Abstract
Although hydrogen peroxide (H2O2) is one of the major antibacterial factors in most honeys, it does not accumulate in medical-grade manuka honey. The goal of this study was to investigate the effect of artificially added methylglyoxal (MGO) on H2O2 accumulation in natural non-manuka honeys. H2O2 concentrations in the honey solutions were determined using a fluorimetric assay. Two, the most potent H2O2 producers honeydew honeys were mixed with MGO at final concentrations of 250, 500, and 1000 mg/kg, and incubated for 4 days at 37°C. Subsequently, H2O2 concentrations were determined in 50% (wt/vol) MGO supplemented honey solutions. In vitro crosslinking of the enzyme glucose oxidase (GOX) after incubation with MGO was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Tested honeys at a concentration of 50% (wt/vol) accumulated up to 495.8±9.1 μM H2O2 in 24 h. The most potent producers were the two honeydew honeys, whose 50% solutions accumulated 306.9±6.8 and 495.8±9.1 μM H2O2, respectively. Levels of H2O2 increased significantly over time in both honey solutions. Contrary to this, the MGO-treated honeys generated significantly lower amounts of H2O2 (P<.001), and this reduction was dose dependent. In addition, MGO-treated GOX formed high molecular weight adducts with increasing time of incubation accompanied by loss of its enzymatic activity. High levels of MGO in manuka honey, by modifying the enzyme GOX, might be responsible for suppressing H2O2 generation. These data highlight the detrimental effect of MGO on significant proteinaceous components of manuka honey.
Key Words: : honey, hydrogen peroxide, methylglyoxal
Currently, honey is considered to be a therapeutic agent, and its successful application in the treatment of chronic wounds and burns has promoted further research into its antibacterial properties. The antibacterial activity of honey has been extensively studied and several honey antibacterial compounds, including hydrogen peroxide (H2O2), methylglyoxal (MGO), and bee defensin 1, have been identified.1–3 H2O2 is one of the major antibacterial components,4,5 and is produced by glucose oxidase (GOX)-mediated conversion of glucose under aerobic conditions in diluted honey. H2O2 accumulation is highest in the range of 30–50% honey.6 Bee-derived GOX is found as a regular but quantitatively variable component of honeys,7 and consequently, H2O2 levels may also differ from honey to honey.8
Interestingly, some honeys, such as manuka honey derived from the nectar of New Zealand manuka trees (Leptospermum scoparium) do not accumulate detectable levels of H2O2 upon dilution.9 It has been documented that the pronounced antibacterial activity (also referred to as “non-peroxide” activity) of manuka honey directly originates from MGO.2 The reason why H2O2 is not generated in these honeys has never been elucidated biochemically.
Several factors affecting H2O2 accumulation in honey have been identified to date: (1) inactivation of the enzyme GOX by exposure to heat, (2) degradation of H2O2 by a catalase enzyme of pollen origin, or (3) low levels of GOX in the honey. Nevertheless, honeys with non-peroxide antimicrobial activity are more closely associated with floral sources, being generally derived from Leptospermum species. Therefore, other factor(s) in manuka honey may need to be considered responsible for the absence of H2O2 accumulation.
Based on current knowledge about the destructive effects of MGO on some proteins in manuka honey,10 we hypothesize that the inability to produce high levels of H2O2 in manuka honey could be associated with high reactivity of MGO with the GOX enzyme. Accordingly, we investigated the effect of artificially added MGO on H2O2 accumulation in natural non-manuka honeys, which are capable of generating high levels of H2O2. Furthermore, we examined the effect of MGO on in vitro crosslinking of GOX and its biological activity.
Honey samples (n=3) were obtained from several regions in Slovakia (Table 1). Commercially available manuka honey with unique manuka factor 15 (UMF 15+), containing around 250 mg/kg MGO, imported from New Zealand, was purchased from Nature's Nectar. Artificial honey was prepared as described elsewhere.11
Table 1.
