Abstract
Female vervet monkeys (Chlorocebus aethiops sabaeus) are used as an experimental model for chronic diseases relevant to women's health. However, reproductive senescence (menopause) has not yet been characterized for vervet monkeys. Here we describe the histologic, hormonal, and menstrual markers of reproductive senescence in vervet monkeys from the Wake Forest Vervet Research Colony. Ovaries from monkeys (age, 0 to 27 y) were serially sectioned (5 μm), stained, and photographed. In every 100th section, the numbers of primordial, primary, and secondary follicles were determined, and triplicate measurements were used to calculate mean numbers of follicles per ovary. Antimüllerian hormone (AMH), follicle stimulating hormone, and menstrual cycle length were measured in additional monkeys. Primordial follicles and AMH decreased significantly with age, and significant correlations between numbers of primordial and primary follicles and between numbers of primary and secondary follicles were noted. Histologic evaluation revealed that ovaries from 4 aged monkeys (older than 23 y) were senescent. One aged monkey transitioned to menopause, experiencing cycle irregularity over 4 y, eventual cessation of menses, and plasma AMH below the level of detection. Finally, with increasing age, the percentage of female vervets with offspring declined significantly. The present study provides insight into ovarian aging and reproductive senescence in vervet monkeys. Results highlight the importance of considering this nonhuman primate as a model to investigate the relationships between ovarian aging and chronic disease risk.
Abbreviations: AMH, antimüllerian hormone; CV, coefficient of variation; FSH, follicle-stimulating hormone; STRAW, Stages of Reproductive Aging Workshop
Women are characterized as being menopausal after 12 consecutive months without a menstrual cycle. Ovarian activity and hormone production (mainly estradiol) during menopause are reduced compared with those during premenopausal years. Postmenopausal women are more vulnerable to cardiovascular disease, osteoporosis, and declining cognitive function than are their premenopausal counterparts. Many of these chronic diseases have been associated with menopause, but controversy remains regarding the role of menopause and changes in reproductive hormones in disease onset and progression.7,20
Among nonhuman primates, macaques have been used extensively to model human reproductive function and aging and the pathobiology of associated chronic diseases (that is, bone loss and atherosclerosis). The use of macaques in these research areas is due to the similarities to women in their reproductive physiology and vulnerability to cardiovascular disease, obesity, and diabetes.17,18,24,30 Similar to macaques, vervets (African green monkeys, Chlorocebus aethiops sabaeus) are vulnerable to obesity and insulin resistance and, in this species, these traits are heritable.16,33 In addition, the vervet menstrual cycle is similar to that in macaques and women, with an average length of approximately 29 d (follicular phase, approximately 12 d; luteal phase, approximately 16 d); midcycle rises in estradiol, follicular stimulating hormone (FSH), and luteinizing hormone; and similar follicular dynamics resulting in ovulation and corpus luteum formation.19,22 Vervets offers additional advantages for reproductive studies, in that this species is characterized by a lack of seasonal anestrus, a straight cervical canal,32 and an inability to act as a host for Macacine herpesvirus 1 (Cercopithecine herpesvirus 1 or B virus, a potentially lethal antropozoonotic virus).9 However, in contrast to macaques, little is known about ovarian aging and reproductive senescence in vervets.
The purpose of the present study was to collect ovarian and reproductive data (hormones and menstrual cyclicity) from female vervet monkeys of known age (6 d to older than 25 y) by using archived ovarian tissues and blood samples, vaginal swabbing, and offspring data. Results of this study provide insights about ovarian aging and reproductive senescence in vervet monkeys and will increase understanding of the interaction between reproductive phenomena and multiple disease processes associated with aging.
Materials and Methods
Animals and housing.
Vervet monkeys (Chlorocebus aethiops sabaeus) and archived tissues and samples used in this study originated from the Vervet Research Colony housed at the Wake Forest School of Medicine. This colony is a multigenerational, pedigreed and genotyped colony that was originally founded in 1975 with 57 animals imported from the islands of St Kitts and Nevis. It has been a closed colony since the mid1980s, with no new animals imported since that time. In early 2008, the colony was transferred from its original location in California to the Wake Forest School of Medicine facilities. All animals involved in this study were colony-born and of known age. All breeding groups are screened annually for SIV, simian retrovirus, simian T-cell leukemia virus, Cercopithecine herpesvirus 1, and measles.14
With the exception of one monkey (no. 979), which was pair-housed, all vervets were housed in social group pens that consisted of a large outdoor area (approximately 1200 ft2) and a divided indoor area (300 ft2). All pens were fitted with elevated perches, platforms, and climbing structures. Each social group contained 1 to 2 adult male vervets and varying numbers of adult female monkeys and immature offspring.
Overview of study design.
