Abstract
Mounting evidence indicates that anomalies in the inflammatory and immune response pathways are essential to tumorigenesis. However, tumor-based innate immunity initiated by transformed breast epithelia tissues has received much less attention. This review summarizes published reports on the role of the toll-like receptor signaling pathway on breast cancer risk, disease progression, survival, and disease recurrence. Specifically, we discuss the underlying biological mechanisms that contribute to the tumorigenic and/or anti-tumorigenic properties of toll-like receptors and their associated agonists in relation to breast tumorigenesis and cancer treatment. Further, we use results from preclinical, clinical, and population-based studies as prompts for the exploration of new and more effective breast cancer therapies. As the knowledge base of innate immunity’s involvement in breast cancer progression increases, current and new immune-modifying strategies will be refined to effectively treat breast cancer.
Keywords: TLR, single nucleotide polymorphism, chemotherapy, radiotherapy, innate immunity
Introduction
Breast cancer, a heterogeneous disease, is a major public health issue for women worldwide. Moreover, it is the leading cause of cancer-related death among women in the USA. In 2012, the American Cancer Society estimated that 226,800 women would receive a breast cancer diagnosis and 39,500 would die from the disease.1 Although there have been improvements in the early detection of breast cancer, a 23% 5-year survival rate plagues women with distant stage disease.2 While breast cancer incidence rates are higher among women of European rather than African descent in the USA, African-American women are more likely to die from this disease. Major breast cancer risk factors include increasing age, overweight/obesity, weight gain after age 18, hormone replacement therapy, alcohol consumption, high breast tissue density, high bone mineral density, exposure to high-dose radiation to the chest, null parity, long menstrual history, having one’s first child after age 30, family history of breast cancer, and inheritance of susceptibilities in high (BRAC1, BRAC2) and low penetrance genes. Recently, studies have suggested that imbalances in inflammatory and immune-associated proteins may also contribute to breast cancer and disease progression.3–6
The immune system consists of both innate and adaptive immune response pathways.7,8 Adaptive immunity is a finely tuned, highly selective response mechanism mediated by antigen-specific T and B lymphocytes in response to infection. In contrast, the evolutionary conserved innate immune system undergoes activation in response to nonspecific exogenous and endogenous insults that possess pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Of the two inflammatory divisions, innate immune responses serve as the first line of defense.
Several cellular mechanisms, including the toll-like receptor (TLR) pathway, play a crucial role in the innate immune system.9,10 In particular, TLR signaling pathways promote survival, proliferation and apoptosis, as well as interferon (IFN), cytokine, and chemokine production. However, there is strong evidence showing that infectious agents activate TLRs and promote tumor progression in gastric cancer, Kaposi’s sarcoma, hematological malignancies, and prostate cancer.11–13 Although pathogenic-induced breast cancer remains inconclusive,14–20 TLR ligand stimulation can either enhance or inhibit tumor growth, as summarized in Table 1.
Table 1.
Impact of compounds that affect toll-like receptor TLR2 and TLR4 activity or expression in breast cancer
| Ligand | Ligand type | Biological function | Impact on breast cancer | References |
|---|---|---|---|---|
| HMGB1 | Endogenous DAMPs | Critical regulator of cell death and survival | Upregulated in most types of tumors; greater expression in tumors than normal epithelium | Tang et al83; Lotze and DeMarco;84 Lotze and Tracey;85 |
| PGN | Exogenous PAMPs | Forms cell wall of bacteria | Promoted breast cancer cell invasiveness and adhesiveness in MDA-MB-231 breast cancer cells | Xie et al86 |
| Serum amyloid A | Endogenous DAMPs | Potent chemoattractant | Elevated in breast cancer patients depending on stage | Hiratsuka et al87; Zhang et al88 |
| Flagellin | Endogenous PAMPs | Forms flagella in bacterial flagellum | Suppresses cell proliferation and growth in breast cancer cells | Sfondrini et al89; Cai et al 201190 |
Abbreviations: DAMPs, damage-associated molecular pattern molecules; HMGB1, high-mobility group protein B1; PAMPs, pathogen-associated molecular patterns; PGN, peptidoglycan.
Recently, single nucleotide polymorphisms (SNPs) within the TLR signaling pathway have garnered a great deal of attention as candidate cancer detection, prognostication, and clinical management tools. It is speculated that genetic susceptibilities in TLR targets may alter the sensitivity of TLRs to their ligands, which may modify downstream signaling, including release of inflammatory and immune responses that favor tumor development. While it is feasible that TLR sequence variants are related to breast cancer outcomes, this research focus remains understudied.
In this review, we evaluate the TLR signaling pathway in relation to TLR breast cancer risk, disease progression, and tumor behavior. In particular, we highlight the clinical impact of synthetic TLR ligands alone or in combination with other therapeutic regimens on breast tumorigenesis. Lastly, we propose future research avenues to explore the contribution of innate immune mechanisms that support tumor progression and new treatment strategies that target these pathways.
