Abstract
Although there have been extensive research efforts to create functional tissues and organs, most successes in tissue engineering have been limited to avascular or thin tissues. The major hurdle in development of more complex tissues lies in the formation of vascular networks capable of delivering oxygen and nutrients throughout the engineered constructs. Sufficient neovascularization in scaffold materials can be achieved through coordinated application of angiogenic factors with proper cell types in biomaterials. This review present the current research developments in the design of biomaterials and their biochemical and biochemical modifications to produce vascularized tissue constructs.
Keywords: Tissue engineering, vascularization, angiogenesis, scaffold, biomaterials
1. INTRODUCTION
There is a tremendous demand for tissue engineered organs. The number of people in the waiting list for organ transplantation surpasses the number of organs donated, and this situation is predicted to get even more serious as the population ages. For example, only 24,422 patients out of 79,512 people on the transplantation wait list received organs in the 2002 while the number of patients who died while still on the wait list exceeded 6,000 [1]. The field of tissue engineering addresses this issue by replacing and restoring various tissues and organs by delivering cells and biomolecules in biomaterials in three dimensional (3D) structures [2]. Use of either autologous or allogenic grafts in traditional transplantation surgeries has serious repercussions such as donor site morbidity and lack of appropriate donor tissue in autologous transplantation, and risk of disease transmission and extended immunosuppression in allogenic transplantation. In contrast, tissue engineering offers to circumvent these problems. In vivo implantation of issues and organs cultured, expanded, and developed ex vivo from small explants of autologous cells could significantly reduce the problems associated with donor site morbidity. With proper scale-up, supplies of tissues and organs might eventually be able to meet the growing demands.
Although there have been tremendous efforts to replace and restore various parts of the body ever since the first FDA approval of tissue engineered epidermis of skin for burn patients [3], most successes have been limited to avascular or thin tissues such as cartilage, skin, or bladder [1,4,5]. In these tissues, oxygen and nutrients can diffuse into the implants and sustain cellular viability. However, as the tissue becomes thicker, cells and tissues located more than a few hundred microns away from nearest capillaries suffer from hypoxia and apoptosis as shown in Figure 1 [6]. Thus, at the current stage, development of more complex tissues or organs such as heart, muscle, kidney, liver and lung still remains a challenge. In order to achieve this goal, there have been numerous attempts to induce vascularization of engineered tissues, and this review will report status of the current research developments relating to the design of biomaterials that promote angiogenesis-like responses.
Fig. (1).
Distribution of oxygen in tissue. Sufficient levels of oxygen and nutrient exchange occur within a few hundred micrometers away from capillaries. Limited oxygen and nutrient delivery throughout thick, implanted tissues contributes to implant failure.
Studies aimed to induce and control vascularization may also advance the clinical utility of therapeutic angiogenesis. Numerous pathological conditions are associated with insufficient blood supply. Chronic wounds such as in diabetic ulcers are caused by inadequate blood supply, leading to recurrent inflammation and infection at the affected sites [7]. Myocardial ischemia as well as peripheral arterial ischemia is a debilitating disease associated with hypoxia and tissue necrosis due to occluded vessels [8]. On the other hand, uncontrolled angiogenesis is also associated with severe pathological conditions including rheumatoid arthritis, macular degeneration, and tumor growth [6]. Therefore, it is critical to develop better understanding of mechanisms associated with angiogenesis and apply that knowledge to guide vessel growth in a regulated manner. Through coordinated application of angiogenic factors delivered in scaffold materials, we can promote sufficient neovascularization in engineered tissues as well as in tissues affected by chronic wounds and ischemia.
2. BIOLOGY OF THE MICROVASCULATURE
Unlike thick vascular walls seen in arteries and veins, a capillary is composed of endothelial cells (EC), basal membrane, and pericytes as shown in Figure 2C. The ECs lining the innermost layer of arteries, veins, and capillaries are one of the major regulators of cardiovascular physiology; ECs provide thrombo-resistance, modulation of leukocyte interactions, control of blood flow and vessel tone, and selective permeability to various molecules [9]. The formation of blood vessels is essential for establishment and maintenance of tissues, and it is classified into two categories: vasculogenesis and angiogenesis.
Fig. (2).
Processes of neovascularization. Primitive endothelial tubes are generated through A) vasculogenesis and B) angiogenesis and eventually become stabilized by recruitment of pericytes to form C) capillary structures.
2.1. Vasculogenesis vs. Angiogenesis
Vasculogenesis refers to the process of differentiation undertaken by mesodermal cells to form new blood vessels. Vasculogenesis involves three stages: 1) differentiation of mesodermal cells into angioblasts or hemangioblasts; 2) differentiation of angioblasts or hemangioblasts into ECs; 3) the organization of new ECs into a primary capillary plexus as shown in Figure 2A [10]. Angiogenesis refers to the formation of new capillary blood vessels by a process of sprouting from pre-existing vessels (Fig. 2B). Recent evidences of EC precursor cells participating in the formation of new blood vessels in postnatal life have extended the definition of angiogenesis. Thus, while vasculogenesis is restricted to embryogenesis, angiogenesis may occur from pre-existing vessels or EC precursor cells, participating in embryogenesis as well as normal and pathological vessel formation in postnatal life [10].
2.2. The Process of Angiogenesis
In their normal, quiescent state, ECs have a very slow turn over rate [11]. However, in an activated state such as in wound healing, inflammation, ischemia, and female reproductive organs, ECs change their phenotype to initiate angiogenesis. Activated ECs release various proteinases into the surrounding area to degrade the basement membrane. Vascular sprouts grow from the existing vessel into the interstitial space. During the sprouting event, ECs situated at the tips of vascular sprouts extend long filapodia, guided by the concentration gradient of chemotactic factors such as vascular endothelial growth factor (VEGF). ECs situated in the vascular stalks proliferate in response to VEGF [12]. ECs undergo vacuole formation by pinocytosis and phagocytosis, and these vacuoles coalesce to form lumen in long extensions of capillaries [13]. In the subsequent resolution phase, the capillary is stabilized by cessation of EC proliferation and synthesis of new basement membrane. The newly formed vessels become mature upon recruitment of mural cells, pericytes and smooth muscle cells (SMCs), in capillaries and larger vessels, respectively. At this point, the ECs regain their normal, quiescent state [6,10].