Ovarian aging and reproductive senescence in vervet monkeys was characterized by using archived ovaries and blood samples (n = 13), menstrual cycle records and opportunistic blood samples (n = 1), prospectively collected blood samples (n = 68), and archived offspring data (n = 247) from the monkeys in the colony. Archived ovaries were derived from monkeys that ranged in age from 6 d to 27.2 y and that had died naturally or were euthanized as part of terminal research studies. Ovaries from these animals were selected to include prepubertal, sexually mature, and postreproductive stages of maturity. In light of previous reports, we assumed that the predicted reproductive lifespan for vervets is approximately 20 y.22,34 Longitudinal measures of menstrual cyclicity were recorded in one aged monkey (no. 979) for about 4 y. Archived blood samples from this monkey (collected for other studies unrelated to the current one) were used to measure reproductive hormones (FSH, estradiol, progesterone, and antimüllerian hormone [AMH]). In addition, AMH was measured in 68 additional female vervets from the colony (age, 5.7 to 25.7 y). Finally, the percentage (according to age) of female vervets in the colony with infants was investigated by using archived records from 247 female vervets with equal exposure to male monkeys during the 2008 breeding season.
All animal procedures related to this study were done in accordance with federal, state, and institutional guidelines and were approved by the IACUC of Wake Forest School of Medicine. Wake Forest School of Medicine is AAALAC-accredited.
Determination of ovarian follicle number and ovarian histopathologic evaluation.
A single archived ovary (right ovary) from each of 13 vervet monkeys was selected for this study. Follicles were quantified from one ovary per monkey due to the follicular symmetry of ovaries.21 Ovaries were fixed in Bouin solution, bisected on the short axis, and embedded in paraffin with the cut side down. Each ovary was serially sectioned (5 μm) and stained with hematoxylin and eosin. By using a sampling technique validated by our group for cynomolgus monkeys3,4 and by others for rodents10,15,31 and ewes,1 every 100th section was digitally captured by using a camera (model DS Fi1, Nikon, Tokyo, Japan) that was mounted on a microscope with a mechanical stage (model BH2, Olympus, Center Valley, PA). To ensure each follicle was counted only once, follicles in each section were quantified by using the tag function of Image-Pro Plus imaging software (version 5.1, Media Cybernetics, Bethesda, MD). Counts obtained from the sections were summed to obtain total follicles counted for each ovary. This procedure was repeated by the same, blinded observer (HA) and was completed a third time by a separate blinded observer (MS). The mean number of follicles per ovary was calculated by using all 3 counts. Intra- and interobserver coefficients of variation (CV) were determined for all follicle counts.21 The intraobserver CV was defined as the SD of duplicate measures made by a single observer divided by the mean of duplicate measures and multiplied by 100. Intraobserver CV and correlations for primordial follicles counts were CV = 9.5%, r = 0.99, and 95% CI = 0.968–0.998); for primary follicles were CV= 25.3%, r = 0.93, and 95% CI = 0.767 to 0.980; and for secondary follicle counts were CV = 22.8%, r = 0.88, and 95% CI = 0.612 to 0.965 (P < 0.01 for all correlations). The interobserver CV was defined as the SD of triplicate measures obtained from 2 observers divided by the mean of triplicate measures and multiplied by 100. Interobserver CV and correlations for primordial follicles were CV = 21.2%, r = 0.99, and 95% CI = 0.971 to 0.998; for primary follicles were CV = 25.8%, r = 0.82, and 95% CI = 0.453 to 0.946; and for secondary follicles were CV = 24.2%, r = 0.97, and 95% CI = 0.908 to 0.993 (P < 0.01 for all correlations).
Primordial, primary, and secondary follicles were categorized by using histologic criteria outlined previously.4,27 A primordial follicle was defined as an oocyte surrounded by a single layer of flattened granulosa cells. Primary follicles were classified as follicles with one or more cuboidal granulosa cells arranged in a single layer around an oocyte. Secondary follicles were classified as oocytes surrounded by 2 or more layers of cuboidal granulosa cells at any point around a follicle that lacked an antrum. Because of their large size and increased likelihood for over-counting, we noted but did not include antral follicles in the data set. Ovaries also were examined for additional histologic features indicative of ovarian aging and histopathology.
Reproductive hormone measurements.
FSH was measured from archived samples collected at or within 6 mo of ovary collection in 9 of the 13 monkeys in the ovary archive study. In one monkey (no. 1099), the only sample available for FSH measurement had been collected approximately 25 mo prior to ovary collection. Monkey FSH radioimmunoassays were performed by the Endocrine Technology and Support Lab (Oregon National Primate Research Center, Beaverton, OR) by using a double-antibody radioimmunoassay procedure.25 The FSH radioimmunoassay kit (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Los Angeles, CA) was a homologous cynomolgus macaque assay with recombinant cynomolgus FSH (catalog no. AFP-6940A, National Hormone and Peptide Program) for both iodination and standards. To complete the assay, the rabbit anticynomolgus FSH (catalog no. AFP-782594, National Hormone and Peptide Program) was used at a final dilution of 1:1,038,462. The standard curve for each tube ranged between 0.005 and 10 ng. The detection limit of the assay for each tube was 0.005 to 0.02 ng. Specificity for cynomolgus FSH was provided with the kit reagents. The intraassay variation for this assay was 6.7%. The interassay variation was less than 12%.
Estradiol, FSH, and AMH were measured using archived samples collected from monkey 979, not timed according to menstrual cycle day. Estradiol was measured using a modified commercially available radioimmunoassay (Diagnostic Products, Los Angeles, CA).26 Progesterone was measured using titrated steroids in an radioimmunoassay procedure.2 AMH was measured at the time of monkey 979’s death (age, 27 y) and in an additional 68 female vervets from the colony. AMH was measured in duplicate using an AMH ELISA assay (catalog no. AL-105-I, Ansh Labs, Webster, TX).
