Abstract
Appropriate animal models for intradermal vaccine delivery are scarce. Given the high similarity of their skin anatomy to that of humans, minipigs may be a suitable model for dermal vaccine delivery. Here we describe the immunization of Göttingen minipigs by using intradermal and intramuscular delivery of hepatitis B surface antigen (HBsAg). Intradermal vaccine delivery by needle and syringe and by needle-free jet injection induced humoral antiHBsAg responses. Priming immunization by using the disposable syringe jet injector (DSJI) resulted in a higher antibody titer than did conventional intradermal immunization and a titer comparable to that after intramuscular vaccination with HBsAg and Al(OH)3 adjuvant. This study highlights the utility of the minipig model in vaccine studies assessing the efficacy of conventional and novel methods of dermal delivery. Moreover, we include suggestions regarding working with minipigs during dermal vaccine delivery studies, thereby fostering future work in this area of vaccinology.
Abbreviations: DSJI, disposable syringe jet injector; HBsAg, hepatitis B surface antigen
In medical science, the use of animal models is obligatory for preclinical evaluation of drugs and therapies. A wide variety of animal models are available, all with different advantages and disadvantages. Pigs and minipigs offer several advantages over other animal models. The anatomy, physiology, and biochemistry of minipigs more closely resembles that of humans than do rodent and nonrodent models.2,14 In addition, minipigs provide a suitable model for various routes of drug administration (for example, oral, parenteral). For these reasons, minipigs have become an animal model of increasing importance and interest in the evaluation of toxicologic and pharmaceutical research questions.2,14 Moreover, the minipig model has been used in diverse pharmacologic studies in vaccine development. Minipigs have been vaccinated by the intramuscular,6 subcutaneous5 and intranasal route12 as well as by particle mediated epidermal delivery.4
Dermal delivery is attractive route for vaccination due to the high prevalence of immunocompetent cells in the skin (for example, Langerhans cells).1,15 Although dermal vaccine delivery has not yet been studied extensively in minipigs, this model could be of particular value. First, the high similarity of skin anatomy between humans and minipigs10 ensures that, as a model for dermal delivery, the minipig holds preference over conventional rodent models. Second, substantial knowledge of the porcine immune system is available compared with that for non-rodents, including dogs and Old World nonhuman primates.2
Conventional dermal delivery—often referred to as the ‘Mantoux method’—involves a needle and syringe. In recent years, there has been an increasing interest in alternative dermal vaccine delivery methods.1 One of the promising alternatives to needle-based intradermal delivery methods is the use of disposable syringe jet injectors (DSJI). DSJI bring many advantages over injection by needle and syringe: delivery into the skin by DSJI is faster than that with needle-based methods, a feature particularly relevant in pediatric applications; DSJI administration facilitates the expression of nucleic acid vaccines without the need for subsequent electroporation; and the lack of a needle eliminates potential needlestick injuries and reduces anxiety in recipients.3,7
In the current study, we tested the utility of the minipig model in the evaluation of intradermal vaccine delivery. In addition, we provide practical details regarding the use of minipigs during dermal vaccine studies. We immunized Göttingen minipigs with a hepatitis B vaccine (HBsAg) by using intradermal DSJI delivery, intradermal inoculation by needle and syringe, and intramuscular inoculation by needle and syringe. Minipigs have previously been used to assess tolerance to a DNA vaccine against hepatitis B after particle-mediated delivery.13 Moreover, the prevalence of a virus similar to human hepatitis B virus has been reported to occur in swine.11 The HBsAg immunization model therefore seems suitable for assessing the utility of the minipig model in dermal vaccine delivery.
Materials and Methods
Animals.
