Abstract
This study characterizes the effect of an excess-calorie, high-fat, high-cholesterol, high-fructose diet on metabolic parameters and reproductive function in female Ossabaw minipigs. Cycling sows were fed a hypercaloric, high-fat, high-cholesterol, and high-fructose diet (obese, n = 4) or a control diet (control, n = 5) for 13 mo. During the final 4 mo, ovarian ultrasonography was done, blood was collected, and weights and measures were taken. Pigs then underwent ovarian stimulation. Cycle length and androstenedione, total testosterone, progesterone, estradiol, follicle-stimulating hormone, luteinizing hormone, insulin, fructosamine, lipid, and glucose levels were measured. In addition, adipose tissue aromatase gene expression was assessed. As compared with control pigs, obese pigs were hyperglycemic and hyperinsulinemic; had elevated total cholesterol, triglyceride, and leptin levels, and demonstrated abdominal adiposity. Visceral adipose tissue of obese pigs, as compared with control pigs, showed increased aromatase gene expression. Obese pigs had longer estrous cycles, higher serum androstenedione, and higher luteal phase serum luteinizing hormone, compared with control pigs. During the luteal phase, obese pigs had more medium, ovulatory, and cystic ovarian follicles, whereas control pigs had more small ovarian follicles. When fed an excess-calorie, high-fat, high-cholesterol, high-fructose diet, female Ossabaw minipigs develop obesity, metabolic syndrome, and abnormal reproductive function. This animal model may be applicable to studies of the effects of obesity on fertility in women.
Abbreviations: CV, coefficient of variation; FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin; LH, luteinizing hormone; MetS, metabolic syndrome; qPCR, real-time quantitative PCR
Adiposity is related to fertility in women14 and female animals.21 Both malnourishment and overnutrition5,25 impair female fertility. Obesity causes perturbations in the hypothalamic–pituitary–ovarian axis via feedback from leptin and insulin,29 which may contribute to the decreased mean serum luteinizing hormone (LH) concentration and amplitude found in obese subjects.18 Furthermore, human epidemiologic studies have demonstrated a relationship between obesity and ovulatory dysfunction,8 miscarriage,6 and gestational complications.19 Although obesity is known to affect fertility outcomes in women and female animals, the underlying mechanisms responsible for obesity-induced infertility remain unknown. For this reason, a suitable animal model is needed in which to study the molecular basis of the relationship between obesity and female reproductive function.
Multiple animal models have been used to study obesity-associated reproductive dysfunction.38 Many of these models involve genetically modified strains of rodents fed high-fat diets and, therefore, may not mimic the disease pathogenesis in humans. Although nonhuman primate models of obesity closely model the disease manifestation in humans,40 the cost of primates and their long lag time to reproductive maturity render them inaccessible to large-scale research. Pigs are a valuable tool for the study of human disease due to their anatomic, physiologic, and biochemical similarities to humans.41 There are considerable similarities between cycle length in humans and pigs,15 and pigs have the same lipoprotein profile as do humans.12
Our goal was to validate Ossabaw minipigs as an animal model for the effects of obesity on metabolic parameters and reproductive function in women. Ossabaw minipigs have a mutation in PRKAG3 that causes increased intramuscular fat, resulting in a thrifty phenotype.11 When fed an excess-calorie, high-fat, high-cholesterol, high-fructose diet, Ossabaw minipigs naturally develop features that mimic metabolic syndrome (MetS) in humans, including visceral obesity, glucose intolerance, and dyslipidemia.11,28
Our study objectives were to characterize the effects of a hypercaloric, high-fat diet on metabolic parameters and reproductive function in female Ossabaw minipigs and to perform ovarian stimulation in female Ossabaw minipigs to compare their in vivo ovarian response to that reported for obese women.
Materials and Methods
All experimental animal procedures were performed in compliance with University of Illinois Urbana-Champaign IACUC regulation, and followed the guidelines outlined in the Guide for the Care and Use of Laboratory Animals.17
Ossabaw husbandry and diet treatment.
Nine multiparous Ossabaw female pigs (Sus scrofa; age, 6 to 8 y) were acquired from Indiana University Purdue University Indianapolis. On the basis of physical exams, animals were deemed healthy and allocated to diet treatment groups. Pigs received their respective diet treatments for 9 mo prior to the initiation of this 4-mo study and throughout the study period. Five pigs were fed 2200 Kcal daily of the control diet (Rund diet, UIUC, Urbana, IL), which was composed of corn (57.5%) and soy (40%) and supplemented with vitamins and minerals, and the remaining 4 pigs were fed 6770 Kcal daily of a high-fat, high-cholesterol, high-fructose diet formulated to induce MetS (obese diet).28 The obese diet was composed of a base pelleted pig feed (LabDiet Swine Diet 20% Fructose 5KA6, Purina Mills, St Louis, MO) supplemented (percentage by weight) with soybean oil (17.1%), cholesterol (2%), cholate (0.7%), corn oil (2.3%), and granular fructose (8.9%).27 During the study, pigs were housed in isolated 100 ft2 group pens, with 2 to 4 sows per pen. Pigs were on a 12:12-h light:dark cycle and had ad libitum access to water. One of the obese pigs died of an unexpected cardiac incident during the night before the ovarian stimulation test. Due to the timing of her death, her tissues could not be preserved for histology. Therefore, 3 obese pigs comprise the cohort from which the obese pig data was generated in the ovarian stimulation test and the histologic assessment of the liver.