The process of angiogenesis is orchestrated by ECs and neighboring mural cell types via various growth factors and extracellular matrix (ECM) proteins. VEGF initiates vessel formation by attracting ECs to the site of angiogenesis and promoting EC proliferation [14]. VEGF also facilitates sprout formation in the presence of angiopoietin-2. Platelet-derived growth factor–BB (PDGF-BB) and angiopoeitin-1 recruit mesenchymal stem cells (MSCs) to the site of neovascularization [15], and transforming growth factor-β (TGF-β) guides the subsequent differentiation into mural cell types. Fully differentiated pericytes serve a number of functions to the newly formed capillaries. Their contractile phenotype allows contraction and relaxation of EC tubes, thus regulating blood flow through capillaries. They also stabilize newly formed vessels by laying down basal ECM. ECM proteins involved in neovascularization include collagen and laminin. Expression of collagen type I in the capillaries is coincident with and required for angiogenesis as ECs from collagen type I knockout mice do not form cords or tubes in culture [16]. Collagen type I fibers seem to provide a scaffold upon which ECs align and form capillary structures. DNA microarray studies performed on ECs undergoing angiogenesis have shown that collagen type IV and laminin are upregulated during EC morphogenesis [17]. In addition, the expression of the α2 integrin subunit, the receptor for collagen type IV and laminin, was upregulated correspondingly, suggesting integral role of collagen type IV and laminin in angiogenesis.
3. OVERVIEW OF APPROACHES TO VASCULARIZE ENGINEERED TISSUES
In extrinsic vascularization methods, vascularization of engineered constructs is promoted ex vivo through material design, culture conditions, and cell source. Scaffolds sufficiently pre-vascularized ex vivo would be transplanted in vivo and encouraged to integrate with the host vasculature. This approach utilizes various biochemical signals embedded in scaffolds to mimic natural microenvironment and aims to maximize angiogenic potential of the seeded cell types. The scaffolds are often modified with ECM proteins and peptides organized in micropatterns to guide angiogenesis, angiogenic factors delivered along with vascular cell types, and ECs co-cultured with secondary supporting cell types.
There have been numerous reports on design and optimization of scaffold materials to promote local angiogenesis directly in vivo and encourage infiltration of host vessels into the scaffolds. Even for scaffolds pre-vascularized ex vivo, successful integration of the implant with the host tissues largely depends on vessel and tissue ingrowth. One major theme guiding this approach is delivery of angiogenic molecules from implanted scaffolds as covered later in this review. Whereas bolus injection of angiogenic factors such as VEGF is associated with negative side effects in non-target tissues (hyperpermeable vessels, hypotension, stimulation of tumor growth, and uncontrolled neovascularization) [18], sustained delivery of angiogenic factors from scaffold materials can be localized to a microenvironment and minimize negative side effects in non-target regions. Furthermore, natural processes of secretion and sequestration of angiogenic factors in ECM beds can be mimicked closely in this system by modulating their release kinetics from the scaffolds.
4. MATERIALS FOR TISSUE ENGINEERING
4.1. Material-Modifications to Promote Neovascularization
4.1.1. Modifications with ECM Proteins
When anchorage-dependent cells are cultured on various biomaterials, ECM proteins such as collagen, fibronectin, fibrin, and gelatin, are frequently used to coat surfaces of various biomaterials to augment their interaction with cells. ECs have been cultured successfully on ECM coated polymers such as hyaluronic acid [19], poly(dimethyl siloxane) (PDMS) [20], poly(L-lysine) [21], poly(L-lactide), and poly(caprolactone) [22]. ECM coating on biomaterials can facilitate neovascularization in vivo. For example, expanded poly(tetrafluoroethylene) (ePTFE) has been adsorbed with ECM secreted by bladder carcinoma cells [23] as well as laminin-5 [24] to render the biomaterials cell-adhesive. When these scaffolds were implanted in vivo, matrix-modification stimulated angiogenesis and accelerated neovascularization in the ePTFE matrices.
Another approach to convert bioinert synthetic polymers to bioactive scaffolds is to impregnate ECM proteins throughout polymer matrices. Fibrinogen was incorporated into poly(ethylene glycol) (PEG) hydrogels to create biosynthetic hybrid scaffolds. The presence of fibrinogen in PEG hydrogels allowed 3D culture of ECs, SMCs [25], cardiomyocytes [26], MSCs [27], and embryonic stem cells [28]. Dacron, commonly used in vascular bypass grafts and prosthesis, has also been impregnated with fibrin in bulk to allow neovascularization and accelerate wound healing [29]. Functioning as reservoir for other angiogenic molecules, fibrin enhanced vascularization and the number of micro-vessels formed throughout the Dacron meshes in a subcutaneous implantation model in mice. These reports testify the importance of cellular interaction with biomaterials in neovascularization and the advantages of using ECM proteins as coating materials.
4.1.2. Modifications with ECM-Derived Peptides
Cellular interactions with ECM proteins are very complex as these proteins present cells with multiple cell binding and growth factor binding domains. To circumvent these problems, peptides, oftentimes only several amino acids long, have been derived from ECM proteins as the most basic subunits required for normal cell adhesion and proliferation. Use of synthetic peptides in cell cultures and engineered tissues can eliminate the need for mass production and purification of ECM proteins from tissue extracts.
Ever since the derivation of Arg-Gly-Asp-Ser (RGDS) peptide from vitronectin and fibronectin as the minimal peptide sequence required for integrin-mediated cell adhesion [30], numerous studies have reported application of RGDS to support cell adhesion across many different cell types, including fibroblasts [31], SMCs [32], preosteoblasts [33], pre-adipocytes [34], and MSCs [35]. In particular, ECs have been successfully cultured on RGDS grafted polymers, including hyaluronic acid hydrogels [36], derivatives of isopropylacrylamides [37,38], and PEG hydrogels [39].