Female Göttingen miniature pigs (age, 12 to 15 wk; Ellegaard Göttingen Minipigs, Dalmose, Denmark) were used for immunization. The pathogen status of the minipigs was established by a thorough screening (http://minipigs.dk/healthmonitoring). The minipigs were transported from Denmark to the Netherlands in a climate-controlled minivan and maintained under standardized conditions in the animal research center of our institution (Institute for Translational Vaccinology, Bilthoven, The Netherlands). Minipigs were housed in groups of 3 in floor pens with softwood sawdust bedding (Lignocell 3-4, Lignocell, Hahn, Germany). The room was environmentally controlled on a 12:12-h light:dark cycle. Approximately 175 g of commercial minipig diet (C Peterson, Gentofte, Denmark) was provided twice daily; drinking water was available free choice. Prior to the study, the minipigs were acclimated for 14 d. The study was approved by the Committee on Animal Experimentation under permit number DPA2010-109. Animals were handled in accordance with relevant Dutch national legislation.
Immunizations.
Three groups of 3 minipigs each received 2 immunizations at a 2-wk interval (that is, on days 0 and 14). Per immunization, each pig received 20 µg HBsAg, which is equivalent to the immunization dose for adult humans.17 Dose and formulation were kept constant between immunizations. Minipigs were either immunized intradermally with 100 µL by needle in the abdominal region, intradermally with 100 µL by DSJI (first-generation device; PharmaJet, Golden, CO) in the abdominal region, or intramuscularly with 100 µL by needle in the gluteal region. The effects of the DSJI device on particle size and antigenicity of HBsAg were determined by using dynamic light scattering and ELISA (Murex, DiaSorin, Dartford, UK) respectively, as described previously.8 Jet injection did not affect the particle size, which suggests that the stress of the jet injection did not induce aggregation. Moreover, jet injection did not affect the antigenicity of the HBsAg, given that full recovery of the antigen was measured by ELISA (data not shown). Just before immunization, the skin at the intradermal injection sites was shaved, wiped with 70% ethanol, and left to dry. All immunizations were performed with freshly prepared vaccine formulations.
Vaccine formulation.
Bulk HBsAg (1.4 mg/mL) was obtained from Serum Institute of India (Pune, India) and dissolved in PBS. Vaccine formulations were prepared in a volume of 1ml. For intramuscular injection (which served as a positive control), 480 µg Al(OH)3 (Brenntag Biosector, Frederickssund, Denmark) was added to each dose. Intradermal immunizations did not include Al(OH)3 adjuvant, because of the risk of granuloma formation.
Anesthesia.
There was no need for the administration of an anesthetic prior to the immunization of minipigs by the DSJI or intramuscular routes. In contrast, intradermal priming immunization by needle induced stress in minipigs; therefore we performed the boosting intradermal immunization by needle in minipigs that had been anesthetized with isoflurane in oxygen. Isoflurane was administered by mask for a period of 3 to 5 min.
Isolation of serum.
Blood samples were collected on days 0, 14, and 28 from minipigs in dorsal recumbancy. Blood samples were withdrawn from the cranial vena cava by using 22-gauge needles. Approximately 5 mL of blood was collected in heparinized tubes (Vacuette, Greiner Bio-one, Kremsmünster, Austria), which subsequently were centrifuged for 15 min at 400 × g Serum samples were stored at −20 °C until further use.
Bleeding and euthanasia of minipigs.
Minipigs were premedicated with azaperone (4 mg/kg IM; Stresnil, Janssen Animal Health, Beerse, Belgium). Anesthesia was induced by using ketamine (12 mg/kg IM; Alfasan, Woerden, The Netherlands ) and pentobarbital (0.2 g/kg IM; Euthasol, AST Farma, Oudewater, The Netherlands). Animals were bled from incisions in the armpits, and the spleen subsequently was resected.
ELISA for IgG, IgG1, IgG2a, and IgM.