Sample collection.
Jugular blood collection and ovarian ultrasonography were performed twice weekly. Transrectal ovarian ultrasonography with a 7.5-mHz linear transducer (model UST 5541-7.5, 38-mm Linear Reproductive Transducer and Prosound SSD-3500SV, Aloka, Wallingford, CT) was conducted in a Panepinto low-stress sling.31 Pigs were weighed weekly. Crown-to-rump length (between the eyes to the tail head), heart girth (in the axilla), height (at the point of the shoulder), and abdominal girth (the widest point of the abdomen) were measured weekly.
Assessment of glucose homeostasis.
The Precision Xtra glucometer (Abbott Laboratories, Bedford, MA) was validated for use in Ossabaw pigs by the University of Illinois-Urbana-Champaign Veterinary Diagnostic Lab. Fasting plasma blood glucose (mg/dL) was monitored twice weekly. Serum fructosamine was assessed every 4 wk (University of Illinois-Urbana-Champaign Veterinary Diagnostic Lab). Serum leptin and insulin concentrations were measured biweekly by using a radioimmunoassay (Linco–Millipore, Billerica, MA). Insulin resistance according to the modified homeostatic model assessment for insulin resistance was calculated.11
Plasma lipids.
Plasma lipid analysis was conducted as previously described.11 Monthly total plasma cholesterol (mg/dL) and triglyceride (mg/dL) concentrations were assayed by using enzymatic kits (Cholesterol EZ, Triglyceride EZ, Sigma–Aldrich, St Louis, MO). HDL (mg/dL) was assayed (Cholesterol EZ), and LDL (mg/dL) was determined by subtracting the HDL concentration and 20% of the triglycerides content from the total cholesterol level.9
Histologic preparation of liver.
Liver tissue was fixed in 10% buffered formalin, embedded in paraffin wax blocks, and serially sectioned (5 µm). Slides were stained with Mayer and eosin Y solution (Sigma–Aldrich).
Ovarian stimulation protocol.
The ovarian stimulation protocol mimicked a human long gonadotropin-releasing hormone agonist protocol.26 Starting in midluteal phase, pigs received a dose of the gonadotropin-releasing hormone agonist triptorelin acetate (100 μg SC; Trp, ProSpec-Tany TechnoGene, Rehovot, Israel) twice daily until no ovulatory size follicles were observed (15 to 19 d).26 At that time, 2 doses (10 to 20 mg SC each) of dinoprost tromethamine (PGF2α; Lutalyse, Pharmacia and Upjohn, Pfizer, New York, NY) were administered 12 h apart to ensure luteolysis. Subsequently, sows received a total daily dose of 100 mg follicle-stimulating hormone (FSH; Folltropin V, Bioniche Animal Health Canada, Belleville, Canada) and 1.67 mg LH (Lutropin V, Bioniche Animal Health Canada) divided equally and given subcutaneously every 8 h for a period of 2 to 7 d, until follicles of ovulatory size (larger than 6.5 mm) were observed.26 Then, a single dose of 2500 IU of human chorionic gonadotropin (hCG; Chorulon, Intervet, Millsboro, DE) was administered intramuscularly.
Serum hormone radioimmunoassay.
Coat-A-Count progesterone, androstenedione, estradiol, and total testosterone kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA) were validated for use with pig serum. Standards were made in charcoal-stripped pig serum for progesterone and androstenedione and in 1% PBS-gel for estradiol and total testosterone by adding a known amount of progesterone (32 to 1000 pg), androstenedione (20 to 640 pg), estradiol (2.5 to 150 pg), or total testosterone (10 to 1600 pg; Steraloids, Newport, RI) and diluting further. To validate each radioimmunoassay described, parallelism and recovery of unlabeled ligand were conducted. Serum samples for estradiol and total testosterone were extracted first with diethyl ether followed by hexane–methanol1 before radioimmunoassay. The progesterone assay sensitivity was 250 pg per tube, with an interassay coefficient of variation (CV) of 4.0% (n = 7) and an intraassay CV of 2.4% (n = 7). The androstenedione assay sensitivity was 60 pg per tube, with an interassay CV of 3.0% (n = 5) and an intraassay CV of 6.9% (n = 5). The estradiol assay sensitivity was 2.5 pg per tube, with an interassay CV of 4.9% (n = 6) and an intraassay CV of 1.2% (n = 6). The total testosterone assay sensitivity was 400 pg per tube, with an interassay CV of 3.2% (n = 3) and an intraassay CV of 1.9% (n = 3).