A laminin-derived peptide sequence, Tyr-Iso-Gly-Ser-Arg, YIGSR, has been used to promote EC-specific cell adhesion on otherwise non-adhesive substrates. Polyurethanes incorporated with YIGSR selectively promoted EC adhesion and proliferation while minimizing platelet adhesion [40,41]. Glass [42] and PEG hydrogels [43] modified with YIGSR also enhanced EC adhesion and migration.
Arg-Glu-Asp-Val, REDV, derived from fibronectin interacts with integrins found on ECs but not on fibroblasts, SMCs, or platelets, promoting specific adhesion by ECs [44]. Recombinant ECM protein containing REDV sequence domains was recently developed and used as vascular graft materials to improve EC adhesion and endothelialization [45]. These studies have demonstrated that peptides oftentimes as short as several amino acids long can substitute bulky ECM proteins as coating materials and augment cellular adhesion and functions on biomaterials. Furthermore, the peptides with EC-specific cell adhesive properties could be micropatterned along with other peptides into specific regions to control spatial organization of ECs as well as other tissue-specific cell types.
4.1.3. Modifications with Signaling Proteins
Covalent immobilization of soluble signaling proteins on biomaterials allows sustained signaling by interfering with cellular internalization of the proteins. By optimizing the immobilization technique, bioactivity of the proteins can be retained on biomaterials. As one of the earliest examples, epidermal growth factor (EGF) covalently coupled to glass slides via a PEG linker resulted in similar level of DNA synthesis in rat hepatocytes as with soluble EGF [46]. In another study, a potent angiogenic factor, VEGF, was covalently incorporated into collagen gels using homobifunctional crosslinking reagent directed to thiol groups in collagen and VEGF. When implanted on chicken chorioallantoic membrane, the collagen gels modified with VEGF enhanced capillary formation and tissue ingrowth [47]. VEGF also has been genetically modified to express N-terminal cysteine, and the recombinant protein was conjugated to fibronectin via thiol-directed bifunctional crosslinking reagent without loss in bioactivity [48]. Another well-studied angiogenic growth factor, basic fibroblast growth factor (bFGF), has been incorporated into PEG hydrogels as an immobilized concentration gradient a gradient maker. The resultant materials guided cell alignment and migration [49].
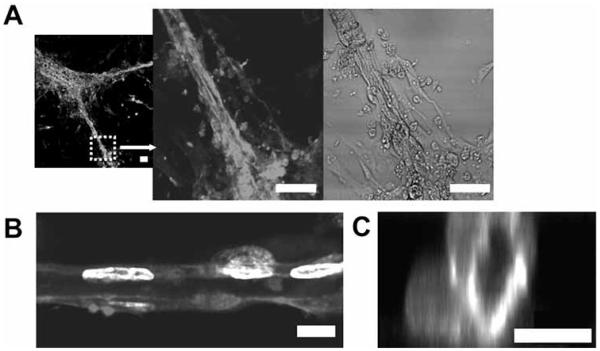
A number of cell-cell interaction proteins have been shown to play important roles in induction of vascularization and may also offer opportunities in design of angiogenic biomaterials. Zisch et al. have coupled a cell membrane protein, ephrin-B2, into fibrin matrices and shown that fibrin-bound ephrin-B2 stimulated EC angiogenic responses [50]. This work has demonstrated that matrix-bound ephrins can evoke prolonged and local signaling events in adjacent cells and tissues. In more recent work, ephrin-A1 was incorporated into PEG hydrogels and was found to stimulate EC adhesion, spreading, and tubule formation as shown in Figure 3. Interestingly, ECs cultured on the surface of these hydrogels spontaneously organized into extensive vasculature-like networks with hollow central lumen with diameters ranging 5-30 μm, resembling capillary beds.
Fig. (3).
Angiogenic responses by ECs cultured on PEG hydrogels grafted with ephrin-A1. A) Ephrin-A1 immobilized on the hydrogels stimulated formation of extensive capillary-like network with lumens. Cells were stained with phalloidin-TRITC and DAPI and visualized with confocal microscopy to reveal B) longitudinal and C) vertical cross-sections. Scale bars = 50 μm in A), and 10 μm in B, C). Adapted and reprinted with permission from Moon J.J. et al. [39] @ 2007 American Chemical Society.
4.1.4. Micropatterning Techniques to Regulate Angiogenesis
Micropatterning techniques such as photolithographic patterning, microcontact printing, micromolding, and laser photolithography can allow control over the presentation of angiogenic biomolecules at cellular length scales as outlined in Fig. 4.
Fig. (4).
Schematic diagrams of various micropatterning techniques. Photolithography, microcontact printing, micromolding, and laser lithography have been used to regulate angiogenesis on biomaterials.
Photolithography
Photolithography provides a convenient method to micropattern molecules onto surfaces of biomaterials. In typical photolithographic patterning, photo-reactive species are exposed to light through masks, creating patterns on substrates. A recently developed method of microscope projection photolithography uses transparencies printed with patterns in high-resolution to develop photoresist, which in turn serves as masters for fabrication of stamps and replica molds [51]. A commercially available liquid crystal display projector (LCDP) was also employed as light source for photolithography [52,53]. Images created in a personal computer were projected through LCDP onto photo-reactive samples, and EC adhesion was restricted to capillary vessel-like networks patterned onto the substrates [54]. An alternative method of photolithography employing conventional confocal microscope system was recently reported [55,56]. Virtual patterns were generated in a confocal scanning software, and photoreactive substrates including photoresists and PEG hydrogels were exposed to the confocal lasers as outlined by the virtual patterns with micron scale resolution easily achieved.