TLR signaling pathway
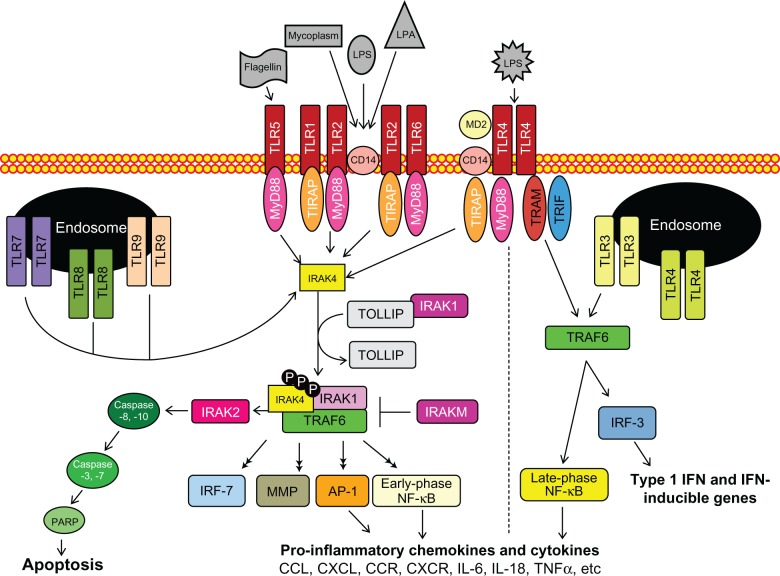
TLRs are essential type I transmembrane glycoproteins that regulate inflammatory responses against harmful pathogens, as shown in Figure 1.21–24 In humans, at least ten members have been identified and categorized as cell surface or intracellular TLRs based on their substrate specificity. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are cell surface receptors, while TLR3, TLR7, TLR8, and TLR9 are embedded in lysosomal, endosomal, as well as endoplasmic reticulum membranes.15 Cell surface receptors primarily recognize lipid or protein PAMPs and DAMPs in response to infection and tissue damage, respectively.25 In contrast, secretory pathway receptors are triggered by DAMPs and nucleic acids, which are presumably released during cell lysis and sequestered in endolysosomes for degradation.26–28 Accessory molecules for TLR ligand recognition, subcellular localization and/or function have been identified for TLR2, TLR3, TLR4, TLR6, TLR7 and TLR9 (reviewed in Lee et al, 2012).29
Figure 1.
The toll-like receptor (TLR) signaling pathway is initiated via activation of TLRs, followed by adaptor complex formation, interleukin-1R-associated kinase (IRAK) and/or tumor necrosis factor receptor-associated factor 6 (TRAF6) activation to induce subsequent mitogen-activated protein kinase, nuclear factor-kappa B (NF-κB), and interferon-regulatory factor (IRF) activation, nuclear translocation, and regulation of pro- or anti-inflammatory gene expression.
Abbreviations: AP-1, activator protein-1; CCL, β-chemokine ligand; CCR, β-chemokine receptor; CD14, cluster of differentiation 14; CXCL, α-chemokine ligand; CXCR, α-chemokine receptor; IFN, interferon; IL, interleukin; IL-1R, interleukin-1 receptor; LPS, lipopolysaccharide; LTA, lipoteichoic acid; MD2, myeloid differentiation protein-2; MMPs, matrix metalloproteinases; MyD88, myeloid differentiation factor 88; PARP, poly-(ADP-ribose) polymerase; PGN, peptidoglycan; TIR, toll/interleukin receptor; TIRAP, toll/interleukin receptor homology domain adaptor protein; TLR, toll-like receptor; TNF, tumor necrosis factor; TRAM, toll/interleukin receptor-domain-containing adapter-inducing interferon-β-related adaptor molecule; TRIF, toll/interleukin receptor-domain-containing adapter-inducing interferon-β; TOLLIP, toll-interacting protein.
With the exception of TLR3, which uses the MyD88-independent pathway and TLR4, which uses both the MyD88-dependent and -independent pathways, activated TLRs signal via the MyD88-dependent pathway. Following stimulation, TLRs bind to the sorting adaptor complex toll/interleukin receptor homology domain adaptor protein/myeloid differentiation factor 88 adapter-like protein (TIRAP/MAL), which recruits MyD88 and subsequently activates regulatory serine/threonine interleukin-1Receptor-associated kinases (IRAKs). Four IRAKs participate in TLR signaling, namely IRAK1, IRAK2, IRAKM (IRAK3), and IRAK4. IRAK4 is the central regulatory, kinase, signaling its own autophosphorylation as well as phosphorylation of IRAK1, IRAK2, and tumor necrosis factor receptor-associated factor 6 (TRAF6), as depicted in Figure 1.30–32 After IRAK1 dissociates from toll-interacting protein (TOLLIP) it binds and activates phospho-TRAF6, which in turn activates mitogen-activated protein (MAP) kinases and nuclear factor-kappa B (NF-κB). As a result, several transcription factors, including NF-κB and activator protein-1, undergo nuclear translocation, resulting in the secretion of proinflammatory chemokines and cytokines (ie, tumor necrosis factor-α, interleukin [IL]-6, and IL-8)33–36 as well as the induction of interferon-regulatory factors (IRFs, IRF1, IRF3, IRF5, IRF7, and IRF8) and matrix metalloproteinases (MMPs). Besides chemokine and cytokine production, IRAK activation stimulates the Fas-associated protein with death domain (FADD) expression and thereby promotes programmed cell death (apoptosis) via activation of caspase-8 and -10.37,38
The MyD88-independent signaling pathway via activated TLR3 and TLR4 recruits the sorting adaptor toll/interleukin receptor domain-containing adaptor inducing interferon (TRIF) and the signaling adaptor toll/interleukin receptor domain-containing adaptor inducing interferon-related adaptor molecule (TRAM). Association of the TRIF/TRAM complex coupled with ligand-bound TLR3 or TLR4 upregulates IRF3/7 via NF-κB activation but intermediates in the MyD88-independent signaling pathway have not been well-characterized. The targeted activation/inhibition of either MyD88-dependent or -independent pathways should prove a useful approach to altering the innate immune system’s complex autocrine and paracrine induction of growth and immune cell recruitment and tumor cell biology.