Hydrogen Peroxide Concentrations in Different Honey Solutions After 24-h Incubation at 37°C
| Honey sample | Principal botanical origin | Sample age (year) | Geographic origin | Hydrogen peroxide concentration (μM) |
|---|---|---|---|---|
| Honeydew 1 | Abies alba Mill | 2 | Bardejov (Slovakia) | 495.8±9.1 |
| Honeydew 2 | A. alba Mill | 2 | Banska Stiavnica (Slovakia) | 306.9±6.8 |
| Acacia | Robinia pseudoacacia | 1 | Šahy (Slovakia) | 36.3±0.7 |
| Manuka | Leptospermum spp. | 3 | New Zealand | 78.9±1.9 |
| Artificial | N/A | 1 | N/A | 16.1±1.2 |
N/A, not applicable.
The honeys with the highest levels of H2O2 were selected for incubation with MGO (Sigma-Aldrich). Two undiluted honeydew honeys were mixed with MGO at final concentrations of 250, 500, and 1000 mg/kg, and incubated for 4 days at 37°C. Subsequently, 50% (w/v) solutions were prepared from MGO-supplemented honeys, and incubated at 37°C for periods of 2, 5, and 24 h.
Each honey sample (0.5 g) was dissolved with distilled water to a final volume of 1 mL until completely fluid. The 50% (w/v) liquid solutions obtained were filtered through a 0.22-μm PES filter (Millipore) and incubated at 37°C for 24 h. H2O2 concentrations in the honey solutions after each indicated time of incubation were determined using a H2O2/peroxidase fluorimetric kit (Cell Biolabs, Inc.) according to the manufacturer's instructions. The fluorescence of the formed product, resorufin, was measured at an excitation wavelength of 530 nm using a 590 nm emission line with a Synergy HT (BioTek Instruments) microplate reader. Each honey sample and standard was tested in triplicate. The results are presented as mean±SD. The data were statistically analyzed using an unpaired Student's t-test; P-values lower than .05 were considered significant.
GOX type II from Aspergillus niger was purchased from Sigma-Aldrich. In case of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analyses, in vitro crosslinking of GOX enzyme was performed at 37°C with increasing time; 2, 24, and 48 h. GOX was dissolved in ultrapure water to a final concentration of 1 mg/mL and was incubated with or without MGO at concentrations corresponding to those found in manuka honey (5, 10, and 15 mM). After treatment, the mixture aliquots (15 μL) were fractionated on 8% SDS-PAGE gels using a Mini-Protean II electrophoresis cell (Bio-Rad). The proteins were stained with SERVA Blue G (Serva) for 60 min and then destained overnight with tap water.
For determination of enzymatic activity, GOX was dissolved in phosphate-buffered saline (PBS) (pH 7.2) to a final concentration of 0.1 mg/mL, and supplemented with 1, 2.5, and 5 mM MGO. Samples were incubated at 37°C for 7 days. To exclude the possibility of MGO interference with fluorimetric assay, samples containing same amounts of MGO, but lacking GOX, were prepared and incubated under given conditions.
Activity of GOX was examined upon H2O2 development. The reactions were performed according to Graf and Penniston12 with several modifications. Briefly, 29.85 and 59.7 μg of glucose was mixed with 1.88 μg of GOX in 1 mL containing 1×PBS (pH 7.2) and incubated for 90 min at 37°C. The level of H2O2 was subsequently evaluated using the H2O2/peroxidase fluorimetric kit as described above except that samples and standards were tested in duplicate. In case of controls containing no GOX, the analyzed volume corresponded to the samples with 0.1 mg/mL of enzyme.
In this work, we present data showing that artificially added MGO affected H2O2 generation in natural honeys with high levels of H2O2. We documented that observed inhibition of H2O2 production is due to structural changes of MGO-treated GOX.