Microcontact Printing
Microcontact printing commonly uses the strong interaction between thiol-containing moieties and a gold substrate to pattern different biomolecules. In this technique, PDMS stamps are fabricated by casting the prepolymer against relief patterns. The stamps are then dipped into solution with alkanethiols and brought into conformal contact with a gold substrate. In one study, self-assembled monolayers (SAMs) of alkalanethiolates on gold were used to form islands of fibronectin [57]. Islands of fibronectin with various geometries were created, and the substrates were plated with ECs. Cells adhered on fibronectin-coated islands that restricted cell size (mean cell area < 500 μm2) underwent apoptosis whereas cells on larger islands that permitted spreading (mean cell area > 1500 μm2) progressed through normal cell cycle. In a subsequent study, ECs were plated on 10 μm and 30 μm wide lines of fibronectin [58]. ECs cultured on 30 μm wide lines spread to mean cell area of 3100 μm cm2 and proliferated whereas ECs cultured on 10 μm wide lines of fibronectin achieved only intermediate cell spreading (approximately 1000 μm2) but initiated capillary morphogenesis. By 72 h in culture, these ECs formed extensive cell-cell contact, and linear cellular cords that had hollow central lumen extending several cell lengths. In another study, cell-resistant anionic copolymer of oligoethyleneglycol methacrylate and methacrylic acid was micropatterned on chitosan and gelatin via microcontact printing method, leaving 20 μm wide ECM lines for cell adhesion [59]. Human microvascular ECs cultured on these substrates formed capillary tube-like structures after 5 days. These studies have demonstrated that well-defined, micropatterned substrates can provide useful tools to regulate the capillary morphogenesis and to investigate the progress of angiogenesis.
Micromolding
Micromolding processes have been applied to create complex tissue architectures in scaffold materials. In typical applications, widely available microfabrication technology is used to create master molds with desired geometry and topography on silicon wafers. A polymer solution is cast and cured on the master mold to produce a substrate with desired micropatterns. As an example, vascular network patterns were created as negative molds on silicon wafers and subsequently replica-molded into polymer substrates, including PDMS [20,60] and poly(glycerol sebacate) [61]. These polymers with molds were bonded with flat basal layers to create microfluidic channels with vascular network patterns and subsequently perfused with ECs to allow endothelialization. A similar approach was taken to create microgrooves in natural polymers. Microgrooves with defined depth and width were created in chitosan and gelatin substrates using PDMS as replica mold [62]. The plateau regions were coated with protein resistant triblock copolymers to achieve selective seeding of ECs on grooves.
Collagen gels were also explored as materials to create micropatterned composite hydrogels. Using PDMS molds, arrays of collagen gels with fibroblasts were fabricated in microscale [63]. Second application of collagen solution with another cell type allows delivery of cells around the preformed arrays of collagen gels, generating coplanar microstructures with two distinct populations of cells. The authors extended on this study to fabricate collagen gels with micropatterned cavities or channels using Matrigel [64] and gelatin layers as sacrificial elements [65]. Treatment with dispase and gelatinase selectively digested Matrigel and gelatin layers, respectively, leaving micropatterned, hollow structures t.
Three Dimensional Laser Photolithography
Laser photolithography exploits selective photopolymerization of biomolecules with lasers to create internally complex 3D materials. In one study, agarose gels were irradiated with a beam of ultraviolet (UV) light to pattern vertical channels of cell binding ligand, RGDS [66]. Ganglia cells seeded on the surface of the agarose gels invaded the gels and underwent guided neurite invasion into the vertical channels of GRGDS. A more recent work examined applicability of multiphoton laser to achieve photopolymerization in microscale in a manner that allows generation of free-form patterns in 3D [67]. A confocal microscope equipped with multiphoton laser was used to generate 3D patterns and gradients of biochemical signals in preformed PEG hydrogels. When HT-1080 fibrosarcoma cells were seeded on PEG hydrogels patterned with RGDS channels, their invasion into the hydrogels was constrained within the channels. This work was expanded to guide 3D migratory pathways of cells in PEG hydrogels [68]. Fibrin gels containing fibroblasts were encapsulated in biodegradable hydrogels, and microscale RGDS migratory pathways were created in the hydrogels with multiphoton laser. As shown in Figure 5, cells underwent guided invasion inside PEG hydrogels along the predetermined pathways decorated with RGDS whereas cells in PEG hydrogels with homogenous distribution of RGDS migrated out in all radial directions [68]. These recent developments in micropatterning technique with multiphoton laser system highlight their potential applications in creation of engineered tissues with complex 3D structures such as microvasculature.
Fig. (5).
Directed cell migration in hydrogels with multi-photon laser lithography. Biodegradable PEG hydrogels were encapsulated with fibroblasts, and RGDS were later covalently conjugated in the hydrogels with multi-photon laser photolithography. Y-shaped RGDS channels were patterned with multi-photon laser lithography and FITC-conjugated RGDS reveals the patterned regions. Staining with phalloidin-TRITC shows that fibroblasts have migrated out of the cell clusters into selective regions of RGDS channels patterned with multi-photon laser lithography [68]. Scale bar = 100 μm.
4.1.5. Formation of Gradients of Immobilized Growth Factors
Various biochemical signals are presented in vivo in concentration gradients to regulate cellular interactions. For example, EC migration is directed by chemotactic factors such as VEGF and bFGF during vessel formation. Biomaterials can be immobilized with concentration gradients of these angiogenic factors to mimic their presentations in vivo and thus guide cell migration and angiogenesis. Using laminar flow in microfluidic channels, smooth concentration gradients of ECM proteins [69] as well as RGDS peptides [70] have been immobilized on substrates to modulate cell adhesion and migration. Photolithographic techniques also have been utilized to graft EGF on surfaces of polymers in concentration gradients, and cell proliferation was enhanced in the regions immobilized with dense EGF concentration [71]. In addition, various methods were applied to create concentration gradients of biomolecules in bulk of biomaterials. Concentration gradients of RGDS [72], bFGF [49], and nerve growth factor [73] have been achieved in photopolymerizable polymers using a gradient maker, and the resulting gradient of growth factors in each hydrogel directed cell migration. Also, endothelial sprouting angiogenesis has been spatially guided in collagen gels modified with gradients of hyaluronic acid [74], demonstrating applicability of these techniques to guide and direct angiogenic responses in tissue engineered constructs.