The TLR signaling pathway and breast tumorigenesis
Several TLRs, expressed in breast cancer cell lines and/or archival tissue, have been evaluated in relation to tumor behavior. Xie et al observed modest mRNA levels of TLR1, TLR2, TLR3, TLR5, TLR6, and TLR8 but not of TLR4, 7, 9, and 10 in MCF-7 and MDA-MB-231 cells.39 Moreover, higher levels of TLR2 or its gene product were present in invasive MDA-MB-231 breast cancer cell lines when compared with MCF-7 cells, based on quantitative reverse transcriptase polymerase chain reaction, (RT-PCR) protein analysis, and flow cytometry. In MDA-MBA-231 cells only, TLR2 inactivation positively influenced cell invasion through a variety of mechanisms including: NF-κB activation, which plays a central role in breast cancer progression;40,41 induction of phosphorylation of nuclear hormone receptor NR2C2 and I kappa B α in the TLR2/NF-κB signaling pathway; and secretion of IL-6, VEGF and, MMP-9. TLR3 activation inhibited tumor growth by inducing pro-apoptotic and inhibiting cell proliferation in in vitro and in vivo studies.42,43 Unlike Xie et al analysis of all TLRs in MDA-MB-231 cells, Yang et al confirmed the presence of only four TLR mRNAs and proteins (TLR4 > TLR6 > TLR5 > TLR7), in this cell line.44 Yang et al also demonstrated that TLR4 silencing inhibited MDA-MB-231 cell proliferation and inflammatory IL-6 and IL-8 secretion.44 Commensurate with Yang’s findings, González-Reyes et al revealed that high expression of TLR4 was associated with disease recurrence, large tumor size, and distant metastatic disease in a case-only study of 74 women.45 In this same study, TLR3 and TLR9 were also linked to tumor size and tumor stage, respectively. In two independent studies, expression and/or ligand stimulation of TLR9 by CpG oligonucleotides promoted: cell migration in an estrogen-receptor negative BT-20 cell line,46 cell invasion mediated by MMP-13 in MDA-MB-231 breast cancer cells but not MCF-7 cells,47 and aggressive tumor behavior.46 Notably, TLR9 expression in 124 breast cancer tissue specimens collected from women correlated with estrogen-receptor negative/progesterone-receptor negative status, high tumor grade, and NF-κB expression.47 Conversely, overexpression of TLR9 in fibroblast cells was associated with low propensity toward metastatic breast cancer.45 The conflicting pro- and anti-tumorigenic properties of TLR9 may be attributed to its role in both cell survival and apoptosis, respectively;48–50 therefore, targeting TLR9 requires evaluation and validation in preclinical studies. The role of TLRs in tumor development and inhibition may depend on the cell type, tumor subtype, tumor microenvironment, and the metabolic condition of the cell.
Collectively, these reports suggest that the TLR signaling pathway in breast tumor cells may play a supporting role in the secretion of proinflammatory cytokines/chemokines, aggressive tumor behavior (eg, NF-κB activity), cell proliferation, cell invasion, cell migration, and metastasis. Future inclusion of an adequate representation of breast tumor subtypes, breast cancer cell lines, transgenic animal models, and downstream signaling TLR markers will enhance our understanding of the biological mechanisms that mediate the role of the innate immunity pathway in breast tumorigenesis. Lastly, studies on the silencing of invasive TLRs (eg, TLR2) hold potential for targeted treatment strategies (see the “TLR pathway and breast cancer treatment” section below).
TLR signaling sequence variants and breast cancer risk
Six published reports have examined the relationship between TLR-associated sequence variants and breast cancer. In a pilot study, Etokebe et al did not observe any significant differences in the genotype frequencies of five TLRSNPs detected in TLR2 (T597C, T1350C), TLR3 (C1377T), TLR4 (rs4986790, A896G, Asp299Gly), and TLR9 (A1635G) between 130 breast cancer cases and 101 controls in a case-control study from Croatia.51 Contrary to the findings of Etokebe et al, Theodoropoulos et al found that inheritance of the TLR4 rs4986790 Asp299Gly allele was linked to a modest increase in breast cancer risk (odds ratio [OR] = 1.67, 95% confidence interval [CI] = 1.17–2.38) among women from Athens, Greece.52 However, in the same study, null findings were observed for the TLR4 Thr399Ile SNP.
In the Greek study, the increased susceptibility affiliated with the TLR4 Asp299Gly SNP may be attributed to a reduction in the recognition of TLR4-associated ligands, such as lipopolysaccharide, which is found in the cell wall of Gram-negative bacteria.53 It is believed that individuals who possess the hyporeactive TLR4 variant allele may have reduced cytokine production, increased susceptibility to acute bacterial infections, and increased mortality.54 Alternatively, the low-functioning TLR4 locus may be linked to a compromised inflammatory response that permits damaging persistent subclinical infection.55–58 In any event, genetic alterations attributed to the TLR4 Asp299Gly SNP may disturb the TLR signaling pathway and enhance proinflammatory networks that favor tumor growth.
