Abstract
Objective: To discuss the physiological bases for using exogenously applied electric field (EF) energy to enhance wound healing with conductive electrical stimulation (ES) devices.
Approach: To describe the types of electrical currents that have been reported to enhance chronic wound-healing rate and closure.
Results: Commercial ES devices that generate direct current (DC), and mono and biphasic pulsed current waveforms represent the principal ES technologies which are reported to enhance wound healing.
Innovation: Wafer-thin, disposable ES technologies (wound dressings) that utilize mini or micro-batteries to deliver low-level DC for wound healing and antibacterial wound-treatment purposes are commercially available. Microfluidic wound-healing chips are currently being used with greater accuracy to investigate the EF effects on cellular electrotaxis.
Conclusion: Numerous clinical trials described in subsequent sections of this issue have demonstrated that ES used adjunctively with standard wound care (SWC), enhances wound healing rate faster than SWC alone.

Luther C. Kloth, MS, PT, FAPTA, CWS, FACCWS
Introduction: Clinical Problem
Chronic wounds represent a major problem to patients, healthcare professionals, and the U.S. healthcare system, involving 5.7–6.5 million patients and costing an estimated 20–25 billion dollars annually.1,2 The encumbrance of treating chronic wounds is increasing rapidly owing to increasing healthcare costs, an aging population, and, in the United States and many other countries, the incidence of diabetes and obesity is increasing precipitously.2 During the past two decades, numerous wound interventions have been developed to facilitate healing by addressing maintenance of wound moisture, reduction of bacterial and nonviable tissue burdens, and management of cytokines and proteases and by stimulating expression of growth factors. To address these and other needs of chronic wounds, frequently standard wound care (SWC) is employed alone or in combination with adjunctive wound treatments that deliver several types of biophysical energy to further enhance wound healing.
Materials and Methods: Background
The use of electric field (EF) energy applied to chronic wounds to enhance healing has been used for decades and is based on the existence of endogenous wound EFs that have been observed to direct cell migration after injury to the integument.3 The strength of the endogenous wound EFs measured in animals and humans that have been observed to direct cell migration (electrotaxis) after wounding have been quantified between 10 and 100 μA/cm2.3,4 Research has verified that EF energy enhances the migration of lymphocytes,5 fibroblasts,6–8 macrophages,9 and keratinocytes.10 Furthermore, in recalcitrant wounds, it seems likely that the endogenous EFs are askew or absent, in which case the wounds often do not respond to SWC. When SWC alone fails to heal chronic wounds, electrical stimulation (ES) combined with SWC has been shown in several clinical trials to enhance healing and closure.11–21 Details of these studies are presented in subsequent sections of this work. The information presented here will define the ES terminology and will describe the types of ES energy and what signal characteristics are reported to enhance wound healing as well as how it is delivered to wounds. Insights on what the future holds for advances in ES wound-healing technology will also be addressed.
Terminology of exogenous and endogenous EFs
The terminology used to describe electric current types, waveforms, and parameters of the ES signals in published wound-healing clinical research is often inconsistent. To appease the reader's puzzlement as to how ES signal (waveform) parameters of amplitude, frequency, and duration influence wound treatment, currents are presented here as adapted from a monograph published by the Section on Clinical Electrophysiology and Wound Management of the American Physical Therapy Association.22
Voltage
Voltage (V) refers to the electromotive force (EMF) which is capable of moving charged particles (ions across cell membranes in wound tissues) that lie between two electrodes applied to the body. The volt is a measure of electrical pressure (analogous to water pressure) and is the EMF (electron [or ion] moving force) that is needed to drive a current of 1 A through a resistance of 1 ohm. The relationship between voltage and amperage is expressed as Ohm's law, V=IR.
To produce directed current flow, there should be a source of free electrons from the ES device, conveyed to the patient via conductive electrodes that are positioned to distribute the flow of a quantity of EF energy (charge) into wound and periwound tissues. With direct current (DC) and monophasic pulsed current (MPC), the two electrodes are polarized with regard to each other, with one being negative (cathode) and the other being positively charged (anode). Currents with polarity are used for wound healing to ostensibly replicate/activate the disturbed endogenous polarized current that is present after wounding of the integument.