Serum LH and FSH were analyzed according to previously described double-antibody radioimmunoassays validated for porcine LH24 and FSH,33 with modifications. Standards were made with purified porcine LH (0.4 to 6.25 ng) and FSH ((0.08 to 5.0 ng; National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA). Aliquots of 5 µg LH or FSH in 5 µL distilled water were iodinated with 2.5 µg of chloramine-T (Chloramine-T hydrate 98%, Sigma–Aldrich) and 0.25 mCi of 125I (Perkin Elmer, Waltham, MA). The contents of the reaction vial were layered on a resin-packed column to separate free 125I from iodinated LH (125I-pLH) and FSH (125I-pFSH). The primary LH antibody was used at a final dilution of 1:4,800,000. The primary FSH antibody was used at a final dilution of 1:400,000. The LH assay was run at an average percentage binding of 29% (n = 4), with an assay sensitivity of 450 pg per tube, an interassay CV of 4.9% (n = 4), and an intraassay CV of 3.6% (n = 4). The FSH assay was run at an average binding of 34% (n = 3), with an assay sensitivity of 627 pg per tube, an interassay CV of 1.9% (n = 4), and an intraassay CV of 2.3% (n = 4). Nondetectable samples were assigned the lower limit of detection.
Follicular description.
Follicles were measured and counted according to a previously established method.23 Follicle size categories were: small antral, less than 3.5 mm; medium antral, 3.5 to 6.5 mm; ovulatory size, 6.5 to 12.5 mm; and cystic, larger than 12.5 mm.23
Real-time quantitative PCR (qPCR).
Transcript abundance of aromatase was analyzed by real-time qPCR. Total RNA was isolated by using the RNeasy Mini kit (Qiagen, Valencia, CA) followed by on-column DNase treatment (RNase-Free DNase Kit, Qiagen). RNA was quantified (Quant-iT RiboGreen, Invitrogen, Carlsbad, CA). Single-strand cDNA was synthesized (MMLV reverse transcriptase, Invitrogen). Primers were designed by using Primer3.36 Melting curve analysis and gel electrophoresis were used to confirm primer specificity. PCR products were cloned into pCR 2.1 TOPO vectors and transformed into E. coli (One Shot TOP10, Invitrogen). Plasmids were digested with EcoR1, sequenced to confirm amplicon identity, quantified (Quant-iT PicoGreen dsDNA Assay, Invitrogen), and serial dilutions prepared for the standard curve. qPCR was run on a 10-fold diluted sample of cDNA in duplicate.
Statistical analysis.
Reproductive data were divided into basal and ovarian stimulation datasets. The first complete estrous cycle for each sow was divided into follicular (approximately days 1 through 7) and luteal (approximately days 8 through 21) phases. Follicular phase was determined by a decrease in serum progesterone to below 2 ng/mL and lack of corpora lutea on ultrasonography. To standardize across pigs, the ovarian stimulation period was subdivided into thirds (first, second, and last third) by percentage of stimulation completion for each pig (initial, first day of stimulation; last third includes the day of hCG administration), and number of days posthCG. Data normality was assessed by using a Levene test of homogeneity and the Shapiro–Wilke test, and nonnormal data were log10-transformed for continuous variables and square-root–transformed for noncontinuous variables. Analysis was done by using the Proc Mixed protocol for repeated measures (version 9.2, SAS Institute, Cary, NC). Proc Mixed results were back-transformed and are presented as least-square mean ± SEM. qPCR data were analyzed by nonparametric ANOVA and the Wilcoxon rank-sum test. In all statistical tests, a P value of less than 0.05 was the criterion for statistical significance.
Results
Metabolic parameters.
Weight (control, 108.5 ± 3.8 kg; obese, 162.8 ± 4.9 kg), thoracic girth (control, 123.7 ± 2.0 cm; obese, 151.2 ± 2.3 cm), and abdominal girth (control, 127.5 ± 2.5 cm; obese, 154.9 ± 2.8 cm) were all significantly (P < 0.05) greater in obese compared with control pigs (Table 1). Height did not differ between treatment groups (control, 70.1 ± 1.8 cm; obese, 73.9 ± 2.3 cm; P > 0.05).
Table 1.
Metabolic parameters in control (n= 5) and obese (n= 4) female Ossabaw pigs
| Control pigs | Obese pigs | P | |
| Weight (kg) | 108.5 ± 3.8 | 162.8 ± 4.9 | 0.0001 |
| Thoracic girth (cm) | 123.7 ± 2.0 | 151.2 ± 2.3 | <0.0001 |
| Abdominal girth (cm) | 127.5 ± 2.5 | 154.9 ± 2.8 | 0.002 |
| Height (cm) | 70.1 ± 1.8 | 73.9 ± 2.3 | 0.8 |
| Glucose (mg/dL) | 59.1 ± 2.8 | 78.7 ± 5.1 | 0.001 |
| Insulin (µU/mL) | 12.0 ± 2.3 | 23.5 ± 2.6 | 0.003 |
| Insulin resistance score | 942.5 ± 322.9 | 1821.4 ± 365.6 | 0.024 |
| Fructosamine (µmol/L) | 282.7 ± 6.0 | 288.0 ± 6.8 | 0.570 |
| Leptin (ng/mL) | 6.6 ± 1.1 | 19.8 ± 1.1 | <0.0001 |
| Total cholesterol (mg/dL) | 102.7 ± 16.7 | 215.4 ± 19.5 | <0.0001 |
| HDL (mg/dL) | 32.6 ± 2.3 | 55.7 ± 6.1 | 0.021 |
| Triglycerides (mg/dL) | 41.6 ± 6.1 | 87.6 ± 14.2 | 0.048 |
| LDL (mg/dL) | 61.7 ± 7.8 | 87.3 ± 34.2 | 0.389 |
| LDL:HDL ratio | 3.6 ± 0.4 | 4.8 ± 1.2 | 0.429 |
Obese pigs were hyperglycemic compared with control pigs (control, 59.1 ± 2.8 mg/dL; obese, 78.7 ± 5.1 mg/dL; Table 1). Obese pigs were hyperinsulinemic (control, 12.0 ± 2.3 µU/mL; obese, 23.5 ± 2.6 µU/mL; Table 1) and had greater insulin resistance scores than did control pigs (control, 942.5 ± 322.9; obese, 1821.4 ± 365.6; Table 1). Obese pigs were not diabetic, as demonstrated by fructosamine levels, which were similar to those of control pigs (control, 282.73 ± 6.01 µmol/L; obese, 288.0 ± 6.8 µmol/L; Table 1). Obese pigs were hyperleptinemic compared with control pigs (control, 6.6 ±1.1 ng/mL; obese, 19.8 ± 1.1 ng/mL; Table 1).