Longitudinal assessment of menstrual cyclicity in an aged vervet female.
Menstrual cycle data (cycle length and variability) were collected from a single, aged vervet (no. 979) over a period of 4 y, beginning at age 22.5 y and ending at 26.8 y. This vervet was housed socially with another monkey in caging that allowed her to be separated from her pair-mate for short periods of time (10 to 15 min). By using well-established techniques,2 positive reinforcement (food rewards) was used to train the monkey to move to the front of the cage and allow a cotton swab to be inserted into the vagina to the level of the cervix and then withdrawn. The training period lasted about 2 wk, and data collection was initiated after training was completed. The presence of blood on the swab was recorded on a scale of 1 to 3: 1, scant; 2, moderate; and 3, heavy. The first day of heavy bleeding was recorded as day 1 of the menstrual cycle. Vaginal swabbing was performed daily every week for 4 y. Menstrual cycle data, FSH, and AMH concentrations were used to assign a reproductive stage according to the 2012 Stages of Reproductive Aging Workshop (STRAW) criteria.11-13
Evaluation of fecundity.
The percentage (by age category: 4 to 10 y, 11 to 15 y, 16 to 20 y, and 21 y and older) of female vervets with infants was investigated by using breeding records from 247 female monkeys with equal exposure to male vervets. Female monkeys were observed throughout a single year, with age and breeding outcomes recorded. In this study, a successful breeding outcome was defined as having an infant that survived more than a week after parturition.
Statistical analyses.
The main outcome variables for quantification of ovarian follicles were mean primordial, primary and secondary follicle numbers counted per ovary and age (years). To meet normality and equality of variance assumptions for correlation analyses, mean follicle numbers and AMH were square-root–transformed. Pearson correlations between the square root of mean follicle numbers and age, among follicle types, between the percentage of female vervets with offspring and age, and between AMH and age were determined by using JMP software (version 9, SAS Institute, Cary, NC). The Kruskal–Wallis test was used for comparisons of follicle numbers and percentage offspring by age category (6 d to 9 y, 10 to 19 y, and 20 to 27 y), and χ2 P values are reported. Both inter- and intraobserver CV were determined for each follicle type. Descriptive statistics (that is, mean ± SE) for menstrual cycle bleeding data were determined by using Excel 2007 (Microsoft, Redmond, WA). For all statistical analyses, a P value of less than 0.05 was considered significant.
Results
Relationships among ovarian follicle number, reproductive hormones and age.
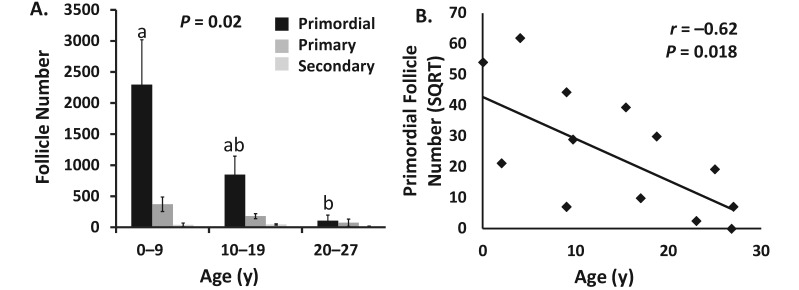
Follicle count, age, and FSH concentrations from each monkey are depicted in Table 1. All follicle types decreased numerically with age. However, only primordial follicle numbers decreased with increasing age category (P = 0.02), with the youngest group (6 d to 9 y) having significantly more primordial follicles than did the monkeys in the oldest group (older than 20 y; Figure 1 A). Similarly, primordial follicle number was inversely associated with age (r = −0.62, P = 0.018; Figure 1 B). Significant correlations were observed between numbers of primordial and primary follicles (r = 0.85, P = 0.03) and between numbers of primary and secondary follicles (r = 0.72, P = 0.01; Table 2).
Table 1.
Vervet age, ovarian follicle numbers, and FSH levels
| No. of |
|||||
| Monkey ID | Age (y) | Primordial folliclesa | Primary folliclesa | Secondary folliclesa | FSHb (ng/mL) |
| 1837 | 0 | 2924 ± 367.5 | 181 ± 26.9 | 0.0 | NA |
| 1294 | 2 | 452.7 ± 65.7 | 165 ± 14.2 | 9.3 ± 5.6 | NA |
| 1023 | 4 | 3838.3 ± 398.5 | 518.7 ± 164.3 | 23.3 ± 8.5 | NA |
| 1162 | 9 | 1967.3 ± 101.4 | 621.7 ± 35 | 127 ± 9.6 | NA |
| 1245 | 9 | 51.7 ± 10.2 | 41.7 ± 3.8 | 2.3 ± 1 | 1.6 |
| 1354 | 10 | 840.3 ± 46.3 | 197 ± 7.8 | 71 ± 24.2 | 1.4 |
| 1071 | 15 | 1555.3 ± 156 | 257.7 ± 69.6 | 47.7 ± 46.4 | 1.4 |
| 1028 | 17 | 98.3 ± 28.6 | 67 ± 25.2 | 17 ± 21 | 1.16 |
| 1254 | 19 | 898.7 ± 56 | 195.7 ± 12.7 | 46.3 ± 14 | 0.07 |
| 1109 | 23 | 6.3 ± 0.33 | 14 ± 2.1 | 14.3 ± 1 | 0.14 |
| 1240 | 25 | 373 ± 25 | 247.3 ± 13.1 | 28.7 ± 15.6 | 0.43 |
| 1339 | 27 | 51 ± 25 | 34 ± 4.6 | 10.3 ± 1 | 0.33 |
| 1099 | 27 | 0.0 | 0.0 | 0.0 | 12.42 |
NA, not available
Numbers of follicles are given as mean ± SE of triplicate measures.