Basic science research has shown that endogenous, measurable EFs are created by transmembrane voltages which are found in cell membranes and that when the epithelium of human skin is wounded, a low resistance pathway is created where the transepithelial potential (voltage) drives current out of the wound. After wounding, in a moist wound environment, there is a lateral voltage gradient of 140 mV/mm at the wound edge that decays to 10 mV/mm at 500–1,000 μm from the wound edge.4 Exogenous ES could provide a directional vector as well as a non-vector activating mechanism to stimulate cells involved in wound healing by enhancing cellular motility in the wound and along the wound edge.4
Waveforms
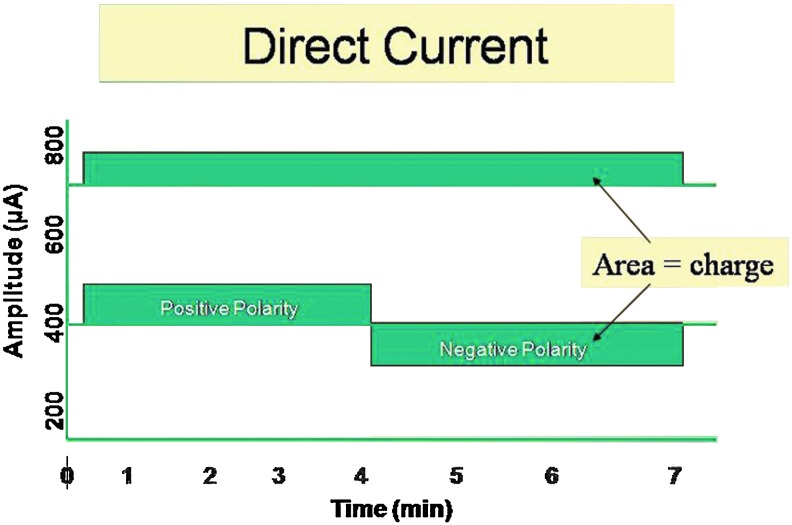
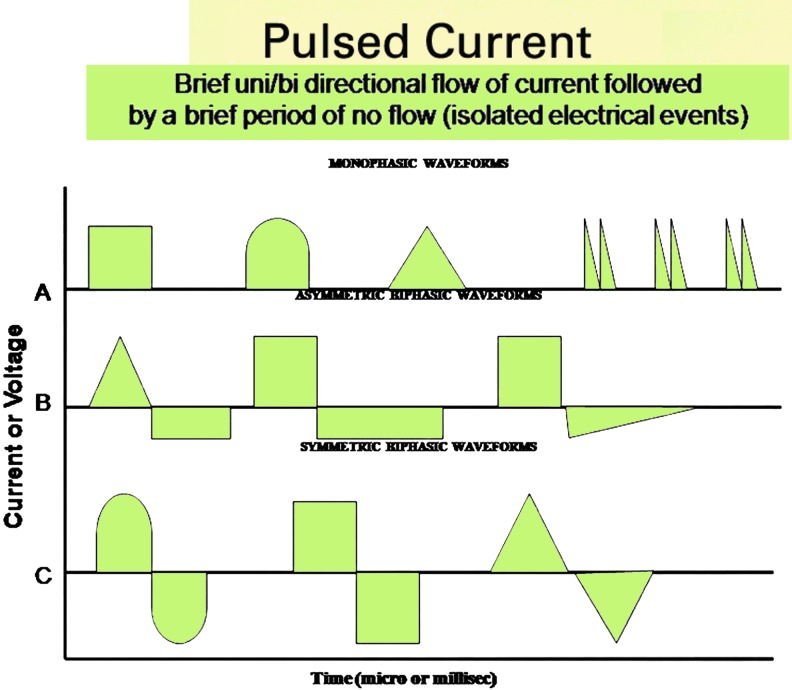
Waveforms are visual representations of voltage or current on an amplitude–time plot. DC has no waveform, because once it leaves the zero base line it continues to flow in one direction for 1 s or longer (for as much as several days for battery driven electrical wound dressings that deliver micro-amperage, continuous DC) (Fig. 1). Pulsed current (PC) waveforms are shown as illustrations of monophasic or biphasic electrical events that begin when the current or voltage leaves the zero (isoelectric) baseline in one direction, then after a finite time either returns to and stops at the same baseline (monophasic waveform) (Fig. 2A) or crosses the baseline in the opposite direction, and ends when the voltage or current returns again to the baseline (biphasic waveform) (Fig. 2B, C).
Figure 1.
DC flows continuously and, therefore, has no waveform but has distinct positive or negative polarity. DC that flows for sufficiently high current levels for significant periods of time may cause electrochemical injury to the skin and wound tissues. At sufficiently low μA levels, exogenous DC current may be used to mimic the endogenous DC current of injury. DC, direct current. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 2.