Obese pigs had elevated total serum cholesterol compared with control pigs (control, 102.7 ± 16.7 mg/dL; obese, 215.4 ± 19.5 mg/dL; Table 1). Although HDL (control, 32.6 ± 2.3 mg/dL; obese, 55.7 ± 6.1 mg/dL) and triglycerides (control, 41.6 ± 6.1 mg/dL; obese, 87.6 ± 14.2 mg/dL; Table 1) were higher in obese compared with control pigs, there was no difference in LDL concentrations (control, 61.7 ± 7.8 mg/dL; obese, 87.3 ± 34.2 mg/dL) or the LDL:HDL ratio (control, 3.6 ± 0.4; obese, 4.8 ± 1.2; Table 1) between treatment groups.
Fixed, stained liver sections from 3 obese and 5 control pigs were examined for steatosis. Multiple areas of steatosis were observed in sections from one obese pig, whereas steatosis was not noted in sections from any of the control pigs.
Reproductive parameters.
Obese pigs had longer estrous cycles than did control pigs (obese, 32.2 ± 1.3 d; control, 25.2 ± 1.0 d; P = 0.002). Obese serum androstenedione was higher than control serum androstenedione in both the follicular and luteal phases, but serum total testosterone was not different between the 2 treatment groups (Table 2).
Table 2.
Basal reproductive hormone concentrations in control (n= 5) and obese (n= 4) Ossabaw pigs
| Control pigs |
Obese pigs |
|||
| Follicular | Luteal | Follicular | Luteal | |
| Androstenedione (ng/mL) | 0.6 ± 0.1a | 0.5 ± 0.1b | 1.2 ± 0.1a | 0.9 ± 0.1b |
| Total Testosterone (ng/mL) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| Estradiol (pg/mL) | 51.8 ± 3.6 | 30.2 ± 1.9 | 50.8 ± 5.2 | 25.5 ± 2.3 |
| Progesterone (ng/mL) | 1.2 ± 6.1 | 30.8 ± 3.9b | 1.7 ± 9.0 | 16.8 ± 4.5b |
| FSH (ng/mL) | 2.6 ± 0.2 | 3.4 ± 0.1 | 3.1 ± 0.3 | 3.5 ± 0.1 |
| LH (ng/mL) | 1.0 ± 0.1 | 0.9 ± 0.03b | 1.0 ± 0.1 | 1.0 ± 0.03b |
For a given hormone, significant (P < 0.05) difference between the values for control and obese pigs during the follicular phase of the estrous cycle.
For a given hormone, significant (P < 0.05) difference between the values for control and obese pigs during the luteal phase of the estrous cycle.