5. DRUG DELIVERY IN TISSUE ENGINEERING
5.1. Controlled Release from Natural ECM Polymers
5.1.1. Simple Loading
Physical entrapment of growth factors in scaffold materials is a simple method of drug delivery. For example, FGF-2 was added during polymerization of photocrosslinkable chitosan hydrogels, and the majority of the added growth factor was released in a sustained fashion during in vivo degradation [75]. Addition of FGF-2 in chitosan scaffolds accelerated wound closure accompanied by increased capillary formation in healing-impaired diabetic mice models. A similar study done with bFGF and VEGF in alginate hydrogels determined that sustained local delivery of VEGF rather than FGF promotes new vessel density, thus identifying VEGF as a more potent mediator of angiogenesis [76].
5.1.2. Heparin Mediated Release
Natural ECM proteins have been modified to increase their angiogenic potential. One prevalent approach taken by many laboratories is to accelerate and maintain neovascularization by achieving sustained release profiles of growth factors from the scaffolds. To this end, heparin-modification has been examined: heparin has inherent ability to bind to various growth factors and release them in response to cellular activities. Heparin also allows prolonged presentation these growth factors by protecting them from proteolytic degradation [77] (Fig. 6). Collagen matrices were covalently immobilized with heparin, and subsequent loading with VEGF [78], bFGF [79], and combination of the two [80] resulted in significantly enhanced neovascularization throughout the matrices in in vivo models. Even in the absence of exogenous growth factors, modification with heparin increased neovascularization, possibly by potentiating endogenous growth factors present in vivo [78-80]. Similar approaches have been taken with alginate [81] and chitosan [82], and these studies corroborated the advantages of incorporating heparin in scaffolds to achieve robust neovascularization.
Fig. (6).

Fibrin matrix containing heparin-binding growth factor delivery system. Linker peptides with a heparin-binding domain were conjugated in fibrin matrices. Addition of heparin and heparin-binding growth factors allows their incorporation into the delivery system. Adapted from Sakiyama-Elbert, S.E. et al. [77].
5.1.3. Ionic Complexation
Another approach to sustain release of growth factors takes advantage of ionic complexation of growth factors with scaffold materials. Various growth factors including bFGF display lysine groups in exterior regions, resulting in overall positive charge in physiological conditions. Acidic gelatin hydrogels with an isoelectric point of 5.0 formed a poly-ion complex with bFGF and induced strong angiogenic responses via slow, sustained release of bFGF [83]. On the other hand, bFGF loaded in basic gelatin hydrogels was released in an initial burst and promoted only transient neovascularization. Release kinetics of VEGF can also be modulated by ionic complexation in poly (lactide-coglycolic) acid (PLGA). PLGA microspheres with free acid end groups retarded initial release of VEGF compared to microspheres without free acid end groups [84]. It is thought that ionic interactions between negatively charged polymer and positively charged growth factor contributed to the slow initial release.
5.1.4. Microparticles
A wide variety of both natural and synthetic materials can be manufactured into microparticles with controllable size and material properties, and research using these microparticles to deliver drugs have shown promising results. Tight control over particle composition, size, wettability, and degradation profile allows regulated release kinetics of many drugs. For example, VEGF loaded in calcium alginate microparticles promoted formation of extensive capillary beds in local regions of implantation in rats [85]. These microparticles can also be incorporated into scaffolds to ensure more localized release of the growth factors. Basic FGF has been encapsulated in PLGA microspheres, which in turn were incorporated into alginate hydrogels to fabricate composite scaffolds [86]. PLGA microspheres loaded with VEGF have also been used to form dextran-based composite hydrogels [87]. When human embryonic stem cells were cultured in these composite hydrogels, differentiation toward vascular cell lineages was significantly favored compared to the level seen in standard embryoid body cultures.
Drug delivery via microparticles allows delivery of multiple drugs with distinct release kinetics governed by particle composition. Dual delivery of VEGF and PDGF from PLGA microspheres encased in PLGA scaffolds promoted new blood vessel formation and recruitment of pericytes to the site of angiogenesis, resulting in mature vessel network [88,89]. Alginate beads [90] as well as gelatin beads [91] have been used as delivery vehicles to modulate release profiles of multiple growth factors. These studies highlight the potential applications of microparticles as vehicles for multiple drug delivery and may provide means to promote strong neovascularization throughout scaffold materials.
5.1.5. Protein Engineering
Fibrin is a natural ECM polymer that provides provisional matrices during wound healing response. In wounds, fibrin promotes natural tissue regeneration accompanied by strong neovascularization at the injury sites. Harnessing the angiogenic potentials of fibrin matrices, Zisch et al. constructed fibrin matrices immobilized with VEGF via proteolytically degradable linkers [92]. The recombinant VEGF was engineered to possess domains for matrix binding catalyzed by factor XIII during fibrin network polymerization as well as metallomatrix proteinase (MMP) sensitive linker domains for controlled release of VEGF triggered by local cellular enzymatic activities. Whereas wildtype, freely diffusible VEGF released from fibrin matrices induced chaotic development of capillary plexus in vivo, recombinant, matrix-bound VEGF induced formation of highly organized, functional vessels as shown in Figure 7 [93,94]. This system has been utilized in a study in which blood vessel formation was monitored in gradients of VEGF imparted by flow conditions [95].
Fig. (7).

Neovascularization of fibrin matrices on chicken chorioallantoic membrane. Fibrin matrices were loaded with A, B) no drug, C, D) freely diffusible VEGF, and E, F) matrix-bound VEGF. Matrix-bound form of VEGF stimulated strong neovascularization with more normal hierarchical organizations compared to freely diffusible VEGF. Scale bars = 1 mm. Adapted and reprinted with permission from Ehrbar, M. et al. [93] @ 2004 American Heart Association.
Another method of introducing angiogenic proteins to scaffold materials is to append chemically reactive species to the proteins of interest via genetic modification. VEGF was engineered to contain cysteine residue at the C-terminal position for covalent conjugation to PEG hydrogels functionalized with thiol-reactive moieties [96,97]. Encapsulated in hydrogels modified with the recombinant VEGF, ECs produced sufficient MMPs to cleave protease-sensitive peptide linkers served as the backbone of the hydrogels and remodeled the matrices [97]. When implanted in vivo, a high degree of cellular ingrowth and neovascularization were observed throughout the bioengineered matrices [96].