The TLR4 Asp299Gly variant allele is 10–20 times more prevalent among women of African ancestry relative to those of other ancestries.53 Moreover, this same variant is two to three times more common among African-Americans than among Caucasians http://www.ncbi.nlm.nih.gov/projects/SNP.59 Despite this disparity and the relatively high breast cancer mortality rate among African-Americans and Africans,59 the influence of the TLR4 Asp299Gly locus on breast cancer outcomes in these subgroups is currently unknown. Consequently, additional studies are warranted to evaluate the impact of polymorphic TLR4 and other innate immunity markers in relation to breast cancer risk and ultimately disease prognosis among women of African ancestry.
In a secondary analysis of data collected by the National Cancer Institute Cancer Genetic Susceptibility Breast Cancer Genome-Wide Association Study, Kimbro et al analyzed 127 TLR SNPs in relation to breast cancer risk among 1145 postmenopausal women of European ancestry with invasive breast cancer and 1142 controls from the Nurses’ Health Study.60 Unfortunately, only one marker (TLR6 rs1039559 C/T) was marginally related to a reduction in breast cancer risk. However, this relationship did not remain significant after adjusting for multiple hypothesis testing. In two other large case-control studies, inheritance of the TLR1/TLR6 rs7696175 CC genotype was associated with a 1.6- and 4-fold increase in breast cancer risk among African-American (OR = 4.11; 95% CI = 1.28–13.24) and Chinese women (OR = 1.63; 95% CI = 1.10–2.41), respectively.61,62 However, this TLR1/TLR6 locus was not an important indicator of disease risk among European-American women.61
An independent study analyzed the relationship between downstream TLR-associated SNPs and breast cancer susceptibility. Among 1536 evaluated SNPs, the additive genetic model of the IRAK3 rs1732877 T1471C SNP was linked to a modest 1.6-fold increase in breast cancer risk (OR = 1.63, 95% CI = 1.14–2.34) among Koreans.63 Unfortunately, this downstream signaling marker did not remain significant after adjusting for multiple comparison bias.
Taken together, the epidemiologic evidence for the impact of genetic alterations in innate immunity markers relative to breast cancer is not very compelling. However, the lack of strong relationships may be partly attributed to the failure to evaluate higher order gene–gene and gene–environment interactions. For instance, exposure to environmental chemicals may alter innate immunity signaling activities and ultimately modify the relationship between genetic susceptibilities and breast cancer risk.64 To date, studies have focused primarily on nine SNPs detected in TLR-associated genes (TLR1–4, TLR9, and IRAK3), ignoring hundreds of known and novel TLR downstream signaling markers. Future studies are needed to consider the full array of TLR-related sequence variants and environmental exposures in relation to breast cancer risk within large and racially/ethnically diverse multicenter studies.
TLR pathway and breast cancer treatment
Over the past 30 years, a few research efforts have taken advantage of genomic anomalies associated with TLRs to both identify candidate therapeutic targets and design treatment strategies against breast cancer. To date, agonists for TLR3 and 7 have been used to directly inhibit tumor growth in preclinical studies or to treat breast cancer in humans.43 In addition, TLR3 and TLR4 expression or functional TLR4 sequence variants appear to influence tumor sensitivity to chemotherapy,43,71 albeit unequivocally.72 Lastly, stimulation of TLR9 is the focus of many ongoing randomized clinical trials to treat breast cancer.
Synthetic double-stranded RNAs (dsRNAs), including polyinosinic:polycytidylic and polyadenylic:polyuridylic acid (Poly A:U) have been used in recent breast cancer preclinical and clinical trial studies.65 The use of dsRNA Poly A:U, which targets TLR3 expressed on breast tumor cells, has resulted in favorable breast cancer outcomes (ie, an increase in overall survival and a decrease in metastasis) in two randomized clinical trials with 14 or 111 months of follow-up.66,67 Similarly, in a randomized trial of dsRNA treatment versus chemotherapy, Salaun et al demonstrated a decrease in metastatic relapse among TLR3 positive but not TLR3 negative breast cancer patients in the Poly A:U arm.43 Among patients with a TLR3 positive tumor, women in the Poly A:U arm were three times more likely to remain metastasis free 15 years post-treatment than those treated with chemotherapy (56% [95% CI = 37–71] vs 20% [95% CI = 8–37]). The anti-tumorigenic properties of Poly A:U were supported with results using a breast adenocarcinoma cell line and an immune-compromised mouse model. Following exposure to Poly A:U, the HCC1806 cells underwent TLR3-dependent cell death. Further, subcutaneous growth of the HCC1806 cell lines was compromised by intravenous injection of Poly A:U within immune-compromised non-obese diabetic/severe combined immunodeficiency (NOD-SCID) mice relative to controls. Although the exact mechanisms are unclear, dsRNAs may elicit their antitumor properties against breast cancer by: reducing cell proliferation; inducing TLR3 (and TRIF)-dependent cell death, independent of dsRNA-dependent protein kinase; and promoting IRAK4 induction of IFN β, NF-κB, and – to a lesser extent – caspase-3 and -8 activation.42,43 Although there are no specific data for breast cancer, we cannot exclude the possibility that dsRNAs may potentiate the capacity of TLR3 to trigger a chemoattractant response (secretion of chemokines, macrophages, neutrophils, and lymphocytes) to eradicate the tumor or alter tumor vascularization.68–70 In any case, participation of innate and adaptive immunity is critical to the success of chemotherapy against breast cancer.