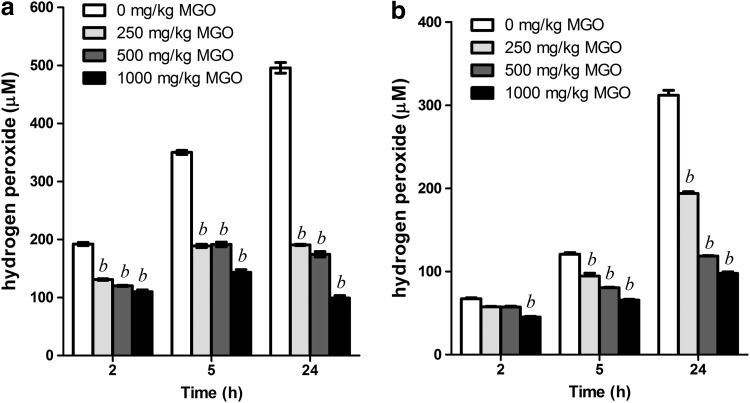
We used four natural honeys for monitoring of H2O2 generation after dilution. Tested honeys at a concentration of 50% (w/v) accumulated up to 495.8±9.1 μM H2O2 in 24 h. The most potent producers were the two honeydew honeys, whose 50% solutions accumulated 306.9±6.8 and 495.8±9.1 μM H2O2, respectively. Significantly lower levels of H2O2 were found in manuka and acacia honey (Table 1). In our very recent study, we documented that high concentrations of MGO present in manuka honeys modify defensin 1, an antibacterial bee-derived peptide and also the dominant protein in honey—MRJP1.10 Due to these MGO-induced structural modifications, defensin 1 lost its antibacterial activity. To evaluate whether MGO has a detrimental effect on the function of GOX, we incubated honeydew honeys with MGO at different concentrations commonly found in manuka honeys. As seen in Figure 1, levels of H2O2 increased significantly over time in both honey solutions. Contrary to this, the MGO-treated honeys generated significantly lower amounts of H2O2, and this reduction was dose dependent. Increasing concentrations of MGO in artificial honey did not affect the H2O2 assay (data not shown). After 24 h of incubation, the untreated honeydew honeys contained three- and fivefold higher concentrations of H2O2 than honeys treated with 1000 mg/kg MGO.
FIG. 1.
Hydrogen peroxide (H2O2) concentrations in honeydew honey solutions (50% w/v) treated with methylglyoxal (MGO) at different concentrations after 24 h of incubation at 37°C. (a) Honeydew honey (Bardejov); (b) honeydew honey (Banska Stiavnica). bP<.001 versus control (0 mg/kg MGO).
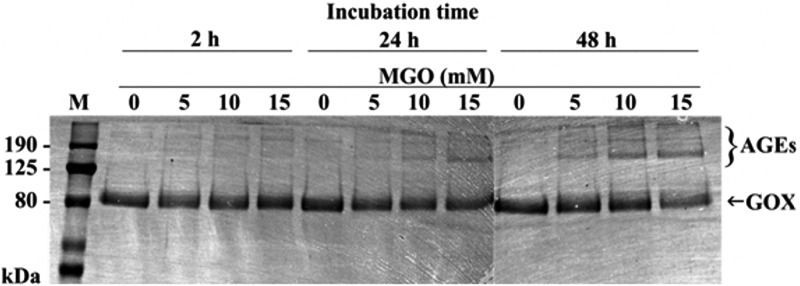
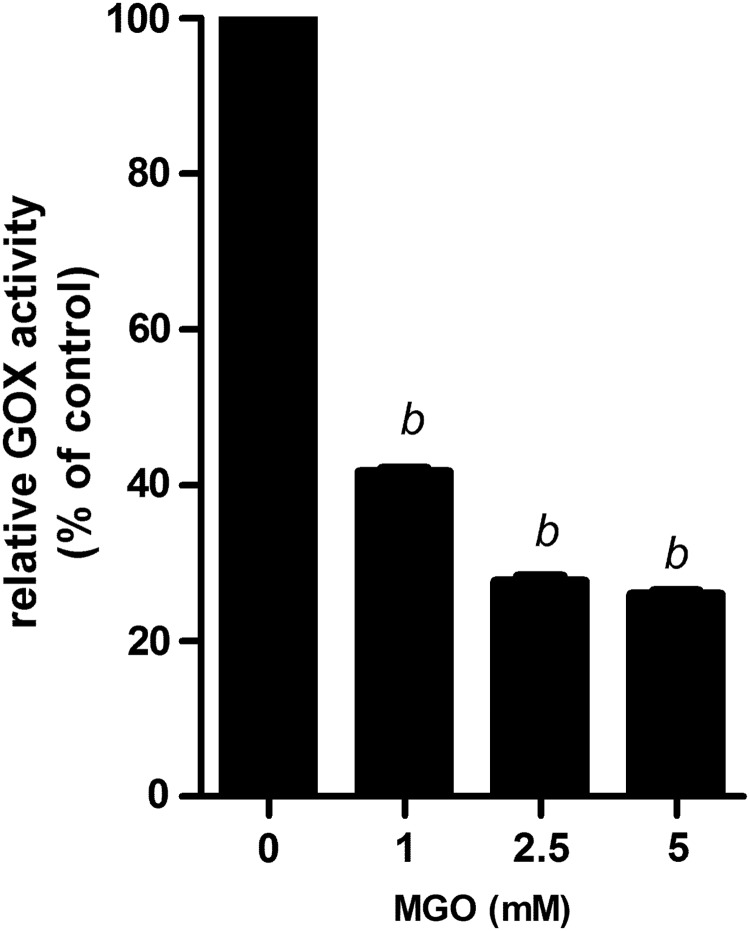
To elucidate observed reduction of H2O2 levels in MGO-treated honeys, we incubated enzyme GOX with different concentrations of MGO for increasing amounts of time. We found that GOX not exposed to MGO migrated as a single band (∼80 kDa), while MGO-treated GOX after 48 h formed high molecular weight adducts—advanced glycation end products (AGEs) (Fig. 