Pulsed electrical currents used in wound healing are either monophasic or biphasic. (A) Examples of monophasic pulses above (or below) the zero baseline. When they are above the baseline, they have positive polarity; when they are below the baseline, they have negative polarity. (B, C) Examples of biphasic pulses, one phase above and one phase below the zero baseline. Their shape may be asymmetric (B) and charge unbalanced with polarity or symmetric (C) and charge balanced without polarity. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Current
Current is the directed flow of electrical energy or charged particles from one place to another within matter. An electrical current (I) is defined as the rate of flow of charged particles (electrons or ions) past a specific point in a specific direction. Current flow in a metal wire conductor occurs as a result of the flow of electrons, whereas current flow in tissues is carried by ions (e.g., Na+, K+, CI−). The unit of measure of current (I) is the ampere (A), which is defined as the rate at which charge flows past a fixed reference point in a conductor or mathematically as I=C/t, where I=amps; C=coulombs; and t=time (s). An ampere is equal to 1 coulomb per second. Coulombs indicate the number of electrons, whereas amperes indicate the rate of electron flow. Exogenous currents delivered to wounds that are intended to mimic the physiological DC tissue currents have an order of magnitude that may be 1,000–1,000,000 times less than 1.0 A, which places them in the milliampere (mA) to microampere (μA) range. When a unidirectional DC or PC is delivered into a wound via electrodes, active cells that participate in the phases of healing (e.g., neutrophils, macrophage, fibroblasts, and keratinocytes) may migrate toward an anodal or cathodal polarized EF (electrotaxis)5–7,9,10 or in the case of fibroblasts, they may be up-regulated to increase their rate of DNA synthesis.7
Types of exogenous wound-healing currents
Two types of electrical current are usually designated as DC and defined as current that flows continuously for 1 s or longer, and alternating current (AC).22 However, the majority of clinical trials that have studied the effects of ES on wound healing have used “pulsatile” waveforms or pulsed current (PC).11–21
The use of the term PC is not meant to insinuate that there is an additional type of basic current (there are still only two, DC and AC). Figures 1 and 2, respectively, show graphic representations of PC and continuous DC that have been used in wound-healing research and practice. Low-frequency AC (1–1,000 Hz [cycles per second (for alternating current)]) has not been used with success in wound healing clinical trials, likely because it lacks polarity. Therefore, sinusoidal AC for wound healing will not be discussed here.
Direct current
Continuous direct current (CDC) is the unidirectional flow of charged particles for 1 s or longer (Fig. 1), whereas pulsed direct current flows for 1 ms to 1 s as a monophasic pulsed waveform.
When delivered to wound tissues, the direction of current flow is determined by the polarity selected. Positive ions move toward the cathode, and negative ions move toward the anode. CDC has no pulses and, subsequently, no waveform. However, since CDC flows for 1 s or longer, at sufficiently high amplitudes, caustic alkaline (NaOH and H2) and acid (HCl) products may form at the cathode and anode, respectively, which may cause observable tissue irritation. CDC studies that are applied between 200 and 800 μA have been reported to have positive wound-healing outcomes in seven clinical trials, of which three are case studies23–29 Wolcott et al.24 reported that after failure of SWC to effect measurable progress toward healing, of 75 ischemic skin ulcers treated with CDC over 15 weeks, 40% closed in a mean of 9.6 weeks; while the healing rate for all wounds was 13.4% per week. Gault and Gatens25 used CDC and the same protocol as Wolcott et al.24 to treat 100 chronic ulcers of the integument that were deemed recalcitrant to SWC. They indicated that 48% of these wounds closed in 4.7 weeks at a rate of 28.4% per week. In a third clinical study, Carley and Wainapel26 randomly assigned 30 patients with indolent skin ulcers below the knee or in the sacral area to treatment with CDC or with SWC. Patients in the treatment groups were matched by age, diagnosis, wound size, and wound etiology. They found that the healing rate over the 5 week study period for the CDC group was 1.5–2.5 times faster than the paired controls. In the fourth clinical study, Wood and Evans27 coordinated a prospective, double-blind, placebo trial conducted at four different academic facilities to evaluate the effects of pulsed DC on healing of stage II and III pressure ulcers that had made no significant improvement to SWC for more than 5 weeks. Unlike the previous three CDC studies that placed one electrode on periwound skin and the other on the wound, they delivered ES at 600 μA to wounds by placing electrodes on opposite sides of the wound∼2 cm from the wound edge. Seventy-four ulcers were treated at the four centers. Forty-three patients were selected for the experimental group, and 31 control subjects were assigned to the sham device (placebo group). In the treatment group, 25 ulcers (58%) closed in 8 weeks; whereas in the placebo group, only 1 ulcer (3%) closed. The difference in healing rates for the two groups was statistically significant (p<0.0001). Despite the positive findings reported from these studies, the availability of CDC devices for wound-healing treatment has ceased to exist in the United States, largely because manufacturers are not including it as a choice in their ES devices. This is unfortunate, as CDC comes close to mimicking the physiological DC EF that occurs after wounding of the integument and subcutaneous tissues. Interestingly, a relatively new bioelectric wound dressing that contains a miniature electric circuit which delivers micro-amperage CDC has been used with some reported success for treating pressure and venous ulcers in the United Kingdom28,29 (Fig. 3). Since the endogenous physiological currents described earlier in this opus are steady DC currents that are measurable in humans along the wound edge,30 exogenous micro-amperage CDC (Fig. 1) or MPC (Fig. 2A) appear to be the best choices for mimicking the measurable, physiological endogenous electrical signals.