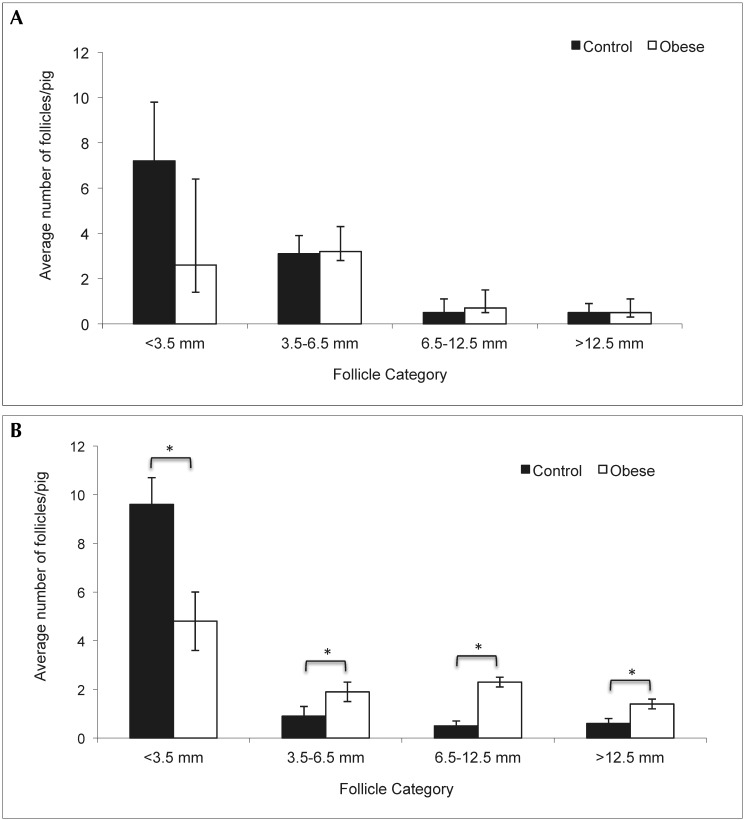
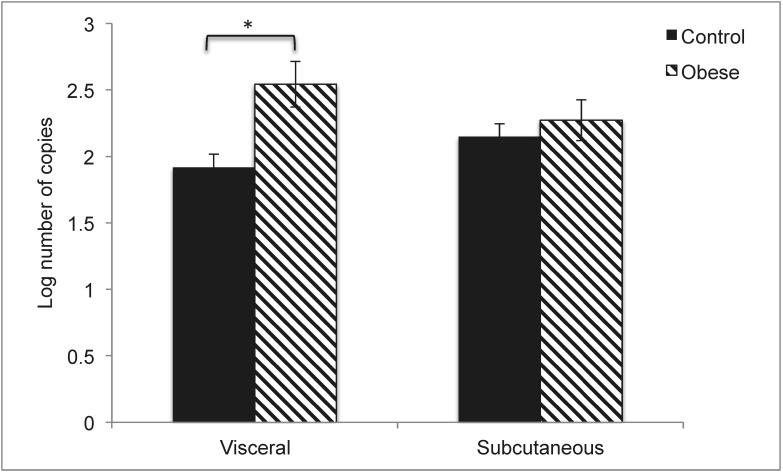
Whereas there were no significant differences in the number of ovarian follicles between the 2 treatment groups during the follicular phase, obese pigs had more medium antral, ovulatory, and cystic follicles than did control pigs during the luteal phase (Figure 1). In contrast, control pigs had more small antral follicles than did obese pigs during the luteal phase (Figure 1). During the luteal phase, obese pigs had lower serum progesterone concentrations but marginally higher serum LH concentrations than did control pigs (Table 2). All other hormone levels were similar between the treatment groups during basal sampling (Table 2). Aromatase gene expression in visceral adipose tissue was greater (P = 0.005) in obese pigs compared with control pigs (Figure 2).
Figure 1.
Number of follicles during the follicular and luteal phases in obese and control pigs. (A) Numbers of follicular-phase follicles. (B) Numbers of luteal-phase follicles. Results are expressed as least-square mean ± SEM (control, n = 5; obese, n = 4). Within a given follicle category, * indicates significant (P ≤ 0.02) difference from control value.
Figure 2.
Aromatase gene expression in visceral and subcutaneous adipose tissue. Results are expressed as least-square mean ± SEM (control, n = 5; obese, n = 4). *, P = 0.005.
Overall, obese and control pigs responded similarly to ovarian stimulation (Table 3). During the stimulation period, 2 of 3 obese pigs and 1 of 5 control pigs developed symptoms of ovarian hyperstimulation syndrome. In response to ovarian stimulation, there were no differences between the 2 treatment groups in serum hormone concentrations or numbers of large follicles. At 2 d post-hCG, obese pigs had significantly (P < 0.05) more cystic follicles than did control pigs (Table 3).
Table 3.
Response of follicular hormones and ovarian follicle growth to ovarian stimulation in control (n= 5) and obese (n= 4) Ossabaw pigs
| Stage of superovulation protocol |
|||||||
| Initial | 1st third | 2nd third | Last third | 1 d after hCG | 2 d after hCG | ||
| Testosterone (ng/mL) | Control | 0.6 ± 0.3 | 0.6 ± 0.3 | 1.0 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.5 ± 0.3 |
| Obese | 0.6 ± 0.4 | 0.7 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.3 | |
| Androstenedione (ng/mL) | Control | 0.8 ± 1.8 | 1.1 ± 1.5 | 2.4 ± 1.5 | 3.2 ± 1.5 | 2.7 ± 1.5 | 2.5 ± 1.5 |
| Obese | 0.8 ± 2.0 | 1.3 ± 2.0 | 4.8 ± 2.0 | 8.5 ± 2.0 | 6.0 ± 2.0 | 3.9 ± 2.0 | |
| Estradiol (pg/mL) | Control | 10.6 ± 68.1 | 24.1 ± 56.3 | 68.6 ± 56.3 | 69.3 ± 56.3 | 67.0 ± 56.3 | 59.3 ± 60.0 |
| Obese | 13.3 ± 112.2 | 29.6 ± 72.7 | 151.4 ± 72.7 | 241.2 ± 72.7 | 66.6 ± 72.7 | 47.3 ± 72.7 | |
| Progesterone (ng/mL) | Control | 1.6 ± 0.5 | 1.4 ± 0.4 | 2.0 ± 0.6 | 4.1 ± 2.3 | 4.5 ± 3.0 | 10.8 ± 7.9 |
| Obese | 1.7 ± 0.5 | 1.3 ± 0.5 | 2.1 ± 0.7 | 4.1 ± 3.0 | 5.2 ± 3.8 | 21.6 ± 10.2 | |
| Ovulatory size follicles | Control | 1.5 ± 0.7 | 0.7 ± 1.0 | 7.1 ± 3.0 | 17.7 ± 11.6 | 21.1 ± 10.2 | 17.0 ± 7.4 |
| Obese | 0.5 ± 0.8 | 1.4 ± 1.2 | 15.4 ± 3.4 | 34.6 ± 13.4 | 39.8 ± 11.8 | 12.2 ± 8.5 | |
| Postovulatory size follicles | Control | 0.0 ± 1.5 | 0.0 ± 1.5 | 0.0 ± 1.5 | 1.6 ± 1.5 | 2.5 ± 1.5 | 3.4 ± 1.5a |
| Obese | 0.0 ± 1.7 | 0.0 ± 1.7 | 0.0 ± 1.7 | 0.0 ± 1.7 | 5.1 ± 1.7 | 8.8 ± 1.7b | |
a,bValues are significantly (P = 0.04) different.