6. CONCLUSIONS AND FUTURE PROSPECTS
In response to the rising demand for engineered tissues and organs, there has been a tremendous investment to fabricate functional tissues in vitro, and such products may revolutionize the current way of healthcare and implantation industry. However, to achieve this daunting task of mimicking functionality and complexity of native tissues, we need to address means to regulate new vessel growth. Organized neovascularization in engineered tissues may allow development of tissues with large mass and complexity. In order to realize this goal, several key issues remain to be addressed.
First, we need a better understanding of biology behind neovascularization. Understanding the natural course of angiogenesis and arteriogenesis provides fundamental basis upon which we can build and optimize new vessel growth in biomaterials. Use of vascular cell precursors may also alleviate cell sourcing problem that can hinder industrial scale-up of engineered tissues. Second, we need to optimize fabrication of scaffold-biomolecule hybrids. There are numerous biomaterials in use today in clinical settings with reasonable biocompatibility. However, performance of these biomaterials in conjunction with incorporated bioactive factors needs to be addressed. For example, organization of biomolecules and cells in biomaterials needs to be optimized to mimic tissue complexity, and micro- and nano-patterning methods may provide solutions. Drug loading conditions and release profiles need to be examined in microenvironment with resident cells to achieve maximum efficacy of the drugs. Finally, we need to integrate pre-vascularized tissue constructs with functional cells of interest. To create functional heart, muscle, lung, etc., the native cells types or their precursors have to be either included or recruited into the scaffolds along with vascular cell types. The resulting interaction among multiple cells types has to be carefully examined so that functional tissues are regenerated with complete network of blood vessels.
ACKNOWLEDGEMENT
This research was financially supported by grants from NIH and NSF.
REFERENCES
- 1.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat. Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Naughton GK. From lab bench to market: critical issues in tissue engineering. Ann. N. Y. Acad. Sci. 2002;961:372–385. doi: 10.1111/j.1749-6632.2002.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 4.Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 1999;17:149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 5.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 6.Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J. Biomater. Sci. Polym. Ed. 2004;15:701–726. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 7.Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast. Reconstr. Surg. 2006;117:143S–149S. doi: 10.1097/01.prs.0000222526.21512.4c. discussion 150S-151S. [DOI] [PubMed] [Google Scholar]
- 8.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifalian AM, Tiwari A, Hamilton G, Salacinski HJ. Improving the clinical patency of prosthetic vascular and coronary bypass grafts: the role of seeding and tissue engineering. Artif. Organs. 2002;26:307–320. doi: 10.1046/j.1525-1594.2002.06841.x. [DOI] [PubMed] [Google Scholar]
- 10.Pepper MS, Mandriota SJ, Montesano R. The New Angiotherapy. Humana Press; Totowa, New Jersey: 2002. Angiogenesis-Regulating Cytokines; pp. 7–40. [Google Scholar]
- 11.Hirschi KK, Skalak TC, Peirce SM, Little CD. Vascular assembly in natural and engineered tissues. Ann. N. Y. Acad. Sci. 2002;961:223–242. doi: 10.1111/j.1749-6632.2002.tb03090.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell. Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 14.Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 15.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell. Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradshaw AD, Sage EH. The New Angiotherapy. Totowa, New Jersey: 2002. Regulation of Vascular Morphogenesis by Extracellular Matrix Proteins; pp. 51–66. [Google Scholar]
- 17.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J. Cell. Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 18.Epstein SE, Kornowski R, Fuchs S, Dvorak HF. Angiogenesis therapy: amidst the hype, the neglected potential for serious side effects. Circulation. 2001;104:115–119. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda J, Khademhosseini A, Yeh J, Eng G, Cheng J, Farokhzad OC, Langer R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials. 2006;27:1479–1486. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Shin M, Matsuda K, Ishii O, Terai H, Kaazempur-Mofrad M, Borenstein J, Detmar M, Vacanti JP. Endothelialized networks with a vascular geometry in microfabricated poly (dimethyl siloxane) Biomed. Microdevices. 2004;6:269–278. doi: 10.1023/B:BMMD.0000048559.29932.27. [DOI] [PubMed] [Google Scholar]
- 21.Wittmer CR, Phelps JA, Saltzman WM, Van Tassel PR. Fibronectin terminated multilayer films: protein adsorption and cell attachment studies. Biomaterials. 2007;28:851–860. doi: 10.1016/j.biomaterials.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchi F, Rosi M, Vozzi G, Emanueli C, Madeddu P, Ahluwalia A. Microfabrication of fractal polymeric structures for capillary morphogenesis: applications in therapeutic angiogenesis and in the engineering of vascularized tissue. J. Biomed. Mater. Res. B. Appl. Biomater. 2007;81:462–468. doi: 10.1002/jbm.b.30685. [DOI] [PubMed] [Google Scholar]
- 23.Kidd KR, Nagle RB, Williams SK. Angiogenesis and neovascularization associated with extracellular matrix-modified porous implants. J. Biomed. Mater. Res. 2002;59:366–377. doi: 10.1002/jbm.1253. [DOI] [PubMed] [Google Scholar]
- 24.Kidd KR, Dal Ponte D, Stone AL, Hoying JB, Nagle RB, Williams SK. Stimulated endothelial cell adhesion and angiogenesis with laminin-5 modification of expanded polytetrafluoroethylene. Tissue Eng. 2005;11:1379–1391. doi: 10.1089/ten.2005.11.1379. [DOI] [PubMed] [Google Scholar]
- 25.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Shapira-Schweitzer K, Seliktar D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater. 2007;3:33–41. doi: 10.1016/j.actbio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12:9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6004–6014. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Fournier N, Doillon CJ. Biological molecule-impregnated polyester: an in vivo angiogenesis study. Biomaterials. 1996;17:1659–1665. doi: 10.1016/0142-9612(96)87645-9. [DOI] [PubMed] [Google Scholar]