Polystyrene 96-well microtiter plates were coated overnight (room temperature) with 0.2 µg/well HBsAg diluted in 8 mM PBS, pH 7.2 (Gibco, Paisley, UK). Plates were washed 4 times with 0.1% Tween 80 in water. Samples were diluted with assay buffer (0.5% BSA and 0.05% Tween 80 in PBS) and added to the coated plates, after which the plates were incubated at 37 °C for 2 h. Plates were washed; horseradish-peroxidase–conjugated goat antipig IgG (Abcam, Cambridge, MA), mouse antipig IgG1, mouse antipig IgG2a (both from Bio-connect, TE Huissen, The Netherlands), or goat antipig IgM (AbD Serotec, Kidlington, UK) was added according to manufacturer's protocol (1:5000 for IgG, 1:100 for IgG1 and IgG2, and 1:10000 for IgM). Plates were incubated for 1.5 h at 37 °C. Next, plates were washed, and samples were incubated with a secondary conjugated goat antimouse IgG1/2a (1:5000; AbD Serotec) for 1.5 h. After washing, 100 µL tetramethylbenzidine was added, and plates were incubated for 10 min. The reaction was stopped with 50 µL 2 M H2SO4, and plates were read at 450 nm. Serum titers on days 14 and 28 were considered positive only when they were higher than the day-0 titer plus 3 SD.
Results
Dermal vaccination of minipigs.
To assess the utility of the minipig as a potential animal model for needle-free dermal vaccine delivery, Göttingen minipigs were immunized with HBsAg intradermally by needle and DSJI device and intramuscularly by injection.
After immunization by intramuscular injection or DSJI delivery, none of the minipigs showed signs of pain or stress. In contrast, minipigs were stressed and showed signs of mild pain after intradermal priming vaccination by needle. Signs consisted of struggling and loud screeching for at least 30 s on advancement of the needle into the skin. As such, it was very challenging to deliver an accurate intradermal dose by needle and syringe without anesthesia of the minipigs. Therefore, the second intradermal vaccination by needle was performed under anesthesia with isoflurane. Other practical considerations regarding intradermal vaccination of minipigs are detailed in Figure 1 and 2. None of the vaccination routes resulted in any local adverse events. In terms of animal tolerance, intradermal vaccination by the DSJI device was clearly better tolerated than was intradermal vaccination by using a needle and syringe.
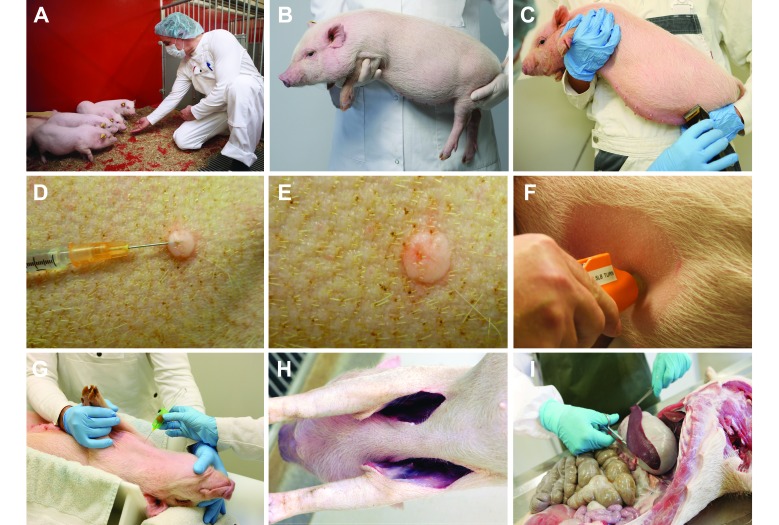
Figure 1.
A guide for handling minipigs during intradermal vaccine studies.
Intradermal delivery of the vaccine by either DSJI (Figure 2 F) or by needle and syringe (Figure 2 D) resulted in a bleb under the surface of the skin (Figure 2 E). After intradermal delivery by either method, minimal leakage originating from the bleb occurred in some of the animals.
Figure 2.