Discussion
The major findings of this study are: 1) mature female Ossabaw minipigs fed a hypercaloric, high-fat, high-cholesterol, high-fructose diet developed MetS (hyperinsulinemia, elevated fasting glucose, dyslipidemia) and hepatic steatosis; were hyperandrogenemic; had longer estrous cycles, and formed more persistent ovarian follicles than did age-matched lean control pigs; and 2) obese female Ossabaw minipigs developed ovarian hyperstimulation syndrome in response to ovarian stimulation but had a relatively similar hormonal and follicular response to that of control pigs. The responses of obese and lean in vitro fertilization patients to ovarian stimulation are similar to our findings in Ossabaw minipigs.37 To our knowledge, this report is the first to describe the effects of obesity with concomitant MetS on female reproductive function in a large animal model. Given that 23% of American women have MetS13 and that 64% are either overweight or obese,30 the obese Ossabaw minipig model may be useful to characterize the mechanisms underlying reproductive dysfunction due to MetS and obesity and may help develop potential treatments for obesity, MetS, and the effects of these diseases on fertility and endocrine function in women.
Obese female Ossabaw minipigs had MetS characterized by abdominal obesity, fasting hyperglycemia, and elevated triglycerides. Obese pigs weighed more and had larger circumferences in both the thoracic and abdominal regions than did control sows. Given that the heights of obese and control pigs were similar, the increased weight of obese pigs can be attributed to android fat deposition. Previous studies have demonstrated that increased abdominal girth in Ossabaw minipigs correlates (R = 0.91) significantly with visceral or android fat distribution.11 In humans, android fat distribution is highly associated with type 2 diabetes, an increased risk for cardiovascular disease,20 and increased androstenedione production,32 which we observed in our obese pigs. Obese pigs had leptin concentrations that were 3-fold higher than those of control pigs. In humans, studies have demonstrated that increased serum insulin concentrations result in increased serum leptin.28 Perhaps the hyperinsulinemia in the obese pigs also contributes to their severe hyperleptinemia. Although obese pigs did not manifest dyslipidemia, they did have elevated triglyceride and total cholesterol concentrations. Furthermore, atherogenic lipid profiles in obese patients are directly correlated with hyperandrogenemia due to accompanying hyperinsulinemia.7 Hyperinsulinemia causes increased VLDL production from the liver, resulting in increased triglyceride secretion3 as well as stimulation of androstenedione production by ovarian theca interna.2 Furthermore, given that 1 of the 3 obese pigs had hepatic steatosis, there is an indication that obese pigs are at risk for increased transport of fatty acids from adipose tissue to the liver.34 Our findings of abdominal obesity, fasting hyperglycemia, and elevated triglycerides in obese female Ossabaw minipigs corroborate our conclusion that these animals manifest MetS with evidence of hyperleptinemia and insulin resistance.