FSH was measured at or within 6 mo of ovary collection (except for monkey 1099, which was measured at 26 mo prior to ovary collection).
Figure 1.
(A) Comparison of numbers of primordial, primary, and secondary follicles for monkeys in the following age categories: 6 d to 9 y (n = 4), 10 to 19 y (n = 4), and 20 to 27 y (n = 4). Only the number of primordial follicles differed significantly by age (overall P = 0.02), with significantly greater numbers of primordial follicles in the youngest group compared with the oldest group (a compared with b, P = 0.03). Data are presented as mean ± SE of follicles counted per ovary. Monkey 1245 is excluded from this data set. (B) Association of mean primordial follicle number per monkey with monkey age (years; n = 13). Data are presented as square root (SQRT) of mean primordial follicle number correlated with age (years).
Table 2.
Follicle correlations
| Age | Primordial follicles (SQRT) | Primary follicles (SQRT) | Secondary follicles (SQRT) | |
| Primordial follicles (SQRT) |
r = -0.62 CI = –0.87 to –0.14 P = 0.018 |
r = 0.86 CI = 0.63 to 0.96 P < 0.001 |
r = 0.40 CI = –0.17 to 0.76 P = 0.15 |
|
| Primary follicles (SQRT) |
r = -0.49 CI = –0.81 to –0.05 P = 0.08 |
r = 0.86 CI = 0.63 to 0.96 P < 0.001 |
r = 0.71 CI = 0.28 to 0.90 P = 0.004 |
|
| Secondary follicles (SQRT) |
r = -0.53 CI = 0.52 to 0.98 P = 0.98 |
r = 0.40 CI = –0.17 to 0.76 P = 0.15 |
r = 0.71 CI = 0.28 to 0.90 P = 0.004 |
CI, confidence interval; SQRT, square root of the mean number of follicles of the indicated type.
FSH was not significantly associated with age in this study (r = 0.3, P = 0.42, n = 9). However, FSH was elevated to a level (12.42 ng/mL) indicative of ovarian senescence (menopause) in monkey 1099 (age, 27 y). Consistent with her FSH levels, this monkey also had no primordial, primary, or secondary follicles present in any of the ovarian sections counted.
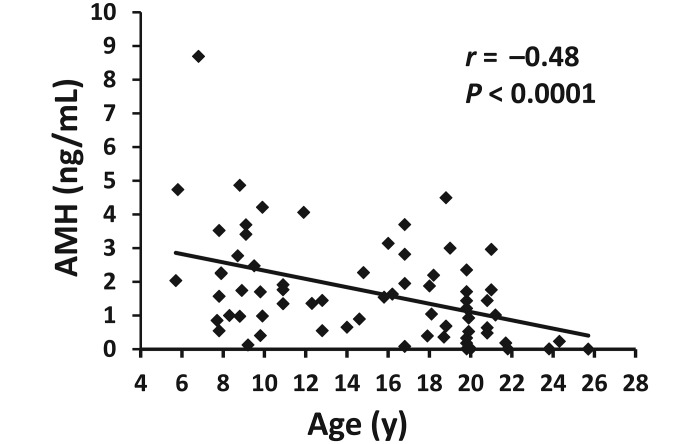
Serum concentrations of AMH were negatively correlated with age (square-root–transformed AMH r = −0.48, P ≤ 0.0001) in 68 additional vervet monkeys that lived in breeding groups in the colony (Figure 2).
Figure 2.
Correlation between serum antiMüllerian hormone (AMH) level and age (5 to 26 y) in female vervet monkeys (n = 68). Correlation between AMH (square-root-transformed) and age: r = −0.48, P < 0.0001. Data presented are AMH levels back-transformed for graphic depiction.
Histologic evidence of menopause.