Figure 3.
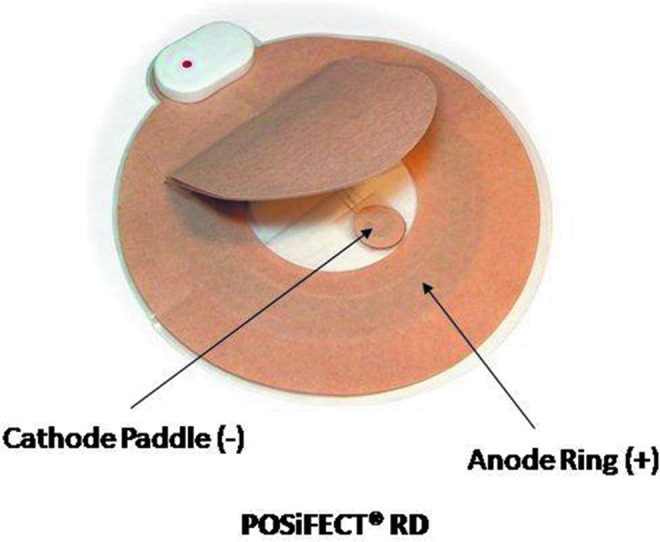
The PosiFect RD™ DC device (BioFisica™, Odiham, Hampshire, United Kingdom and Atlanta, GA), with anode ring and cathode tab. Shown with permission of Rafael Andino, President of Biofisica. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Pulsed current
PC is the brief unidirectional or bidirectional flow of electrons or ions in which each pulse is separated by a longer off period with no current flow. Thus, each pulse is an isolated electrical event that is separated from each of a series or train of pulses by a finite off time. PC is described by its waveform, amplitude, duration, and frequency. PC can have two waveforms: monophasic or biphasic.
Monophasic pulsed current
As shown graphically in Figures 4 and 5,31 a monophasic pulse represents a very brief movement of electrons or ions away from the isoelectric line, returning to the zero line after a finite period of time (<1 ms). MPC waveforms described in the clinical wound-healing literature include the rectangular or square waveform of low-voltage MPC12–14,17,18 and the twin-peaked waveform of high-voltage, monophasic PC (HVPC).15,16,19–21 The duration of MPC pulses is always less than 1 ms; therefore, unlike CDC, which by definition flows for 1 s or longer and frequently causes irritating pH changes on skin, MPC does not cause pH changes and will not harm skin or tissues.32 In addition, similar to the endogenous, DC physiological EFs, both exogenous CDC and PC have polarity while sinusoidal AC has no polarity (charge balanced) and, therefore, cannot mimic the physiological endogenous DC signal.