Guidelines for intradermal immunization of minipigs. (A) Frequent interaction between personnel and minipigs is recommended during the 14-d acclimation period before study inititation. (B) Minipigs can be restrained by holding them. (C) Intradermal immunization requires shaving of the abdomen. (D) Intradermal vaccination by using a 25-gauge, beveled needle requires isoflurane or sevoflurane anesthesia of pigs, to maximize accuracy and minimize stress. (E) Intradermal bleb after intradermal vaccine delivery. (F) Intradermal vaccination by using DSJI device. (G) Blood sampling of minipig in dorsal recumbancy. (H) Incisions in the armpits of a minipig after blood sampling. (I) Resection of the spleen after blood sampling.
Evaluation of vaccine immunogenicity.
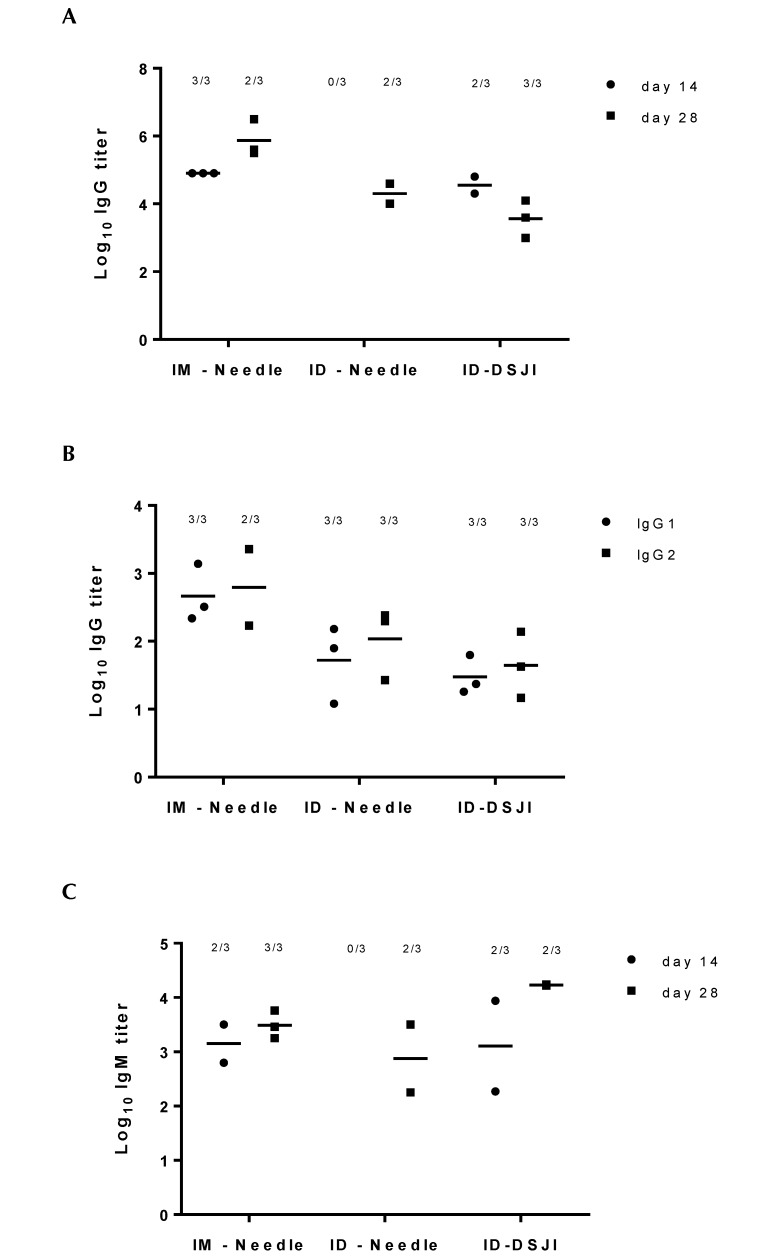
To evaluate the immunogenicity of intradermally delivered HBsAg, antibody titers in serum samples on day 0 (prior to the priming immunization), day 14 (prior to the booster immunization), and day 28 (after the booster immunization) were determined by ELISA. Serum antibody titers on days 14 and 28 were corrected for the background values of serum from the minipigs on day 0, which did not differ from background signaling. Priming immunization resulted in antiHBsAg-specific IgG responses (at day 14) in both IM-needle and ID-DSJI immunized animals. In contrast, no HBsAg specific IgG response was detected in minipigs immunized by ID-needle injection. Following a booster immunization, all 3 groups showed an IgG response (Figure 3 A). Further specification of IgG subclass titers on day 28 showed that both IgG1 and IgG2a were produced in all immunization groups (Figure 3 B). The trend of the IgM response was similar to that of the IgG response (Figure 3 C). The minimal leakage of the inoculum after delivery by DSJI did not seem to influence antibody titers in immunized minipigs (data not shown). The sample size per group was too small to enable statistical evaluation of differences in antibody titers according to inoculation route.