Obesity in women is associated with variable oligomenorrhea with a lengthened follicular phase, decreased mean serum LH, decreased LH pulse amplitude,39 decreased luteal progesterone,18 and variable elevations in androgens.35 Our obese female Ossabaw minipigs had elevated serum androstenedione but not total testosterone. In obese women, although the ovary, adrenal gland and adipose tissue may all contribute to androstenedione production, the ovary is the primary source of testosterone via 17β-hydroxysteroid dehydrogenase type 5 steroidogenic activity. There is very little information known about the existence of 17β-hydroxysteroid dehydrogenase type 5 in the pig ovary. A single study has demonstrated its presence in some sows during early estrus.22 In our study, testosterone did increase marginally during the ovarian stimulation protocol, indicating the potential presence of 17β-hydroxysteroid dehydrogenase type 5 in the ovary of Ossabaw pigs. Serum androstenedione was elevated nonsignificantly in obese pigs during ovarian stimulation. This functional increase in ovarian androstenedione production suggests an active role of the ovary in the production of androstenedione in obese pigs. However, because adrenal androgens like dehydroepiandrosterone sulfate and androstenedione are known to increase in parallel with obesity,16 it is possible that the basal elevation of androstenedione in obese Ossabaw minipigs is due to both adrenal and ovarian dysfunction. Furthermore, adipose tissue can convert androstenedione to testosterone and vice versa via 17β-hydroxysteroid dehydrogenase type 5,4 with omental adipose favoring androstenedione production and subcutaneous adipose favoring testosterone production.32
Obese female Ossabaw minipigs demonstrated a protracted average estrous cycle length similar to the oligomenorrhea seen in obese women. Obese pigs had lower progesterone concentrations during the luteal phase, indicating possible luteal insufficiency similar to that found in obese women.18 In future studies, numerical determination of corpora lutea would indicate whether obese pigs are oligoovulatory and thus corroborate potential luteal insufficiency. Obese pigs had a marginally significant increase in serum LH during the luteal phase compared with that in control pigs, which does not mimic LH pathology in obese women. However, given that LH was measured only twice weekly and that LH tonic secretion is episodic, this result should be interpreted judiciously. Moreover, the increased numbers of cystic follicles in obese pigs may indicate the lack of an effective LH surge or an inappropriate response of the follicle to the LH surge in obese animals. The presence of large numbers of medium antral, ovulatory, and cystic follicles during the luteal phase in obese pigs is suggestive of a hyper-responsiveness of growing follicles to FSH during this phase, coupled with a lack of appropriate responsiveness to LH during the follicular phase. Assessment of anti-Müllerian hormone in control and obese pigs would be prudent, given that anti-Müllerian hormone controls the responsiveness of antral follicles to FSH.10
In conclusion, when fed an excess-calorie, high-fat, high-cholesterol, high-fructose diet, female Ossabaw minipigs develop obesity, MetS, and ovarian and endocrine abnormalities. The inherent thrifty genotype of this animal model results in the development of diet-induced obesity and associated reproductive pathologies without a reliance on genetic manipulations that do not mimic the pathogenesis of obesity in humans. This pig model provides the opportunity to characterize the molecular mechanisms underlying obesity-induced metabolic and reproductive pathologies, which affect reproductive function and fertility in women.
Acknowledgments
We thank Robert V Knox for his assistance with follicular dynamics analysis and donation of Folltropin V and Lutropin V, Jim Wenzel and Jim Byrd for their assistance with pig handling and tissue sampling, and Kevin Armstrong for advice on statistical analysis. This work was supported by the University of Illinois, College of Agricultural, Consumer and Environmental Sciences. The authors have no competing interests.
References
- 1.Bahr JM, Wang SC, Huang MY, Calvo FO. 1983. Steroid concentrations in isolated theca and granulosa layers of preovulatory follicles during the ovulatory cycle of the domestic hen. Biol Reprod 29:326–334 [DOI] [PubMed] [Google Scholar]
- 2.Baptiste CG, Battista M-C, Trottier A, Baillargeon J-P. 2010. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol 122:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basciano H, Federico L, Adeli K. 2005. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blouin K, Veilleux A, Luu-The V, Tchernof A. 2009. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol 301:97–103 [DOI] [PubMed] [Google Scholar]
- 5.Booth PJ. 1990. Metabolic influences on hypothalamic–pituitary–ovarian function in the pig. J Reprod Fertil Suppl 40:89–100 [PubMed] [Google Scholar]
- 6.Boots C, Stephenson MD. 2011. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med 29:507–513 [DOI] [PubMed] [Google Scholar]
- 7.Castelo-Branco C, Steinvarcel F, Osorio A, Ros C, Balasch J. 2010. Atherogenic metabolic profile in PCOS patients: role of obesity and hyperandrogenism. Gynecol Endocrinol 26:736–742 [DOI] [PubMed] [Google Scholar]
- 8.Chavarro JE, Rich-Edwards JWM, Rosner BA, Willett WC. 2007. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol 110:1050–1058 [DOI] [PubMed] [Google Scholar]
- 9.Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. 1999. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol 19:2981–2992 [DOI] [PubMed] [Google Scholar]
- 10.Durlinger ALL, Visser JA, Themmen APN. 2002. Regulation of ovarian function: the role of antiMullerian hormone. Reproduction 124:601–609 [DOI] [PubMed] [Google Scholar]