- 30.Pytela R, Pierschbacher MD, Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc. Natl. Acad. Sci. USA. 1985;82:5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 32.Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045–3051. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 33.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc. Natl. Acad. Sci. USA. 2005;102:4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel PN, Gobin AS, West JL, Patrick CW., Jr. Poly (ethylene glycol) hydrogel system supports preadipocyte viability, adhesion, and proliferation. Tissue Eng. 2005;11:1498–1505. doi: 10.1089/ten.2005.11.1498. [DOI] [PubMed] [Google Scholar]
- 35.Shin H, Temenoff JS, Bowden GC, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg-Gly-Asp modified hydrogels without dexamethasone and beta-glycerol phosphate. Biomaterials. 2005;26:3645–3654. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 36.Baier Leach J, Bivens KA, Patrick CW, Jr., Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 37.Hatakeyama H, Kikuchi A, Yamato M, Okano T. Biofunctionalized thermoresponsive interfaces facilitating cell adhesion and proliferation. Biomaterials. 2006;27:5069–5078. doi: 10.1016/j.biomaterials.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Ebara M, Yamato M, Aoyagi T, Kikuchi A, Sakai K, Okano T. Temperature-responsive cell culture surfaces enable “on-off” affinity control between cell integrins and RGDS ligands. Biomacromolecules. 2004;5:505–510. doi: 10.1021/bm0343601. [DOI] [PubMed] [Google Scholar]
- 39.Moon JJ, Lee SH, West JL. Synthetic biomimetic hydrogels incorporated with ephrin-A1 for therapeutic angiogenesis. Biomacromolecules. 2007;8:42–49. doi: 10.1021/bm060452p. [DOI] [PubMed] [Google Scholar]
- 40.Jun HW, West JL. Modification of polyurethaneurea with PEG and YIGSR peptide to enhance endothelialization without platelet adhesion. J. Biomed. Mater. Res. B. Appl. Biomater. 2005;72:131–139. doi: 10.1002/jbm.b.30135. [DOI] [PubMed] [Google Scholar]
- 41.Taite LJ, Yang P, Jun HW, West JL. Nitric oxide-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J. Biomed. Mater. Res. B. Appl. Biomater. 2007 doi: 10.1002/jbm.b.30850. [DOI] [PubMed] [Google Scholar]
- 42.Kouvroukoglou S, Dee KC, Bizios R, McIntire LV, Zygourakis K. Endothelial cell migration on surfaces modified with immobilized adhesive peptides. Biomaterials. 2000;21:1725–1733. doi: 10.1016/s0142-9612(99)00205-7. [DOI] [PubMed] [Google Scholar]
- 43.Fittkau MH, Zilla P, Bezuidenhout D, Lutolf MP, Human P, Hubbell JA, Davies N. The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials. 2005;26:167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Massia SP, Hubbell JA. Vascular endothelial cell adhesion and spreading promoted by the peptide REDV of the IIICS region of plasma fibronectin is mediated by integrin alpha 4 beta 1. J. Biol. Chem. 1992;267:14019–14026. [PubMed] [Google Scholar]
- 45.Heilshorn SC, DiZio KA, Welsh ER, Tirrell DA. Endothelial cell adhesion to the fibronectin CS5 domain in artificial extracellular matrix proteins. Biomaterials. 2003;24:4245–4252. doi: 10.1016/s0142-9612(03)00294-1. [DOI] [PubMed] [Google Scholar]
- 46.Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat. Med. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- 47.Koch S, Yao C, Grieb G, Prevel P, Noah EM, Steffens GC. Enhancing angiogenesis in collagen matrices by covalent incorporation of VEGF. J. Mater. Sci. Mater. Med. 2006;17:735–741. doi: 10.1007/s10856-006-9684-x. [DOI] [PubMed] [Google Scholar]
- 48.Backer MV, Patel V, Jehning BT, Claffey KP, Backer JM. Surface immobilization of active vascular endothelial growth factor via a cysteine-containing tag. Biomaterials. 2006;27:5452–5458. doi: 10.1016/j.biomaterials.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 49.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 50.Zisch AH, Zeisberger SM, Ehrbar M, Djonov V, Weber CC, Ziemiecki A, Pasquale EB, Hubbell JA. Engineered fibrin matrices for functional display of cell membrane-bound growth factor-like activities: study of angiogenic signaling by ephrin-B2. Biomaterials. 2004;25:3245–3257. doi: 10.1016/j.biomaterials.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Love JC, Wolfe DB, Jacobs HO, Whitesides GM. Microscope projection photolithography for rapid prototyping of masters with micron-scale features for use in soft lithography. Langmuir. 2001;17:6005–6012. [Google Scholar]
- 52.Itoga K, Yamato M, Kobayashi J, Kikuchi A, Okano T. Micropatterned surfaces prepared using a liquid crystal projector-modified photopolymerization device and microfluidics. J. Biomed. Mater. Res. A. 2004;69:391–397. doi: 10.1002/jbm.a.30010. [DOI] [PubMed] [Google Scholar]
- 53.Itoga K, Yamato M, Kobayashi J, Kikuchi A, Okano T. Cell micropatterning using photopolymerization with a liquid crystal device commercial projector. Biomaterials. 2004;25:2047–2053. doi: 10.1016/j.biomaterials.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 54.Itoga K, Kobayashi J, Yamato M, Kikuchi A, Okano T. Maskless liquid-crystal-display projection photolithography for improved design flexibility of cellular micropatterns. Biomaterials. 2006;27:3005–3009. doi: 10.1016/j.biomaterials.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 55.Hahn MS, Miller JS, West JL. Laser scanning lithography for surface micropatterning on hydrogels. Adv. Mater. 2005;17:2939–2942. [Google Scholar]
- 56.Miller JS, Bethencourt MI, Hahn M, Lee TR, West JL. Laser-scanning lithography (LSL) for the soft lithographic patterning of cell-adhesive self-assembled monolayers. Biotechnol. Bioeng. 2006;93:1060–1068. doi: 10.1002/bit.20809. [DOI] [PubMed] [Google Scholar]
- 57.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol. Prog. 1998;14:356–363. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- 58.Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In. Vitro. Cell. Dev. Biol. Anim. 1999;35:441–448. doi: 10.1007/s11626-999-0050-4. [DOI] [PubMed] [Google Scholar]
- 59.Co CC, Wang YC, Ho CC. Biocompatible micropatterning of two different cell types. J. Am. Chem. Soc. 2005;127:1598–1599. doi: 10.1021/ja044382a. [DOI] [PubMed] [Google Scholar]
- 60.Borenstein JT, Terai H, King KR, Weinberg EJ, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication technology for vascularized tissue engineering. Biomed. Microdev. 2002;4:167–175. [Google Scholar]
- 61.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 62.Wang YC, Ho CC. Micropatterning of proteins and mammalian cells on biomaterials. FASEB J. 2004;18:525–527. doi: 10.1096/fj.03-0490fje. [DOI] [PubMed] [Google Scholar]