2). Similarly, GOX exposed to the sugars (glucose, fructose, and ribose) exhibited a clear alteration in electrophoretic behavior and formed products that failed to enter the gel.13 In addition to MGO-induced structural changes of GOX, we determined the catalytic activity of GOX after incubation with/without MGO, which was examined by H2O2 development. A 58% and 70% reduction of enzyme activity was observed after incubation of GOX with 1 and 5 mM MGO, respectively (Fig. 3).
FIG. 2.
In vitro crosslinking of glucose oxidase (GOX) by MGO. GOX at concentration of 1 mg/mL was incubated with or without MGO at concentrations of 5, 10, and 15 mM for an indicated time period. After treatment, the mixture aliquots (15 μL) were fractioned on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. The gels were stained with Serva Blue G. High molecular weight GOX adducts (AGEs) were detected after 48 h of incubation with MGO. M, molecular weight marker; AGEs, advanced glycation end products.
FIG. 3.
Effect of MGO on enzymatic activity of GOX. GOX (0.1 mg/mL) was incubated with 1, 2.5, and 5 mM MGO at 37°C for 7 days. Activity of GOX was examined upon H2O2 development using the H2O2/peroxidase fluorimetric assay. Results were expressed as the percentage of relative GOX activity. bP<.001 versus control (0 mM MGO).
It has recently been found that MGO at concentration of 10 mM caused significant inhibition of antioxidant enzymes possible due to modification of protein molecules.14 On the other hand, incubation of human catalase with 10 mM MGO did not affect the enzyme activity.
Catalase is a variable natural component of honey.15 The enzyme that originates in the pollen, has a destroying effect on H2O2. Therefore, catalase may also take part in the elimination of H2O2 in manuka honey, but the potential negative effect of MGO on catalase in manuka honey remains to be investigated.
In conclusion, our data, along with those from our previous study, suggest that MGO induces modification of significant proteinaceous components of honey, including those contributing to its antibacterial activity.