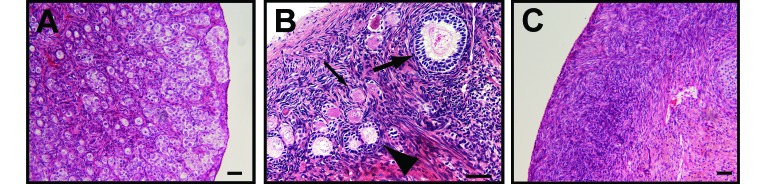
Histopathology was assessed for all ovarian sections by a pathologist blinded to monkey age and follicle counts. The ovary from the youngest animal (no. 1837, 6 d) had an ovarian cortex and medulla that lacked distinction and that were filled with myriad primordial follicles and fewer primary follicles within scant stroma (Figure 3 A). There were occasional apoptotic cells and occasional mitosis in follicles, as expected in a neonatal ovary. Vervets between the ages of 4 and 16 y (Figure 3 B) typically had as many as 7 antral follicles in various stages of development or atresia, and several remnants of corpora lutea. In the 4 oldest animals (23 to 27 y; Figure 3 C), primordial and primary follicles were greatly decreased in number (that is, virtually absent). In all of the oldest animals, remnants of corpora lutea were common, hilar arteries were often surrounded by abundant dense connective tissue with occasional thickening of artery walls by eosinophilic hyalinized material, and aggregates of adipocytes frequently occurred within the stroma and hilus, similar changes to those reported for aged macaques.5 One animal (no. 1099; age, 27 y) had smooth muscle hyperplasia in the ovarian stroma, and 3 of the 4 oldest animals had small foci of ovarian ectopic decuidua.
Figure 3.
Ovarian histologic sections illustrating decreasing primordial follicle populations with age. (A) Neonatal (6 d) vervet monkey shows a myriad of primordial follicles characterized by a flattened single granulosa cell layer and occasional apoptotic oocytes and mitotic oocytes. (B) Reproductive-age (9 y) animal demonstrates abundant primordial follicles (thin arrow), primary follicles (arrowhead), secondary follicles (thick arrow), and antral follicles (not shown). (C) Aged (23 y) vervet monkey has paucity of all follicle types in the ovarian cortex. Bar, 50 μm.
In the ovarian histopathology study, monkey no. 1245 had unusually low numbers of follicles for her age of 9 y (Figure 4). Primordial, primary, and secondary follicles were rare in both ovaries (Figure 4 B). A large, dominant, antral follicle (diameter, 5 mm) with well-developed theca interna and externa occupied about 50% of the right ovary (Figure 4 A). In addition, smaller antral follicles were present in the right and left ovaries, as were the remnants of a few corpora lutea. There was a 1-mm focus subjacent to the germinal epithelium that was composed of a mix of hair, deciduous-like very large polygonal to round cells, and cells with less than 1 μm dark-brown to black cytoplasmic granules (that is, a benign teratoma). Within the uterus of monkey no. 1245, the glandular epithelium frequently was pseudostratified with frequent mitoses, and the endometrial stroma contained mitoses and abundant edema (not shown), consistent with the proliferative phase of the menstrual cycle. The superficial endometrium contained abundant hemorrhage. The uterus and ovarian findings in monkey no. 1245 were consistent with the follicular or proliferative phase with midcycle bleeding.
Figure 4.
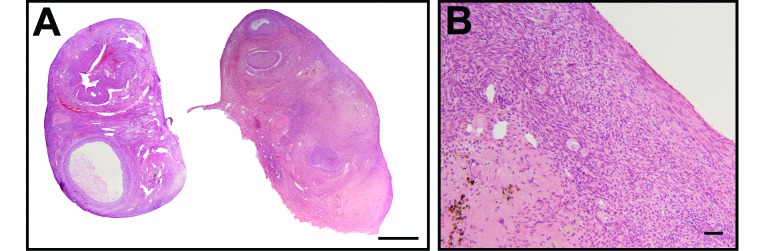
(A) Right and left ovaries (bar, 1 mm) and (B) ovarian cortex (bar, 50 μm) from a reproductive-age monkey (9.1 y) with suspected premature ovarian failure. Primordial, primary, and secondary follicles are rare.
Menstrual cyclicity and hormones in an aged vervet female.
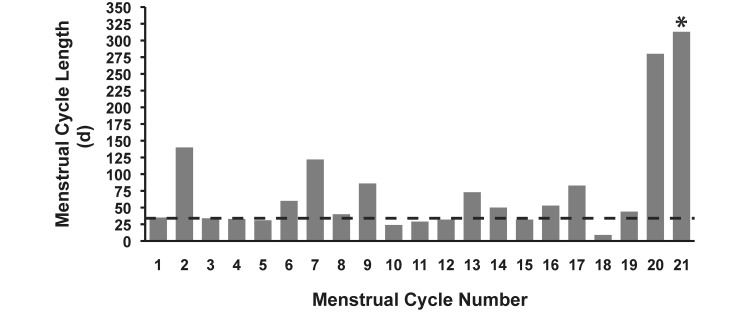
Menstrual cycle data was collected during 20 complete cycles from monkey no. 979 (age, 22.5 to 26.8 y; Figure 5). The cycle length (mean ± 1 SD) during this approximately 4-y period was 64.5 ± 60.6 d (SE = 13.5 d), and the length of each menses was 2.8 ± 2.3 d (SE = 0.5 d). Several cycles (7 of 20) were longer than 60 d, and the last recorded complete cycle was 280 d. After this final cycle, the monkey failed to show signs of menstruation until her death (more than 300 d later). Between the ages of 22.5 and 26 y, blood samples were collected for other studies, and from those, 5 samples were available for hormone measurement. During the 4-y period, FSH increased 3-fold (from 0.79 to 2.27 ng/mL), estradiol concentrations were low (less than 25 pg/mL) or below the level of detection (4 of 5 samples), and progesterone was below detectable levels (3 of 5 samples). Finally, a sample collected at the time of her death indicated that AMH was below the level of detection for the assay and thus confirmed ovarian senescence in monkey no. 979.