Figure 3.
AntiHBsAg IgG1, IgG2a, and IgM titers in immunized minipigs after various routes of vaccine administration. The numbers of responding animals per total numbers of immunized animals are depicted above the titer dots. (A) IgG titers of minipigs on days 14 and 28 after priming and booster immunization with HBsAg intramuscularly by needle (IM-needle) and intradermally by needle (ID-needle) and DSJI (ID-DSJI). (B) IgG1 and IgG2a titers in minipigs on day 28 after priming and booster immunization with HBsAg. (C) IgM titers of minipigs on days 14 and 28 after priming and booster immunization with HBsAg intramuscularly by needle and intradermally by needle and DSJI.
Discussion
By using HBsAg vaccination as a proof of principle, the current study shows that minipigs can be used for evaluation of needle-free dermal vaccine delivery.
Regardless of the route used, immunization of minipigs with HBsAg resulted in a marked humoral immune response. DSJI immunization of minipigs induced less handling-associated stress in animals than did conventional intradermal vaccination. Moreover, inoculation with DSJI resulted in higher antibody titers after priming than did conventional intradermal delivery. Although the small group size precluded statistical analysis regarding potential differences in the efficacy of the various vaccination routes, our data clearly show that the minipig model is useful for studying in dermal immunization. Although antibody titers could be detected, cellular immune responses in isolated PBMCs and splenocytes were not apparent (data not shown). This lack may reflect suboptimal conditions of HBsAg splenocyte restimulation and a low cellular immunity elicited by HBsAg.
Recent advances in dermal immunology hint to the high potency of dermal vaccine delivery.9 Minipigs offer an interesting model for assessing new vaccine-delivery methods, such as jet injection, especially because of the thickness of rodent skin, DSJI is not feasible in conventional murine models. In fact, we found that HBsAg immunization by DSJI proved impossible in mice due to the thickness of their skin (data not shown). With an increasing interest in DSJI vaccine applications, minipigs likely will become an animal model of increasing importance, particularly because the delivery device and route tested in an animal safety study should be identical to those intended to be used clinically16 and because rodent species seem unfit for DSJI. Although pigs are used more frequently to evaluate vaccine efficacy, minipigs may be preferable for various reasons (for example, husbandry, ease of handling). In addition, the guidelines we share here for handling minipigs in vaccine delivery studies may benefit future research on vaccine design, development, and delivery.
Acknowledgments
We thank Dirk Elberts, Piet van Schaaik, Tanja Schouten, and Christine Soputan from the Intravacc Animal Research Center.