- 11.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. 2006. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56:35–45 [PubMed] [Google Scholar]
- 12.Etherton TD, Kris-Etherton PM. 1980. Characterization of plasma lipoproteins in swine with different propensities for obesity. Lipids 15:823–829 [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Giles WH, Dietz WH. 2002. Prevalence of the metabolic syndrome among US adults: Findings from the Third National Health and Nutrition Examination survey. JAMA 287:356–359 [DOI] [PubMed] [Google Scholar]
- 14.Frisch RE, McArthur JW. 1974. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 185:949–951 [DOI] [PubMed] [Google Scholar]
- 15.Hall JA, Meisterling EM, Benoit AM, Cooper DA, Coleman DA, Lerner SP, Lewis PE, Dailey RA. 1993. Factors contributing to the formation of experimentally induced ovarian cysts in prepubertal gilts. Domest Anim Endocrinol 10:141–155 [DOI] [PubMed] [Google Scholar]
- 16.Hamatani T, Carter MG, Sharov AA, Ko MSH. 2004. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell 6:117–131 [DOI] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research 2011. Guide to the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 18.Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B, Santoro N. 2007. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab 92:2468–2473 [DOI] [PubMed] [Google Scholar]
- 19.Jungheim ES, Moley KH. 2010. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol 203:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanasaki K, Koya D. 2011. Biology of obesity: lessons from animal models of obesity. J Biomed Biotechnol 2011:197636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knauer M, Stalder KJ, Serenius T, Baas TJ, Berger PJ, Karriker L, Goodwin RN, Johnson RK, Mabry JW, Miller RK, Robison OW, Tokach MD. 2010. Factors associated with sow stayability in 6 genotypes. J Anim Sci 88:3486–3492 [DOI] [PubMed] [Google Scholar]
- 22.Knobil E, Neill J. 1998. Encyclopedia of reproduction. San Diego (CA): Academic Press [Google Scholar]
- 23.Knox RV. 2005. Recruitment and selection of ovarian follicles for determination of ovulation rate in the pig. Domest Anim Endocrinol 29:385–397 [DOI] [PubMed] [Google Scholar]
- 24.Kraeling RR, Rampacek GB, Cox NM, Kiser TE. 1982. Prolactin and luteinizing hormone secretion after bromocryptine (CB154) treatment in lactating sows and ovariectomized gilts. J Anim Sci 54:1212–1220 [DOI] [PubMed] [Google Scholar]
- 25.Kruip TA, Kemp B. 1999. Nutrition and fertility in agricultural domestic animals. Tijdschr Diergeneeskd 124:462–467 [PubMed] [Google Scholar]
- 26.Lainas TG, Petsas GK, Zorzovilis IZ, Iliadis GS, Lainas GT, Cazlaris HE, Kolibianakis EM. 2007. Initiation of GnRH antagonist on day 1 of stimulation as compared to the long agonist protocol in PCOS patients. A randomized controlled trial: effect on hormonal levels and follicular development. Hum Reprod 22: 1540–1546 [DOI] [PubMed] [Google Scholar]
- 27.Lee L, Alloosh M, Romil S, Van Alstine W, Watkins BA, Klaunig JE, Sturek M, Chalasani N. 2009. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology 50:56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malmström R, Taskinen MR, Karonen SL, Yki-JÄrvinen H. 1996. Insulin increases plasma leptin concentrations in normal subjects and patients with NIDDM. Diabetologia 39:993–996 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell M, Armstrong DT, Robker RL, Norman RJ. 2005. Adipokines: implications for female fertility and obesity. Reproduction 130:583–597 [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health 2012. Overweight and obesity statistics, p 1–6 Bethesda (MD): US Department of Health and Human Services [Google Scholar]
- 31.Panepinto LM, Phillips RW, Norden S, Pryor PC, Cox R. 1983. A comfortable, minimum-stress method of restraint for Yucatan miniature swine. Lab Anim Sci 33:95–97 [PubMed] [Google Scholar]
- 32.Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. 2004. Androgen generation in adipose tissue in women with simple obesity—a site-specific role for 17β-hydroxysteroid dehydrogenase type 5. J Endocrinol 183:331–342 [DOI] [PubMed] [Google Scholar]
- 33.Rayford P, Brinkley HJ, Young EP, Reichert LE., Jr 1974. Radioimmunoassay of porcine FSH. J Anim Sci 39:348–354 [DOI] [PubMed] [Google Scholar]
- 34.Reddy JK, Sambasiva Rao M. 2006. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 290:G852–G858 [DOI] [PubMed] [Google Scholar]
- 35.Rosenfield RL, Bordini B. 2010. Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity. Brain Res 1364:186–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozen S, Skaletsky HJ. 1999. Bioinformatics: methods and protocols, p 500 In: Misener S, Krawetz S. Methods in molecular biology. Totowa (NJ): Humana Press [Google Scholar]
- 37.Souter I, Baltagi LM, Kuleta D, Meeker JD, Petrozza JC. 2011. Women, weight, and fertility: the effect of body mass index on the outcome of superovulation/intrauterine insemination cycles. Fertil Steril 95:1042–1047 [DOI] [PubMed] [Google Scholar]
- 38.Szukiewicz D, Uilenbroek JTJ. 1998. Polycystic ovary syndrome —searching for an animal model. J Med 29:259–275 [PubMed] [Google Scholar]
- 39.Tadros W, Lipshitz HD. 2009. The maternal-to-zygotic transition: a play in 2 acts. Development 136:3033–3042 [DOI] [PubMed] [Google Scholar]
- 40.Tardif SD, Power ML, Ross CN, Rutherford JN. 2013. Body mass growth in common marmosets: toward a model of pediatric obesity. Am J Phys Anthropol 150:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welsh MJ, Rogers CS, Stoltz DA, Meyerholz DK, Prather RS. 2009. Development of a porcine model of cystic fibrosis. Trans Am Clin Climatol Assoc 120:149–162 [PMC free article] [PubMed] [Google Scholar]