To the Editor
Allergic transfusion reactions (ATRs) occur in up to 3% of platelet transfusions1, are under-reported, and range in severity from mild itching and hives to fatal anaphylaxis. Clinical data have broadly defined factors that may be involved, such as a pathogenic role for plasma. Retrospective data of plasma reduction maneuvers to prevent ATRs to blood products are confounded by selection bias: patients always receive unmanipulated platelets before being switched to volume reduced or washed platelets following ATRs. An individual's risk of ATRs might decrease with increasing numbers of platelet transfusions, independent of platelet product manipulation. Thus, plasma reduction may temporally coincide with, but not cause, a reduction in ATR risk2.
To address the question of whether ATR risk varies with increasing platelet transfusion number, we analyzed severe urticarial reaction data from the Trial to Reduce Alloimmunization to Platelets (TRAP)3. There were 31 severe urticarial reactions reported in 24 subjects in the TRAP study (n=8,770 transfusions). Details about minor allergic reactions or the criteria for defining a severe urticarial reaction were not recorded in the study. Anaphylaxis was an evaluable outcome, but anaphylaxis was not reported after any platelet transfusion. However, three severe urticarial reactions were accompanied by dyspnea or bronchospasm. These reactions meet current criteria for likely anaphylaxis4.
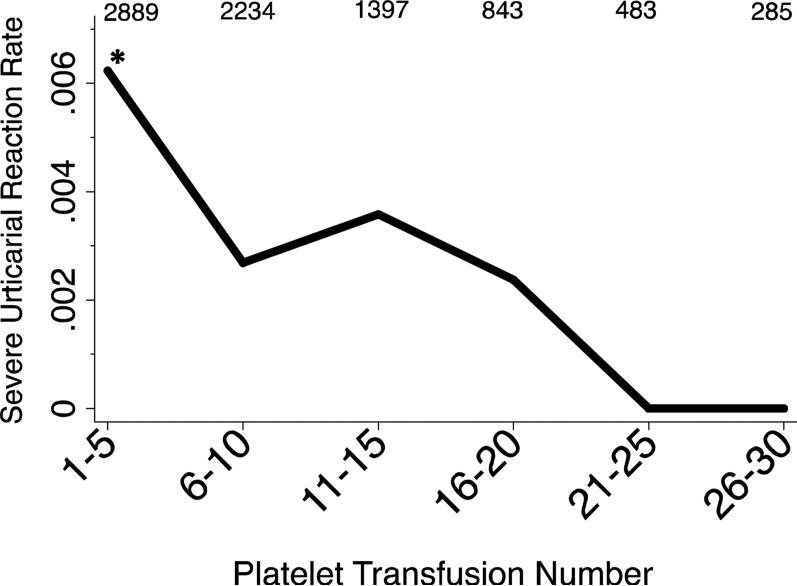
The figure shows the frequency of severe urticarial reactions by transfusion number. The risk for a severe urticarial reaction is not constant and is highest among the first 5 transfusions: 18 severe urticarial reactions in transfusions 1-5 (n=2889) vs. 13 severe urticarial reactions in the remaining transfusions (n=5881; P=0.006, Fisher exact test). We conducted a logistic regression analysis using a clustered sandwich estimator to account for subjects receiving different total numbers of transfusions. The odds ratio for a severe urticarial reaction for every additional platelet transfusion is 0.91 (95% CI 0.85-0.98, P=0.007). Five percent of platelets in TRAP were volume reduced. When these transfusions are excluded from the analysis, the findings do not materially change (OR 0.92; 95% CI 0.86-0.98).
Figure. The rate of severe urticarial reactions to platelets in the Trial to Reduce Alloimmunization to Platelets decreases over time.
Platelet transfusion exposure was grouped into bins of 5 transfusions. The number of platelet transfusions in each bin is indicated above the graph. The asterisk indicates when the three likely anaphylactic reactions occurred: two occurred at transfusion 3, and one at transfusion 4.
It is possible that subjects with an increased ATR predisposition received fewer platelet transfusions than subjects with no predisposition to ATRs, and this would bias the results. Indeed, the 24 subjects who experienced a severe urticarial reaction had a median (range) of 7 (1-32) platelet transfusions during the study period vs. 9 (1-92) for the subjects who did not have a severe reaction (P<0.001). Nevertheless, even if the analysis is restricted to the 24 subjects who had a severe urticarial reaction, the risk still decreases with each transfusion (OR 0.94, 95% CI 0.88-0.99, P=0.03). Additionally, the three likely anaphylactic reactions occurred in the first four transfusions, while the majority of platelet transfusions in this susceptible group occurred after platelet transfusion number four.
A second possible confounder might be that subjects suffering from ATRs benefited from premedication in subsequent transfusions. We consider this unlikely because multiple observational studies and trials have failed to show a reduction in ATRs with premedication5. The TRAP study did not record premedications for transfusion, so an analysis of premedications cannot be performed.
The observation that severe urticarial and anaphylactic reactions may occur earlier during serial platelet transfusion has several implications. First, it provides some reassurance that the risk of severe allergic reactions diminishes over time, regardless of intervention. Second, any study evaluating ATRs should account for the risk of ATRs varying with platelet transfusion number. Third, it supports the hypothesis that recipient susceptibility is critical for the risk for ATRs. If platelet product and donor factors were primarily responsible, one would expect a relatively constant rate of ATRs and anaphylaxis over the course of TRAP. Finally, it leads to a hypothesis that serial exposure to platelets may desensitize susceptible platelet recipients, at least to severe reactions, similar to widely used allergy desensitization methods that initiate desensitization with allergen immunotherapy (“allergy shots”) 1-3 times per week. Patients who have allergic transfusion reactions have been shown to be more atopic than those who do not6, consistent with this hypothesis. An alternative hypothesis is that ongoing immunosuppression in leukemia patients blunts allergic responses. Understanding of the pathophysiology of ATRs is critical for developing strategies to reduce the clinical burden of these reactions.
Acknowledgments
Supported by an American Society of Hematology Scholar Award
This manuscript was prepared using TRAP Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TRAP or the NHLBI.
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R21HL107828. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: The authors declare no conflicts of interest.
References
- 1.Heddle NM, Klama L, Meyer R, et al. A randomized controlled trial comparing plasma removal with white cell reduction to prevent reactions to platelets. Transfusion. 1999;39(3):231–238. doi: 10.1046/j.1537-2995.1999.39399219278.x. [DOI] [PubMed] [Google Scholar]
- 2.Vamvakas EC. A patient-centric approach to preventing allergic reactions to platelet transfusions. Transfusion. 2011;51(8):1651–1653. doi: 10.1111/j.1537-2995.2011.03246.x. [DOI] [PubMed] [Google Scholar]
- 3.Enright H, Davis K, Gernsheimer T, McCullough JJ, Woodson R, Slichter SJ. Factors influencing moderate to severe reactions to PLT transfusions: experience of the TRAP multicenter clinical trial. Transfusion. 2003;43(11):1545–1552. doi: 10.1046/j.1537-2995.2003.00529.x. [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. The Journal of allergy and clinical immunology. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 5.Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007;47(6):1089–1096. doi: 10.1111/j.1537-2995.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 6.Savage WJ, Tobian AA, Savage JH, Hamilton RG, Ness PM. Atopic predisposition of recipients in allergic transfusion reactions to apheresis platelets. Transfusion. 2011;51(11):2337–2342. doi: 10.1111/j.1537-2995.2011.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]