Abstract
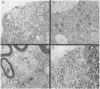
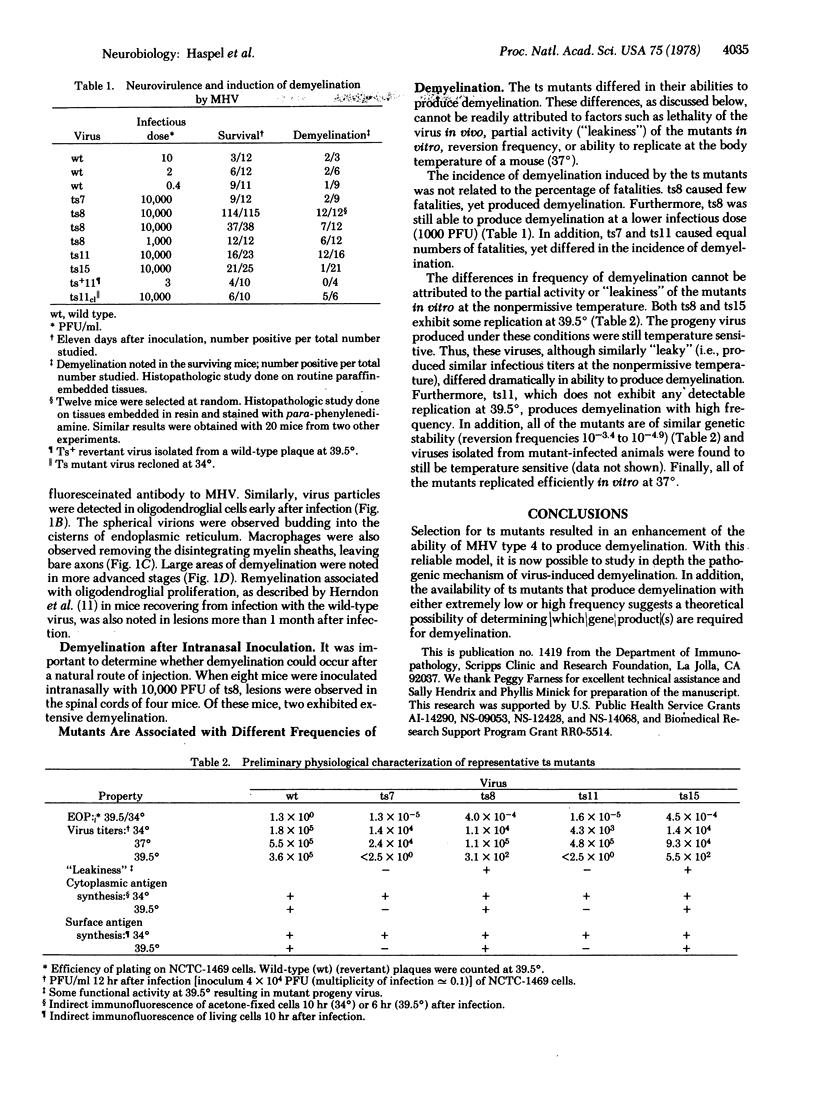
Mutagenesis of mouse hepatitis virus with 5-azacytidine or 5-fluorouracil yielded several temperature-sensitive mutants. Mutants have been isolated that dramatically enhance the production of demyelinating disease over that previously noted with the wild-type virus. This reproducible model should now make possible the precise elucidation of the pathogenic mechanism and molecular basis of this virus-induced demyelination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breschkin A. M., Haspel M. V., Rapp F. Neurovirulence and induction of hydrocephalus with parental, mutant, and revertant strains of measles virus. J Virol. 1976 May;18(2):809–811. doi: 10.1128/jvi.18.2.809-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N. Genetic manipulation of reovirus--a model for modification of disease. N Engl J Med. 1972 Nov 16;287(20):1026–1033. doi: 10.1056/NEJM197211162872007. [DOI] [PubMed] [Google Scholar]
- HARTLEY J. W., ROWE W. P. Tissue culture cytopathic and plaque assays for mouse hepatitis viruses. Proc Soc Exp Biol Med. 1963 Jun;113:403–406. doi: 10.3181/00379727-113-28378. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Rapp F. Measles virus: an unwanted variant causing hydrocephalus. Science. 1975 Feb 7;187(4175):450–451. doi: 10.1126/science.1111114. [DOI] [PubMed] [Google Scholar]
- Herndon R. M., Price D. L., Weiner L. P. Regeneration of oligodendroglia during recovery from demyelinating disease. Science. 1977 Feb 18;195(4279):693–694. doi: 10.1126/science.190678. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Sims J. K., Kniazeff A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol. 1973 Mar 30;24(1):76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz S. G., Dal Canto M. C., Johnson T. C. Comparison of central nervous system disease produced by wild-type and temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1976 Apr;13(4):1242–1249. doi: 10.1128/iai.13.4.1242-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKSMAN B. H., ADAMS R. D. Infectious leukoencephalitis. A critical comparison of certain experimental and naturally-occurring viral leukoencephalitides with experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1962 Oct;21:491–518. [PubMed] [Google Scholar]
- Weiner L. P., Johnson R. T., Herndon R. M. Viral infections and demyelinating diseases. N Engl J Med. 1973 May 24;288(21):1103–1110. doi: 10.1056/NEJM197305242882106. [DOI] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]