Although attention has been given to targeting the TLR signaling pathway for breast cancer treatment, it is important to note that this pathway influences sensitivity to some, but not all, chemotherapeutic strategies. In a breast cancer murine model, Stimulation of dendritic cell TLR4 in combination with systemic chemotherapy or local radiotherapy was shown to reduce tumor growth and prolong survival of tumor-bearing mice.71 Apetoh et al demonstrated that breast cancer patients who carried a TLR4 loss of function SNP (TLR4299 Asp/Gly + Gly/Gly) were more likely to experience metastatic recurrence after chemotherapy and local radiation relative to carriers of the referent genotype.71 In contrast, Szkandera et al did not observe a significant relationship between two commonly reported TLR4 SNPs (Asp299Gly and Thr399Ile) and pathological response to neoadjuvant anthracycline-based chemotherapy among breast cancer patients.72 However, this null finding may be due to the small sample size (70 Caucasian female patients) as well as failure to consider other TLR-associated sequence variants.
In a subcutaneous setting, the administration of TLR5 ligand (Salmonella typhimurium flagellin) in the D2F2 mouse mammary tumor cell line and its antigenic sub-line with stably transfected ErbB-2 (DF2/E2) resulted in varied breast outcomes depending on the dosing regimen.70 Tumor growth was inhibited only in the D2F2 cell line when flagellin was administered 8–10 days after tumor inoculation. However, accelerated tumor growth was observed for both cell lines when the ligand was administered at the time of inoculation. These findings suggest that the impact of TLR5 in relation to breast tumorigenesis is influenced by interactions among the tumor, the tumor microenvironment, and the host immune system.73 In addition, a combination of TLR5 and TLR9 agonists (i.e., CpG oligonucleotides) [CpG ODN] administered 8–10 days after inoculation, inhibited tumor growth in D2F2/E2 cell lines. Additional studies are warranted on the stimulation of multiple TLRs and their joint effects on abrogating tumor growth.
TLR7 recognizes single-stranded RNA and triggers innate immune signaling in response to viral infections.73 TLR7 is also activated by several low molecular weight compounds, including imiquimod, which has anti-tumorigenic properties: induction of cytokines (IFN-γ, IL-12), activation of tumor antigen-specific cytotoxic T-lymphocytes, and activation of myeloid dendritic cells with cytotoxic activity.75–77 A topical cream formulation of imiquimod has been approved by the US Food and Drug Administration for the successful treatment of superficial basal cell carcinoma, actinic keratosis, and genital warts.65 Within ongoing cancer trials, topical application of imiquimod targets several cancers including breast cancer. For instance, the New York University School of Medicine is currently running a Phase II/III clinical trial with imiquimod for breast cancer patients with chest wall recurrence and skin metastasis. In addition, the Fred Hutchinson Cancer Research Center recently completed a Phase III trial of imiquimod in conjunction with a paclitaxel albumin-stabilized nanoparticle formulation.
Despite the conflicting roles of TLR9 in tumorigenesis, TLR9 agonists (eg, CpG ODN) have been studied as antitumor drugs either alone or in concert with other therapeutic strategies in preclinical and clinical studies.65 Stimulation of TLR9 in CpG DNA triggers tumor invasion and migration in breast cancers using in vitro assays.46 Currently, several clinical trials are capitalizing on the anti-tumorigenic properties of the TLR9 agonist CpG 7909 alone or in conjunction with trastuzumab.65,78–81 It is speculated that TLR9 ligands may stimulate anti-tumorigenic activity by inducing apoptotic signals or by interfering with blood vessel formation and tumor growth; however, the exact mechanism for breast cancer remains unclear.82
The growing understanding that TLR-associated targets are involved in breast tumorigenesis offers some justification using larger pharmacogenetic studies. However, such observational studies need to consider the full cadre of possible targets involved in the TLR signaling pathway (TLRs, adaptor/accessory proteins, IRAKs, MMPs, chemokines, cytokines, and caspases). Collectively, continuing efforts targeting the TLR signaling pathway may lead to more effective, noninvasive strategies that will improve breast cancer survival rates.
Conclusion
Breast cancer is a heterogeneous disease whose incidence and progression may vary according to genetic status and function of the tumor innate immunity system. Basic research on key proteins and genetic variants in the TLR signaling pathway will likely offer insights into the processes that promote and/or prevent breast tumorigenesis. Therapeutic targeting of the TLR signaling pathway may be used in conjunction with new or existing chemotherapy, radiation, or immunotherapy approaches to treat breast cancer. Future studies targeting multiple TLRs may lead to more effective therapeutic targets for drug development and treatment of breast cancer. Such effort may ultimately improve breast cancer survival rates among all women.
