.
Gelatin has been used to immobilize PEI-siRNA complexes to alter surface compatibility of vascular stents.[10] In contrast, the data presented here shows how therapeutic amounts of siRNA can be deposited on PET based grafts materials after simple complexation with PEI.
In future experiments, maximal siRNA loading dose and release rate from ePET will be evaluated. Layer-by-layer deposition of PEI-siRNA complexes as well as partial crosslinking of PEI may represent additional protocol modifications that could increase total siRNA deposition and modulate siRNA release from ePET fibers, respectively.
5. CONCLUSION
This report illustrates direct incorporation of therapeutic amounts of siRNA onto a prosthetic vascular graft material using PEI without exogenous binder agents. These complexes remained functional and lead to targeted gene silencing in infiltrating human aortic smooth muscle cells. This protocol could be easily transitioned into a clinical setting given its simple technical requirements. The technique could also be modified should there be a need for silencing of multiple target genes simultaneously or a prolonged siRNA release.
Supplementary Material
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health (NIH 5R01 HL021796, NIH 2R01 HL086741 and T32 HL007734) and the William J. von Liebig Foundation.
Footnotes
CONFLICT OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cordero JA, Jr, Quist WC, Hamdan AD, Phaneuf MD, Contreras MA, LoGerfo FW. Identification of multiple genes with altered expression at the distal anastomosis of healing polytetrafluoroethylene grafts. J Vasc Surg. 1998;28:157–66. doi: 10.1016/s0741-5214(98)70211-3. [DOI] [PubMed] [Google Scholar]
- 2.Hamdan AD, Aiello LP, Quist WC, Ozaki CK, Contreras MA, Phaneuf MD, et al. Isolation of genes differentially expressed at the downstream anastomosis of prosthetic arterial grafts with use of mRNA differential display. J Vasc Surg. 1995;21:228–34. doi: 10.1016/s0741-5214(95)70264-4. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida S, Nabzdyk CS, Pradhan L, LoGerfo FW. Thrombospondin-2 gene silencing in human aortic smooth muscle cells improves cell attachment. J Am Coll Surg. 2011;213:668–76. doi: 10.1016/j.jamcollsurg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S, Salacinski HJ, Hamilton G, Seifalian AM. The mechanical properties of infrainguinal vascular bypass grafts: their role in influencing patency. Eur J Vasc Endovasc Surg. 2006;31:627–36. doi: 10.1016/j.ejvs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Willis DJ, Kalish JA, Li C, Deutsch ER, Contreras MA, LoGerfo FW, et al. Temporal gene expression following prosthetic arterial grafting. J Surg Res. 2004;120:27–36. doi: 10.1016/j.jss.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Junaid Y, Malek TSM, Andersen Nicholas D, Wu Sheng-Qian, Contreras Mauricio A, LoGerfo Frank W. Selective isolation of anastomotic intimal hyperplasia for microarray analysis by laser capture microdissection. N Engl Soc Vasc Surg Ledyard, Connecticut. 2007 [Google Scholar]
- 7.Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc Natl Acad Sci U S A. 1999;96:4449–54. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009;3:215–25. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu Q, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 2009;104:688–98. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 10.Nolte A, Walker T, Schneider M, Kray O, Avci-Adali M, Ziemer G, et al. Small-interfering RNA-eluting surfaces as a novel concept for intravascular local gene silencing. Mol Med. 2011;17:1213–22. doi: 10.2119/molmed.2011.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monahan TS, Andersen ND, Martin MC, Malek JY, Shrikhande GV, Pradhan L, et al. MARCKS silencing differentially affects human vascular smooth muscle and endothelial cell phenotypes to inhibit neointimal hyperplasia in saphenous vein. FASEB J. 2009;23:557–64. doi: 10.1096/fj.08-114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monahan TS, Andersen ND, Panossian H, Kalish JA, Daniel S, Shrikhande GV, et al. A novel function for cadherin 11/osteoblast-cadherin in vascular smooth muscle cells: modulation of cell migration and proliferation. J Vasc Surg. 2007;45:581–9. doi: 10.1016/j.jvs.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Nabzdyk CS, Lancero H, Nguyen KP, Salek S, Conte MS. RNA interference-mediated survivin gene knockdown induces growth arrest and reduced migration of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2011;301:H1841–9. doi: 10.1152/ajpheart.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen ND, Monahan TS, Malek JY, Jain M, Daniel S, Caron LD, et al. Comparison of gene silencing in human vascular cells using small interfering RNAs. J Am Coll Surg. 2007;204:399–408. doi: 10.1016/j.jamcollsurg.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Liu K, Shen L, Wu H, Jing H. Small interfering RNA to c-myc inhibits vein graft restenosis in a rat vein graft model. J Surg Res. 2011;169:e85–91. doi: 10.1016/j.jss.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 16.Nabzdyk CS, Chun M, Pradhan Nabzdyk L, Yoshida S, LoGerfo FW. Differential susceptibility of human primary aortic and coronary artery vascular cells to RNA interference. Biochem Biophys Res Commun. 2012;425:261–5. doi: 10.1016/j.bbrc.2012.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen ND, Chopra A, Monahan TS, Malek JY, Jain M, Pradhan L, et al. Endothelial cells are susceptible to rapid siRNA transfection and gene silencing ex vivo. J Vasc Surg. 2010;52:1608–15. doi: 10.1016/j.jvs.2010.06.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doshi J, Reneker D. Electrospinning process and application of electrospun fibers. J Electrostat. 1995;35:151–60. [Google Scholar]