References
- 1.Amorij JP, Kersten GF, Saluja V, Tonnis WF, Hinrichs WL, Slutter B, Bal SM, Bouwstra JA, Huckriede A, Jiskoot W. 2012. Towards tailored vaccine delivery: needs, challenges, and perspectives. J Control Release 161:363 –376 [DOI] [PubMed] [Google Scholar]
- 2.Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, Sims J. 2010. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods 62:196–220 [DOI] [PubMed] [Google Scholar]
- 3.De Maria N, Idilman R, Colantoni A, Van Thiel DH. 2001. Increased effective immunogenicity to high-dose and short-interval hepatitis B virus vaccination in individuals with chronic hepatitis without cirrhosis. J Viral Hepat 8:372–376 [DOI] [PubMed] [Google Scholar]
- 4.Dincer Z, Jones S, Haworth R. 2006. Preclinical safety assessment of a DNA vaccine using particle-mediated epidermal delivery in domestic pig, minipig and mouse. Exp Toxicol Pathol 57:351 –357 [DOI] [PubMed] [Google Scholar]
- 5.Fan PC, Chung WC, Lin CY, Wu CC. 2003. Vaccination trials against Taenia solium eggs in pigs injected with frozen oncospheres of T. solium or Taenia saginata asiatica. J Microbiol Immunol Infect 36:96 –100 [PubMed] [Google Scholar]
- 6.Fomsgaard A, Karlsson I, Gram G, Schou C, Tang S, Bang P, Kromann I, Andersen P, Andreasen LV. 2011. Development and preclinical safety evaluation of a new therapeutic HIV1 vaccine based on 18 T-cell minimal epitope peptides applying a novel cationic adjuvant CAF01. Vaccine 29:7067–7074 [DOI] [PubMed] [Google Scholar]
- 7.Gorres JP, Lager KM, Kong WP, Royals M, Todd JP, Vincent AL, Wei CJ, Loving CL, Zanella EL, Janke B, Kehrli ME, Jr, Nabel GJ, Rao SS. 2011. DNA vaccination elicits protective immune responses against pandemic and classic swine influenza viruses in pigs. Clin Vaccine Immunol 18:1987–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschberg H, van Kuijk S, Loch J, Jiskoot W, Bouwstra J, Kersten G, Amorij JP. 2012. A combined approach of vesicle formulations and microneedle arrays for transcutaneous immunization against hepatitis B virus. Eur J Pharm Sci 46:1 –7 [DOI] [PubMed] [Google Scholar]
- 9.Kis EE, Winter G, Myschik J. 2012. Devices for intradermal vaccination. Vaccine 30:523–538 [DOI] [PubMed] [Google Scholar]
- 10.Lavker RM, Dong G, Zheng PS, Murphy GF. 1991. Hairless micropig skin. A novel model for studies of cutaneous biology. Am J Pathol 138:687–697 [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, She R, Liu L, You H, Yin J. 2010. Prevalence of a virus similar to human hepatitis B virus in swine. Virol J 7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascua PN, Song MS, Lee JH, Park KJ, Kwon HI, Baek YH, Hong SP, Rho JB, Kim CJ, Poo H, Ryoo TS, Sung MH, Choi YK. 2009. Evaluation of the efficacy and cross-protectivity of recent human and swine vaccines against the pandemic (H1N1) 2009 virus infection. PLoS ONE 4:e8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilling AM, Harman RM, Jones SA, McCormack NA, Lavender D, Haworth R. 2002. The assessment of local tolerance, acute toxicity, and DNA biodistribution following particle-mediated delivery of a DNA vaccine to minipigs. Toxicol Pathol 30:298–305 [DOI] [PubMed] [Google Scholar]
- 14.Svendsen O. 2006. The minipig in toxicology. Exp Toxicol Pathol 57:335 –339 [DOI] [PubMed] [Google Scholar]
- 15.van der Maaden K, Jiskoot W, Bouwstra J. 2012. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release 161:645 –655 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization 2003. Guidelines on nonclinical evaluation of vaccines. WHO Technical Report Series; No. 927, Annex 1. Geneva (Switzerland): World Health Organization [Google Scholar]
- 17.Ziegler A. 2007. [Needle-free injection--science fiction or comeback of an almost forgotten drug delivery system?]. Med Monatsschr Pharm 30:297–303 [Article in German] [PubMed] [Google Scholar]