Figure 5.
Menstrual cycle lengths (days) of an aged vervet monkey (ID = 979) transitioning to menopause. Menses was recorded between age 22.5 and 26.8 y by using daily vaginal swabbing. Cycle length (days; mean ± SE) for all complete cycles is 64.5 ± 13.5, with a SD of 60.6 d (minimum, 9; maximum, 280). The dashed line represents the upper range of normal menstrual cycle length for vervet monkeys (approximately 35 d). *, This bar represents the number of days (313) since the monkey's last recorded vaginal bleed until the time she died at age 26.8 y.
Decreasing fecundity with increasing age.
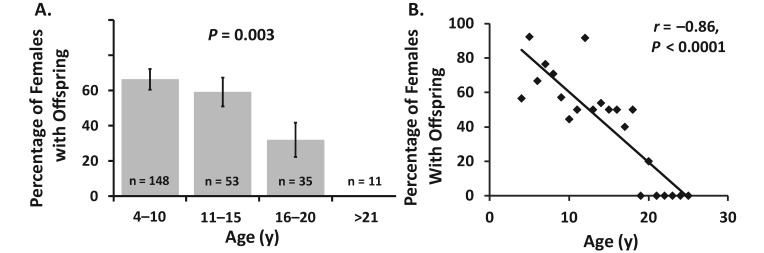
Depicted in Figure 6 A are the percentages of female vervets with offspring according to the following age categories: 4 to 10 y (n = 148), 11 to 15 y (n = 53), 16 to 20 y (n = 35), and older than 20 y (n = 11). There were was a significant (P = 0.003) effect of age category on the percentage of female vervets with infants, with percentages declining in monkeys 16 to 20 y old, and no monkeys older than 20 y had infant offspring. In addition, using continuous data revealed that the percentage of female vervets in the colony with infant offspring declined significantly with increasing age (r = −0.87, P < 0.001; Figure 6 B). Because all female monkeys had access to intact male vervets during this single breeding season, any female vervet that did not have an infant either did not get pregnant or did not have a successful birth event.
Figure 6.
(A) Percentage of female vervets with offspring according to age category. (B) Correlation between age and percentage of female monkeys with offspring. Data are presented for a single breeding season (2008) by using female monkeys with equivalent male exposure time.
Discussion
In the present study, we noted that reproductive and ovarian senescence in vervet monkeys resembles that reported for women12 and other Old World macaques.5,24 Although sample sizes for the various data types (ovarian tissue) were relatively small, the resulting data were consistent in showing decreased numbers of ovarian follicles with increasing age. Specifically, primordial follicles declined significantly with age, and histologic evidence of ovarian senescence was described in the oldest monkeys. In addition, we found that the numbers of primordial and primary follicles and of primary and secondary follicles are highly correlated. Given that follicles develop in a hierarchical manner, it is likely that primary and secondary follicles also decrease in number with age in vervets. In addition, the menstrual cycle data collected daily for a single monkey between the ages of 22.5 and 26.8 y suggest that intermenstrual cycle length and variability increase with age. Furthermore, this study provided evidence that AMH and FSH are useful markers of reproductive aging in vervet monkeys. Finally, fertility decreased monotonically with age in the colony, with almost no animals bearing a live infant after 20 y of age. These data further support the existence of ovarian senescence and menopause in vervet monkeys.
The criteria for staging menopausal transition in women have recently been updated, with menstrual cycle variation as the principal criterion for staging.11,13 The STRAW guidelines suggest that variable cycle length (7 d or greater difference in length of consecutive cycles) is indicative of the early menopausal transition and prolonged intervals of amenorrhea (minimum, 60 d) are the hallmark signs of late menopausal transition. In women, additional supportive criteria for the late menopausal transition STRAW stage may include an elevation in FSH (25 IU/L or greater). Our data provide evidence that the aforementioned menopause-associated changes in menstrual cyclicity occur similarly in vervets as in women. During approximately 4 y of observation of an aged vervet female (age, approximately 23 to 27 y), menstrual cycles became increasingly irregular, with numerous cycles exceeding 60 d in length. The last recorded interval between menses was greater than 275 d, and at the time of her death, 313 d had passed without menses. In addition, FSH rose 3-fold, comparable to the 2-fold increase in follicular-phase FSH concentration observed between the premenopausal and late perimenopausal phases in women.11,29 Although the relationship between FSH levels and menopause in vervets has not yet been established fully, it is known that FSH levels increase during the menopausal transition in both human and rhesus macaque females.8 Finally, monkey 979 had a serum AMH concentration below the detectable limit (23 pg/mL) at the time of her death (27 y), confirming ovarian senescence. Other STRAW criteria that would be supportive of menopausal transition but that are not available for this monkey include inhibin B levels and antral follicle count.