- 19.Hohman MM, Shin G, Ruteledge G, Brenner MP. Electrospinning, electrospraying and the instability of electrically forced jets, part 1. Physics of Fluids. 2001;13:2201–20. [Google Scholar]
- 20.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 21.Annis D, How TV, Fisher AC. Recent advances in the development of artificial devices to replace diseased arteries in man: a new elastomeric synthetic artery. In: Planck H, Egbers G, Syre I, editors. Polyurethanes in Biomedical Engineering. Elsevier Science Publishers B.V; Netherlands: 1984. [Google Scholar]
- 22.Phaneuf MD, Quist WC, Bide MJ, LoGerfo FW. Modification of polyethylene terephthalate (Dacron) via denier reduction: effects on material tensile strength, weight, and protein binding capabilities. J Appl Biomater. 1995;6:289–99. doi: 10.1002/jab.770060410. [DOI] [PubMed] [Google Scholar]
- 23.Zhong T, Bide MJ, Ukponmwan J, Phaneuf MD, LoGerfo FW, Quist WC. Bifunctional surface modification of polyester. American Association of Textile Chemists and Colorists; Greenville, South Carolina: 2003. p. 24. [Google Scholar]
- 24.Nimesh S. Polyethylenimine as a promising vector for targeted siRNA delivery. Curr Clin Pharmacol. 2012;7:121–30. doi: 10.2174/157488412800228857. [DOI] [PubMed] [Google Scholar]
- 25.Goyal R, Tripathi SK, Tyagi S, Sharma A, Ram KR, Chowdhuri DK, et al. Linear PEI nanoparticles: efficient pDNA/siRNA carriers in vitro and in vivo. Nanomedicine. 2012;8:167–75. doi: 10.1016/j.nano.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Zintchenko A, Philipp A, Dehshahri A, Wagner E. Simple modifications of branched PEI lead to highly efficient siRNA carriers with low toxicity. Bioconjug Chem. 2008;19:1448–55. doi: 10.1021/bc800065f. [DOI] [PubMed] [Google Scholar]
- 27.Nimesh S, Chandra R. Polyethylenimine nanoparticles as an efficient in vitro siRNA delivery system. Eur J Pharm Biopharm. 2009;73:43–9. doi: 10.1016/j.ejpb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Dettinger GB, Bowers WF. Tissue response to orlon and dacron sutures; a comparison with nylon, cotton, and silk. Surgery. 1957;42:325–35. [PubMed] [Google Scholar]
- 29.Narat JK, Cangelosi JP, Belmonte JV. Evaluation of dacron suture material for general surgery. Surg Forum. 1957;7:176–8. [PubMed] [Google Scholar]
- 30.Phelan JT, Botham RJ, Young WP, Schmidt ER. The effect of suture material in determining the patency of small artery grafts; a comparison study using silk, nylons, and dacron arterial suture material. Surgery. 1958;43:969–73. [PubMed] [Google Scholar]
- 31.Latimer EO, Werr JA. Clinical experience with dacron as a nonabsorbable suture material. Surg Gynecol Obstet. 1961;112:373–4. [PubMed] [Google Scholar]
- 32.Shumacker BH, Jr, Mandelbaum I. Clinical evaluation of dacron suture material. Arch Surg. 1961;83:647–9. doi: 10.1001/archsurg.1961.01300170003001. [DOI] [PubMed] [Google Scholar]
- 33.Henshaw RE. An appraisal of dacron suture material for general surgical use. J Am Osteopath Assoc. 1964;63:734–6. [PubMed] [Google Scholar]
- 34.Coda A, Bendavid R, Botto-Micca F, Bossotti M, Bona A. Structural alterations of prosthetic meshes in humans. Hernia. 2003;7:29–34. doi: 10.1007/s10029-002-0089-6. [DOI] [PubMed] [Google Scholar]
- 35.Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater. 2005;74:570–81. doi: 10.1002/jbm.b.30247. [DOI] [PubMed] [Google Scholar]
- 36.Roll S, Muller-Nordhorn J, Keil T, Scholz H, Eidt D, Greiner W, et al. Dacron vs. PTFE as bypass materials in peripheral vascular surgery--systematic review and meta-analysis. BMC Surg. 2008;8:22. doi: 10.1186/1471-2482-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdollahi A, Maddah GH, Mehrabi BM, Jangjoo A, Forghani MN, Sharbaf N. Prosthetic incisional hernioplasty: clinical experience with 354 cases. Hernia. 2010;14:569–73. doi: 10.1007/s10029-010-0685-9. [DOI] [PubMed] [Google Scholar]
- 38.Mehrabi M, Jangjoo A, Tavoosi H, Kahrom M, Kahrom H. Long-term outcome of Rives-Stoppa technique in complex ventral incisional hernia repair. World J Surg. 2010;34:1696–701. doi: 10.1007/s00268-010-0426-3. [DOI] [PubMed] [Google Scholar]
- 39.Wehbe MA, Ciccarello RM, Reitz JL. Trapezium resection with mersilene suspension sling. Hand Clin. 2013;29:27–35. doi: 10.1016/j.hcl.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Stein AJ, Schofield JL, Marsh M, Paulo J. Ligament reconstruction tendon interposition with mersilene augmentation. Tech Hand Up Extrem Surg. 2011;15:12–5. doi: 10.1097/BTH.0b013e3181e71728. [DOI] [PubMed] [Google Scholar]
- 41.Bouillot JL, Poghosyan T, Corigliano N, Canard G, Veyrie N. Management of voluminous abdominal incisional hernia. J Visc Surg. 2012;149:e53–8. doi: 10.1016/j.jviscsurg.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Kadakol AK, Nypaver TJ, Lin JC, Weaver MR, Karam JL, Reddy DJ, et al. Frequency, risk factors, and management of perigraft seroma after open abdominal aortic aneurysm repair. J Vasc Surg. 2011;54:637–43. doi: 10.1016/j.jvs.2011.03.258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.