Acknowledgments
The preparation of this article, together with the program of research reported, was supported in part by the following grant/research support: Clinical Translational Science Pilot Grant to LRK; the JGBCC Bucks for Brains “Our Highest Potential” in Cancer Research Endowment to LRK; and grant P20-MD000175 NIH NCMHD to KSK.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.American Cancer Society . Cancer Facts and Figures 2013. Atlanta, Georgia: American Cancer Society; 2013. Available at http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. Accessed. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed A, Wang JH, Redmond HP. Silencing of TLR4 Increases Tumor Progression and Lung Metastasis in a Murine Model of Breast Cancer. Ann Surg Oncol. 2012 Aug 14; doi: 10.1245/s10434-012-2595-9. Epub. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Hagemann A, DeMichele A. Immuno-modulatory gene polymorphisms and outcome in breast and ovarian cancer. Immunol Invest. 2009;38(3–4):324–340. doi: 10.1080/08820130902910567. [DOI] [PubMed] [Google Scholar]
- 5.Pensa S, Watson CJ, Poli V. Stat3 and the inflammation/acute phase response in involution and breast cancer. J Mammary Gland Biol Neoplasia. 2009;14(2):121–129. doi: 10.1007/s10911-009-9124-x. [DOI] [PubMed] [Google Scholar]
- 6.Hojilla CV, Wood GA, Khokha R. Inflammation and breast cancer: metalloproteinases as common effectors of inflammation and extracellular matrix breakdown in breast cancer. Breast Cancer Res. 2008;10(2):205. doi: 10.1186/bcr1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9(1):4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 8.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5(10):971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 9.Kutikhin AG. Association of polymorphisms in TLR genes and in genes of the Toll-like receptor signaling pathway with cancer risk. Hum Immunol. 2011;72(11):1095–1116. doi: 10.1016/j.humimm.2011.07.307. [DOI] [PubMed] [Google Scholar]
- 10.Kutikhin AG, Yuzhalin AE. Inherited variation in pattern recognition receptors and cancer: dangerous liaisons? Cancer Manag Res. 2012;4:31–38. doi: 10.2147/CMAR.S28688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad H, Gubbels R, Ehlers E, et al. Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF) J Biol Chem. 2011 Mar 11;286(10):7865–7872. doi: 10.1074/jbc.M110.191452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayi A, Kohler E, Toller IM, et al. TLR-2-activated B cells suppress Helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J Immunol. 2011 Jan 15;186(2):878–890. doi: 10.4049/jimmunol.1002269. [DOI] [PubMed] [Google Scholar]
- 13.Kundu SD, Lee C, Billips BK, et al. The toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate. 2008 Feb 1;68(2):223–229. doi: 10.1002/pros.20710. [DOI] [PubMed] [Google Scholar]
- 14.Prinz C, Schwendy S, Voland P. H pylori and gastric cancer: shifting the global burden. World J Gastroenterol. 2006;12(34):5458–5464. doi: 10.3748/wjg.v12.i34.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10(4):369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42(3):161–170. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 17.Kundu SD, Lee C, Billips BK, et al. The toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate. 2008;68(2):223–229. doi: 10.1002/pros.20710. [DOI] [PubMed] [Google Scholar]
- 18.Amarante MK, Watanabe MA. The possible involvement of virus in breast cancer. J Cancer Res Clin Oncol. 2009;135(3):329–337. doi: 10.1007/s00432-008-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson JS, Glenn WK, Whitaker NJ. Breast cancer as an infectious disease. Womens Health (Lond Engl) 2010;6(1):5–8. doi: 10.2217/whe.09.73. [DOI] [PubMed] [Google Scholar]
- 20.Joshi D, Buehring GC. Are viruses associated with human breast cancer? Scrutinizing the molecular evidence. Breast Cancer Res Treat. 2012;135(1):1–15. doi: 10.1007/s10549-011-1921-4. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010;49(1):1–9. doi: 10.1016/j.cyto.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 23.Jing ZZ, He XB, Fang YX, Jia HJ, Zhou T. Modulation of the host toll-like receptor signaling pathways by virus infection. Bing Du Xue Bao. 2012;28(4):453–461. Chinese. [PubMed] [Google Scholar]
- 24.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im1412s77. Chapter 14:Unit 14.12. [DOI] [PubMed] [Google Scholar]
- 25.Pisetsky DS. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol. 2012;144(1):32–40. doi: 10.1016/j.clim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 27.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 28.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Lee C, Avalos A, Ploegh H. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12:168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motshwene PG, Moncrieffe MC, Grossmann JG, et al. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem. 2009;284(37):25404–25411. doi: 10.1074/jbc.M109.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465(7300):885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gay NJ, Gangloff M, O’Neill LA. What the Myddosome structure tells us about the initiation of innate immunity. Trends Immunol. 2011;32(3):104–109. doi: 10.1016/j.it.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300(5625):1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 34.Snoussi K, Mahfoudh W, Bouaouina N, Ahmed SB, Helal AN, Chouchane L. Genetic variation in IL-8 associated with increased risk and poor prognosis of breast carcinoma. Hum Immunol. 2006;67(1–2):13–21. doi: 10.1016/j.humimm.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 36.