- 63.Tang MD, Golden AP, Tien J. Molding of three-dimensional microstructures of gels. J. Am. Chem. Soc. 2003;125:12988–12989. doi: 10.1021/ja037677h. [DOI] [PubMed] [Google Scholar]
- 64.Tang MD, Golden AP, Tien J. Fabrication of collagen gels that contain patterned, micrometer-scale cavities. Adv. Mater. 2004;16:1345–1348. [Google Scholar]
- 65.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab. Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 66.Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 2004;3:249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 67.Hahn MS, Miller JS, West JL. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv. Mater. 2006;18:2679–2684. [Google Scholar]
- 68.Lee SH, Moon JJ, West JL. Three-dimensional patterning of bioactive hydrogels via two-photon laser scanning lithography for guided 3D cell migration. 2007. Submitted. [DOI] [PMC free article] [PubMed]
- 69.Dertinger SK, Jiang X, Li Z, Murthy VN, Whitesides GM. Gradients of substrate-bound laminin orient axonal specification of neurons. Proc. Natl. Acad. Sci. USA. 2002;99:12542–12547. doi: 10.1073/pnas.192457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burdick JA, Khademhosseini A, Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir. 2004;20:5153–5156. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- 71.Chen G, Ito Y. Gradient micropattern immobilization of EGF to investigate the effect of artificial juxtacrine stimulation. Biomaterials. 2001;22:2453–2457. doi: 10.1016/s0142-9612(00)00432-4. [DOI] [PubMed] [Google Scholar]
- 72.DeLong SA, Gobin AS, West JL. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J. Control. Release. 2005;109:139–148. doi: 10.1016/j.jconrel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Kapur TA, Shoichet MS. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J. Biomed. Mater. Res. A. 2004;68:235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- 74.Borselli C, Oliviero O, Battista S, Ambrosio L, Netti PA. Induction of directional sprouting angiogenesis by matrix gradients. J. Biomed. Mater. Res. A. 2007;80:297–305. doi: 10.1002/jbm.a.30896. [DOI] [PubMed] [Google Scholar]
- 75.Obara K, Ishihara M, Ishizuka T, Fujita M, Ozeki Y, Maehara T, Saito Y, Yura H, Matsui T, Hattori H, Kikuchi M, Kurita A. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials. 2003;24:3437–3444. doi: 10.1016/s0142-9612(03)00220-5. [DOI] [PubMed] [Google Scholar]
- 76.Lee KY, Peters MC, Mooney DJ. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J. Control. Release. 2003;87:49–56. doi: 10.1016/s0168-3659(02)00349-8. [DOI] [PubMed] [Google Scholar]
- 77.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J. Control. Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 78.Steffens GC, Yao C, Prevel P, Markowicz M, Schenck P, Noah EM, Pallua N. Modulation of angiogenic potential of collagen matrices by covalent incorporation of heparin and loading with vascular endothelial growth factor. Tissue Eng. 2004;10:1502–1509. doi: 10.1089/ten.2004.10.1502. [DOI] [PubMed] [Google Scholar]
- 79.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJ, Brouwer LA, Veerkamp JH, van Kuppevelt TH. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J. Biomed. Mater. Res. 2002;62:185–194. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 80.Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–1131. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 81.Chinen N, Tanihara M, Nakagawa M, Shinozaki K, Yamamoto E, Mizushima Y, Suzuki Y. Action of microparticles of heparin and alginate crosslinked gel when used as injectable artificial matrices to stabilize basic fibroblast growth factor and induce angiogenesis by controlling its release. J. Biomed. Mater. Res. A. 2003;67:61–68. doi: 10.1002/jbm.a.10061. [DOI] [PubMed] [Google Scholar]
- 82.Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, Yura H, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials. 2004;25:699–706. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 83.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20:2169–2175. doi: 10.1016/s0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 84.Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, Cuthbertson A. Development of poly-(D,L-lactide–coglycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J. Control. Release. 2001;72:13–24. doi: 10.1016/s0168-3659(01)00258-9. [DOI] [PubMed] [Google Scholar]
- 85.Elcin YM, Dixit V, Gitnick G. Extensive in vivo angio-genesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif. Organs. 2001;25:558–565. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- 86.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. A. 2003;65:489–497. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 87.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28:2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 89.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatiotemporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm. Res. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 90.Peirce SM, Price RJ, Skalak TC. Spatial and temporal control of angiogenesis and arterialization using focal applications of VEGF164 and Ang-1. Am. J. Physiol. Heart. Circ. Physiol. 2004;286:H918–925. doi: 10.1152/ajpheart.00833.2003. [DOI] [PubMed] [Google Scholar]
- 91.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release. 2005;101:111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 92.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF–fibrin matrices for endothelialization. J. Control. Release. 2001;72:101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 93.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, Wood J, Burri PH, Hubbell JA, Zisch AH. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res. 2004;94:1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 94.Ehrbar M, Metters A, Zammaretti P, Hubbell JA, Zisch AH. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J. Control. Release. 2005;101:93–109. doi: 10.1016/j.jconrel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 95.Helm CL, Fleury ME, Zisch AH, Boschetti F, Swartz MA. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl. Acad. Sci. USA. 2005;102:15779–15784. doi: 10.1073/pnas.0503681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointer-active cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 97.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J. Biomed. Mater. Res. 2004;68A:704–716. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]