One unexpected observation involved monkey 1245. The reproductive tissue histology described in the present study was consistent with the hypothesis that premature ovarian failure occurs in vervet monkeys. In women, premature ovarian failure is characterized by the complete, or nearly complete, dysfunction or depletion of primordial and developing follicles, with antral follicles in varying stages of atresia in women younger than 40 y. The cause of premature ovarian failure is unknown, but potential etiologies include destruction of follicles due to autoimmune oophoritis, cytotoxic drugs, radiation, viral infection, and chromosomal abnormalities and fewer ovarian germ cells at birth.23 The paucity of primordial, primary, and secondary follicles in monkey 1245 at 9 y of age is indicative of premature follicular depletion (Figure 4). To our knowledge, monkey 1245 had not been exposed to cytotoxic drugs or radiation that may have induced premature follicular depletion. In addition, there was no evidence of oophoritits. The only notable abnormalities were follicle depletion and a presumed benign teratoma present near the section of ovary. A teratoma is a tumor, usually benign, containing tissues identified as originating from all 3 embryonic germ cell layers. Whether the teratoma influenced follicle depletion is unknown.
Three potential limitations of this study deserve mention. The use of a manual method of follicle quantification potentially decreases the repeatability of the ovarian follicle counting. It is challenging to histologically quantifying any type of particle within tissues, and all commonly used methods (including the manual computer-based tagging method we adopted) provide only an estimate of the number of follicles present.6 Nonetheless, the current methodology improved the ease and repeatability of follicle quantification, compared with purely microscope-based counts. The intraobserver CV for primordial follicle counts were less than 10%. The higher CV for primary and secondary follicles (greater than 15%) were likely due to variation in follicle type classification by each observer. A second potential limitation is the cross-sectional nature of the ovary study. The follicle numbers represent the number of follicles present at death but do not provide insight into the ovarian reserve at birth. Because it is not possible to count follicles histologically over time, indirect markers of follicle number such as AMH are used. We found that there was a negative association between AMH and age in vervet monkeys, similar to findings in women (decreased AMH and primordial follicles with age).28 A third limitation was the relatively small number of animals assessed for FSH concentration and ovarian reserve and the use of a single animal to track the course of the menopausal transition.
Despite any shortcomings in sample size or methodology, the several types of data presented here all converge to present a unified picture linking ovarian aging and reproductive dysfunction in vervet monkeys to increased chronologic age, terminating in menopause around 20 to 23 y (as indicated by the absence of births after 20 y of age and follicle counts). The results of this study are strengthened by the known age, consistent nutritional environment, menstrual cycle history, and reproductive history of the research-colony–based monkeys evaluated.
In conclusion, vervets experience ovarian senescence similar to that observed in women and other Old World nonhuman primates. The present study provides insight into ovarian aging and reproductive senescence in vervet monkeys. Results highlight the importance of considering this nonhuman primate as a model to investigate the relationships between ovarian aging and chronic disease risk.
Acknowledgments
This study was supported by NIH grant T35OD010946 and by NIH grants RR019963 and OD010965 (PI, Jay R Kaplan) and NIA RO1 AG 027847 (PI, Susan E Appt), with additional funding provided by Wake Forest School of Medicine, UCLA, and the Veterans Administration. We acknowledge J. Mark Cline for his contributions to the histopathologic classification of aging ovaries, Francis KY Pau (Director, Endocrine Technology Support Core Lab, Oregon National Primate Research Center) for help with the FSH assay, Anthony Morrison (Ansh Labs) for help with the AMH assay, and Debbie Golden for her technical support.
References
- 1.Al-Gubory KH, Martinet J. 1986. Comparison of the total ovarian follicular populations at day 140 of pregnancy and at day 5 postpartum in ewes. Theriogenology 25:795–808 [Google Scholar]
- 2.Adams MR, Kaplan JR, Koritnik DR. 1985. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav 35:935–940 [DOI] [PubMed] [Google Scholar]
- 3.Appt SE, Chen H, Goode AK, Hoyer PB, Clarkson TB, Adams MR, Wilson ME, Franke AA, Kaplan JR. 2010. The effect of diet and cardiovascular risk on ovarian aging in cynomolgus monkeys (Macaca fascicularis). Menopause 17:741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appt SE, Clarkson TB, Chen H, Adams MR, Christian PJ, Hoyer PB, Wilson ME, Kaplan JR. 2009. Serum antimüllerian hormone predicts ovarian reserve in a monkey model. Menopause 16:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buse E, Zöller M, Esch EV. 2008. The macaque ovary, with special reference to the cynomolgus macaque (Macaca fascicularis). Toxicol Pathol 36:24S–66S [Google Scholar]
- 6.Charleston JS, Hansen KR, Thyer AC, Charleston LB, Gougeon A, Siebert JR, Soules MR, Klein NA. 2007. Estimating human ovarian nongrowing follicle number: the application of modern stereology techniques to an old problem. Hum Reprod 22:2103–2110 [DOI] [PubMed] [Google Scholar]