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7(5):353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 37.Hsu LC, Park JM, Zhang K, et al. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature. 2004;428(6980):341–345. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- 38.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19(13):3325–3326. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie W, Wang Y, Huang Y, Yang H, Wang J, Hu Z. Toll-like receptor 2 mediates invasion via activating NF-kappaB in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2009;379(4):1027–1032. doi: 10.1016/j.bbrc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Park BK, Zhang H, Zeng Q, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13(1):62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 41.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 42.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176(8):4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 43.Salaun B, Zitvogel L, Asselin-Paturel C, et al. TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res. 2011;71(5):1607–1614. doi: 10.1158/0008-5472.CAN-10-3490. [DOI] [PubMed] [Google Scholar]
- 44.Yang H, Zhou H, Feng P, et al. Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. J Exp Clin Cancer Res. 2010;29:92. doi: 10.1186/1756-9966-29-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.González-Reyes S, Marín L, González L, et al. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 2010;10(1):665. doi: 10.1186/1471-2407-10-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger R, Fiegl H, Goebel G, et al. Toll-like receptor 9 expression in breast and ovarian cancer is associated with poorly differentiated tumors. Cancer Sci. 2010;101(4):1059–1066. doi: 10.1111/j.1349-7006.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merrell MA, Ilvesaro JM, Lehtonen N, et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res. 2006;4(7):437–447. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- 48.József L, Khreiss T, Filep JG. CpG motifs in bacterial DNA delay apoptosis of neutrophil granulocytes. FASEB J. 2004;18(14):1776–1778. doi: 10.1096/fj.04-2048fje. [DOI] [PubMed] [Google Scholar]
- 49.El Andaloussi A, Sonabend AM, Han Y, Lesniak MS. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia. 2006;54(6):526–535. doi: 10.1002/glia.20401. [DOI] [PubMed] [Google Scholar]
- 50.Fischer SF, Rehm M, Bauer A, et al. Toll-like receptor 9 signaling can sensitize fibroblasts for apoptosis. Immunol Lett. 2005;97(1):115–122. doi: 10.1016/j.imlet.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Etokebe GE, Knezević J, Petricević B, Pavelić J, Vrbanec D, Dembić Z. Single-nucleotide polymorphisms in genes encoding toll-like receptor -2, -3, -4, and -9 in case-control study with breast cancer. Genet Test Mol Biomarkers. 2009;13(6):729–734. doi: 10.1089/gtmb.2009.0045. [DOI] [PubMed] [Google Scholar]
- 52.Theodoropoulos GE, Saridakis V, Karantanos T, et al. Toll-like receptors gene polymorphisms may confer increased susceptibility to breast cancer development. Breast. 2012;21(4):534–538. doi: 10.1016/j.breast.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Ferwerda B, McCall MB, Verheijen K, et al. Functional consequences of toll-like receptor 4 polymorphisms. Mol Med. 2008;14(5–6):346–352. doi: 10.2119/2007-00135.Ferwerda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schröder NW, Schumann RR. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect Dis. 2005;5(3):156–164. doi: 10.1016/S1473-3099(05)01308-3. [DOI] [PubMed] [Google Scholar]
- 55.Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002;186(10):1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 56.Child NJ, Yang IA, Pulletz MC, et al. Polymorphisms in Toll-like receptor 4 and the systemic inflammatory response syndrome. Biochem Soc Trans. 2003;31(Pt 3):652–653. doi: 10.1042/bst0310652. [DOI] [PubMed] [Google Scholar]
- 57.Faber J, Meyer CU, Gemmer C, et al. Human toll-like receptor 4 mutations are associated with susceptibility to invasive meningococcal disease in infancy. Pediatr Infect Dis J. 2006 Jan;25(1):80–81. doi: 10.1097/01.inf.0000195595.22547.fe. [DOI] [PubMed] [Google Scholar]
- 58.Fraudendorf H, Gelbrich W, Reimer W, Hering B. Effect of experimentally induced changes in blood flow on the heat and electrical conductivity of the skin. Acta Biol Med Ger. 1975;34(5):923–931. German. [PubMed] [Google Scholar]
- 59.Sherry ST, Ward WH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimbro KS, Oprea GM, Burns BG, et al. Innate immunity-related sequence variants as predictors of breast cancer risk among women of African descent. J Clin Oncol. 2010;28(Suppl):15s. Abstract 1581. [Google Scholar]
- 61.Barnholtz-Sloan JS, Shetty PB, Guan X, et al. FGFR2 and other loci identified in genome-wide association studies are associated with breast cancer in African-American and younger women. Carcinogenesis. 2010;31(8):1417–1423. doi: 10.1093/carcin/bgq128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan M, Ji S, Liaw C, et al. Association of common genetic variants with breast cancer risk and clinicopathological characteristics in a Chinese population. Breast Cancer Res Treat. 2012;136(1):1–12. doi: 10.1007/s10549-012-2234-y. [DOI] [PubMed] [Google Scholar]
- 63.Lee JY, Park AK, Lee KM, et al. Candidate gene approach evaluates association between innate immunity genes and breast cancer risk in Korean women. Carcinogenesis. 2009;30(9):1528–1531. doi: 10.1093/carcin/bgp084. [DOI] [PubMed] [Google Scholar]