Acknowledgments
We thank Mr. Jozef Volansky (Medar s.r.o., Bardejov, Slovakia) and Dr. Jan Kopernicky (Institute of Apiculture, Liptovsky Hradok, Slovakia) for providing honey samples from the different apiaries in Slovakia. We also thank Dr. Peter Ditte (Institute of Virology, Slovak Academy of Sciences) for his help with data analysis. This work had been funded by the Operational Program of Research and Development and cofinanced with the European Fund for Regional Development (EFRD). Grant: ITMS 26240220030: Research and development of new biotherapeutic methods and its application in some illnesses treatment, and partially by the Center of Excellence for Glycomics, ITMS 26240120031, supported by the Research and Development Operational Program funded by the ERDF.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.White JW, Subers MH, Schepartz AI: The identification of inhibine, the antibacterial factor in honey, as hydrogenperoxide and its origin in a honey glucose-oxidase system. Biochim Biophys Acta 1962;73:57–70 [DOI] [PubMed] [Google Scholar]
- 2.Mavric E, Wittmann S, Barth G, Henle T: Identification and quantification of methylglyoxal as the dominant antibacterial constituent of manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res 2008;52:483–489 [DOI] [PubMed] [Google Scholar]
- 3.Kwakman PH, te Velde AA, De Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA: How honey kills bacteria. FASEB J 2010;24:2576–2582 [DOI] [PubMed] [Google Scholar]
- 4.Brudzynski K, Abubaker A, St-Martin L, Castle A: Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front Microbiol 2011;2:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brudzynski K, Lannigan R: Mechanism of honey bacteriostatic action against MRSA and VRE involves hydroxyl radicals generated from honey's hydrogen peroxide. Front Microbiol 2012;3:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang LM, Bantting C, Molan PC: The effects of dilution rate on hydrogen peroxide production in honey and its implications for wound healing. J Altern Complement Med 2003;9:267–273 [DOI] [PubMed] [Google Scholar]
- 7.Pernal SF, Currie RW: Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 2000;31:387–409 [Google Scholar]
- 8.Brudzynski K: Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can J Microbiol 2006;52:1228–1237 [DOI] [PubMed] [Google Scholar]
- 9.Kwakman PH, Te Velde AA, De Boer L, Vandenbroucke-Grauls CM, Zaat SA: Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS One 2011;6:e17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majtan J, Klaudiny J, Bohova J, et al. : Methylglyoxal-induced modifications of significant honeybee proteinous components in manuka honey: possible therapeutic implications. Fitoterapia 2012;83:671–677 [DOI] [PubMed] [Google Scholar]
- 11.Majtan J, Majtan V: Is manuka honey the best type of honey for wound care? J Hosp Infect 2010;73:305–306 [DOI] [PubMed] [Google Scholar]
- 12.Graf E, Penniston JT: Method for determination of hydrogen peroxide, with its application illustrated by glucose assay. Clin Chem 1980;26:658–660 [PubMed] [Google Scholar]
- 13.Jairajpuri DS, Fatima S, Saleemuddin M: Complexing of glucose oxidase with anti-glucose oxidase antibodies of the F(ab)′2/F(ab)′ fragments derived thereforom protects both the enzyme and antibody/antibody fragments against glycation. Biochemistry (Mosc) 2008;73:1235–1241 [DOI] [PubMed] [Google Scholar]
- 14.Lankin VZ, Konovalova GG, Tikhaze AK, Nedosugova LV: The effect of natural dicarbonyls on activity of antioxidant enzymes in vitro and in vivo. Biochemistry (Mosc) 2012;6:81–86 [DOI] [PubMed] [Google Scholar]
- 15.Dustmann JH: Über die Katalaseaktivität in Bienenhonig aus der Tracht der Heidekrautgewächse (Ericaceae) [Activity of catalase in honey of Ericaceae-flow]. Z Lebensm Unters Forsch 1971;145:294–295 [Google Scholar]