- 7.Davies E, Mangongi NP, Carter CL. 2013. Is timing everything? A meeting report of the Society for Women's Health Research roundtable on menopausal hormone therapy. J Womens Health (Larchmt) 22:303–311 [DOI] [PubMed] [Google Scholar]
- 8.Downs JL, Urbanski HF. 2006. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta). Biol Reprod 75:539–546 [DOI] [PubMed] [Google Scholar]
- 9.Elmore D, Eberle R. 2008. Monkey B virus (Cercopithecine herpesvirus 1). Comp Med 58:11–21 [PMC free article] [PubMed] [Google Scholar]
- 10.Haas JR, Christian PJ, Hoyer PB. 2007. Effects of impending ovarian failure induced by 4-vinylcyclohexene diepoxide on fertility in C57BL/6 female mice. Comp Med 57:443–449 [PubMed] [Google Scholar]
- 11.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. 2007. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab 92:3060–3067 [DOI] [PubMed] [Google Scholar]
- 12.Hansen KR, LaTasha BC, Zavy MT, Klein NA, Soules MR. 2012. Ovarian primordial and nongrowing follicle counts according to the Stages of Reproductive Aging Workshop (STRAW) staging system. Menopause 19:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. 2012. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19:387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen MJ, Rudel LL, Nudy M, Kaplan JR, Clarkson TB, Pajewski NM, Schnatz PF. 2012. 25(OH)D3 and cardiovascular risk factors in female nonhuman primates. J Womens Health (Larchmt) 21:959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao SW, Sipes IG, Hoyer PB. 1999. Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol 13:67–75 [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD. 2007. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 15:1666–1674 [DOI] [PubMed] [Google Scholar]
- 17.Kaplan JR, Manuck SB. 2004. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J 45:89–115 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan JR, Wagner JD. 2006. Type 2 diabetes—an introduction to the development and use of animal models. ILAR J 47:181–185 [DOI] [PubMed] [Google Scholar]
- 19.Kundu MC, May MC, Chosich J, Bradford AP, Lasley B, Gee N, Santoro N, Appt SE, Polotsky AJ. 2013. Assessment of luteal function in the vervet monkey as a means to develop a model for obesity-related reproductive phenotype. Syst Biol Reprod Med 59:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maki PM. 2013. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 20:695–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller PB, Charleston JS, Battaglia DE, Klein NA, Soules MR. 1999. Morphometric analysis of primordial follicle number in pigtailed monkey ovaries: symmetry and relationship with age. Biol Reprod 61:553–556 [DOI] [PubMed] [Google Scholar]
- 22.Molskness TA, Hess DL, Maginnis GM, Wright JW, Fanton JW, Stouffer RL. 2007. Characteristics and regulation of the ovarian cycle in vervet monkeys (Chlorocebus aethiops). Am J Primatol 69:890–900 [DOI] [PubMed] [Google Scholar]
- 23.Nelson LM. 2009. Primary ovarian insufficiency. N Engl J Med 360:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. 2005. Ovarian senescence in the rhesus monkey (Macaca mulatta). Hum Reprod 20:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niswender GD, Spies HG. 1973. Serum levels of luteinizing hormone, follicle-stimulating hormone, and progesterone throughout the menstrual cycle of rhesus monkeys. J Clin Endocrinol Metab 37:326–328 [DOI] [PubMed] [Google Scholar]
- 26.Pazol K, Kaplan JR, Abbott D, Appt SE, Wilson ME. 2004. Practical measurement of total and bioavailable estradiol in female macaques. Clin Chim Acta 340:117–126 [DOI] [PubMed] [Google Scholar]
- 27.Pedersen T, Peters H. 1968. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 17:555–557 [DOI] [PubMed] [Google Scholar]
- 28.Rosen MP, Johnstone E, McCulloch CE, Schuh-Huerta SM, Sternfeld B, Reijo-Pera RA, Cedars MI. 2012. A characterization of the relationship of ovarian reserve markers with age. Fertil Steril 97:238–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santoro N, Crawford SL, Lasley WL, Luborsky JL, Matthews KA, McConnell D, Randolph JF, Jr, Gold EB, Greendale GA, Korenman SG, Powell L, Sowers MF, Weiss G. 2008. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab 93:1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelton KA, Clarkson TB, Kaplan JR. 2012. Nonhuman primate models of atherosclerosis, p 385–411. In: Abee C, Mansfield K, Tardif S, Morris T. Nonhuman primates in biomedical research, 2nd ed San Diego (CA): Academic Press [Google Scholar]
- 31.Smith BJ, Mattison DR, Sipes IG. 1990. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol Appl Pharmacol 105:372–381 [DOI] [PubMed] [Google Scholar]
- 32.Sparman ML, Ramsey CM, Thomas CM, Mitalipov SM, Fanton JW, Maginnis GM, Stouffer RL, Wolf DP. 2007. Evaluation of the vervet (Chlorocebus aethiops) as a model for assisted reproductive technologies. Am J Primatol 69:917–929 [DOI] [PubMed] [Google Scholar]
- 33.Voruganti VS, Jorgensen MJ, Kaplan JR, Kavanagh K, Rudel LL, Temel R, Fairbanks LA, Comuzzie AG. 2013. Significant genotype by diet (G × D) interaction effects on cardiometabolic responses to a pedigree-wide, dietary challenge in vervet monkeys (Chlorocebus aethiops sabaeus). Am J Primatol 75:491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitten PL, Turner TR. 2009. Endocrine mechanisms of primate life history trade-offs: growth and reproductive maturation in vervet monkeys. Am J Hum Biol 21:754–761 [DOI] [PubMed] [Google Scholar]