- 64.Bi X, Hameed M, Mirani N, Pimenta EM, Anari J, Barnes BJ. Loss of interferon regulatory factor 5 (IRF5) expression in human ductal carcinoma correlates with disease stage and contributes to metastasis. Breast Cancer Res. 2011;13(6):R111. doi: 10.1186/bcr3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goutagny N, Estornes Y, Hasan U, Lebecque S, Caux C. Targeting pattern recognition receptors in cancer immunotherapy. Target Oncol. 2012;7(1):29–54. doi: 10.1007/s11523-012-0213-1. [DOI] [PubMed] [Google Scholar]
- 66.Lacour J, Lacour F, Ducot B, et al. Polyadenylic-polyuridylic acid as adjuvant in the treatment of operable breast cancer: recent results. Eur J Surg Oncol. 1988;14(4):311–316. [PubMed] [Google Scholar]
- 67.Laplanche A, Alzieu L, Delozier T, et al. Polyadenylic-polyuridylic acid plus locoregional radiotherapy versus chemotherapy with CMF in operable breast cancer: a 14 year follow-up analysis of a randomized trial of the Fédération Nationale des Centres de Lutte contre le Cancer (FNCLCC) Breast Cancer Res Treat. 2000;64(2):189–191. doi: 10.1023/a:1006498121628. [DOI] [PubMed] [Google Scholar]
- 68.Paone A, Galli R, Gabellini C, et al. Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha. Neoplasia. 2010;12(7):539–549. doi: 10.1593/neo.92106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmer S, Steinmetz M, Asdonk T, et al. Activation of endothelial toll-like receptor 3 impairs endothelial function. Circ Res. 2011;108(11):1358–1366. doi: 10.1161/CIRCRESAHA.111.243246. [DOI] [PubMed] [Google Scholar]
- 70.Bergé M, Bonnin P, Sulpice E, et al. Small interfering RNAs induce target-independent inhibition of tumor growth and vasculature remodeling in a mouse model of hepatocellular carcinoma. Am J Pathol. 2010;177(6):3192–3201. doi: 10.2353/ajpath.2010.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 72.Szkandera J, Absenger G, Dandachi N, et al. Analysis of functional germline polymorphisms for prediction of response to anthracycline-based neoadjuvant chemotherapy in breast cancer. Mol Genet Genomics. 2012;287(9):755–764. doi: 10.1007/s00438-012-0715-7. [DOI] [PubMed] [Google Scholar]
- 73.Maruyama K, Selmani Z, Ishii H, Yamaguchi K. Innate immunity and cancer therapy. Int Immunopharmacol. 2011;11(3):350–357. doi: 10.1016/j.intimp.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 74.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 75.Rechtsteiner G, Warger T, Osterloh P, Schild H, Radsak MP. Cutting edge: priming of CTL by transcutaneous peptide immunization with imiquimod. J Immunol. 2005;174(5):2476–2480. doi: 10.4049/jimmunol.174.5.2476. [DOI] [PubMed] [Google Scholar]
- 76.Prins RM, Craft N, Bruhn KW, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176(1):157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 77.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204(6):1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pfizer . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2008. [Accessed February 21, 2013]. PF-3512676 (CpG 7909) injection for patients who completed an oncology study using PF-3512676 (CpG 7909) [updated March 11, 2009]. Available from: http://clinicaltrials.gov/show/NCT00043368. NLM identifier: NCT00043368. [Google Scholar]
- 79.Pfizer . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2002. [Accessed February 21, 2013]. CPG 7909 plus Herceptin® in patients with metastatic breast cancer. [updated March 11, 2009]. Available from: http://clinicaltrials.gov/show/NCT00043394. NLM identifier: NCT00043394. [Google Scholar]
- 80.Pfizer . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2002. [Accessed February 21, 2013]. Safety and efficacy study of the combination of CpG 7909 and Herceptin in patients with metastatic breast cancer. [updated May 26, 2011]. Available from: http://clinicaltrials.gov/show/NCT00031278. NLM identifier: NCT00031278. [Google Scholar]
- 81.Ohio State University Comprehensive Cancer Center . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2009. [Accessed February 21, 2013]. Agatolimod and trastuzumab in treating patients with locally advanced or metastatic breast cancer. [updated November 9, 2011]. Available from: http://clinicaltrials.gov/show/NCT00824733. NLM identifier: NCT00824733. [Google Scholar]
- 82.Vabulas RM, Braedel S, Hilf N, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277(23):20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 83.Tang D, Kang R, Cheh CW, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29(38):5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lotze MT, DeMarco RA. Dealing with death: HMGB1 as a novel target for cancer therapy. Curr Opin Investig Drugs. 2003;4:1405–1409. [PubMed] [Google Scholar]
- 85.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 86.Xie W, Huang Y, Guo A, Wu W. Bacteria peptidoglycan promoted breast cancer cell invasiveness and adhesiveness by targeting toll-like receptor 2 in the cancer cells. PLoS One. 2010;5:e10850. doi: 10.1371/journal.pone.0010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10(11):1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 88.Zhang G, Sun X, Lv H, Yang X, Kang X. Serum amyloid A: A new potential serum marker correlated with the stage of breast cancer. Oncol Lett. 2012;3:940–944. doi: 10.3892/ol.2012.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sfondrini L, Rossini A, Besusso D, et al. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J Immunol. 2006;176(11):6624–6630. doi: 10.4049/jimmunol.176.11.6624. [DOI] [PubMed] [Google Scholar]
- 90.Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011 Apr 1;71(7):2466–2475. doi: 10.1158/0008-5472.CAN-10-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]