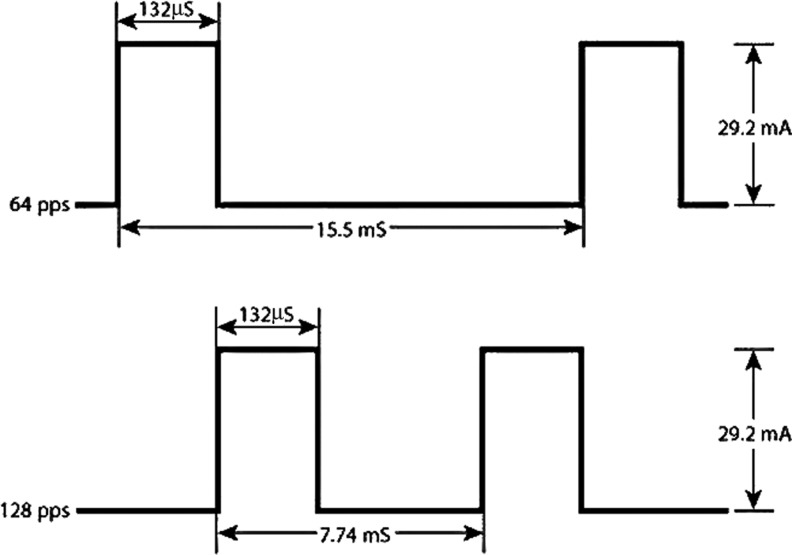
Figure 4.
Waveform of LVMPC used in several clinical trials12,17,18,20 that reported positive healing outcomes after treatment of chronic wounds in conjunction with standard care. LVMPC, low-voltage monophasic pulsed current.
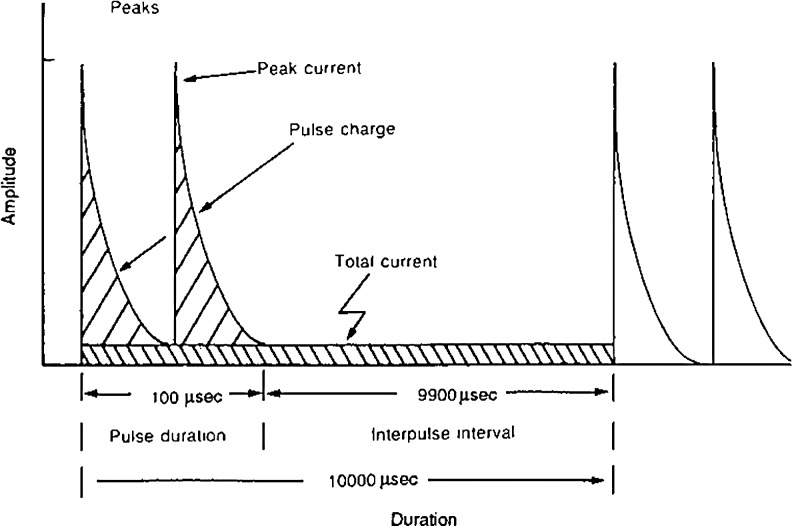
Figure 5.
Waveform of high-voltage monophasic pulsed current. Clinical voltage levels used to deliver low levels of current to wound tissues (∼3.2 μC/pulse) will not cause tissue injury from electrochemical pH changes. (From Nelson and Currier,31 with permission.)
Biphasic pulsed currents
The biphasic PC waveform is bidirectional and consists of two phases. One phase leaves the isoelectric line, and after a finite time returns to baseline. Typically without delay, the second phase leaves the isoelectric line in the opposite direction, and after a brief time returns to baseline. The biphasic waveform may be asymmetric or symmetric about the isoelectric line (Fig. 2B, C, respectively). In the symmetric biphasic waveform, the phase charges are electrically equal or balanced, which is an undesirable attribute, as there is no polarity. Asymmetric biphasic waveforms may be electrically balanced or unbalanced. The use of biphasic symmetrical (charge balanced)33 (Fig. 2C) and asymmetrical (charge unbalanced)13,14 (Fig. 2B) waveforms have been described in clinical wound-healing literature with the latter two studies reporting positive treatment outcomes when treated with an asymmetrical, charge unbalanced (polarized) waveform. Thus, as previously suggested, it seems logical that the therapeutic ES signal should come from a polarized source of DC or PC if the intent of the exogenous ES signal is to boost the disturbed, polarized, endogenous DC signal.
Electrodes: delivery of exogenous electric signals to wound tissue
Electrodes are the conductive elements of an electrical circuit that deliver a dosage of electrical energy into the wound tissues. There are two electrode placement methods that may be used to deliver exogenous electrical currents into wound tissues. One method utilizes a treatment electrode of selected polarity placed in direct contact with the wound and the return electrode placed on intact periwound skin.11,12,15–18,20,21,23–26 (Fig. 6). Figure 7 shows the indirect electrode placement method in which one lead wire is bifurcated to two electrodes of the same polarity placed bestride the wound on intact periwound skin with the return electrode of opposite polarity on intact skin.13,14,34–37
Figure 6.
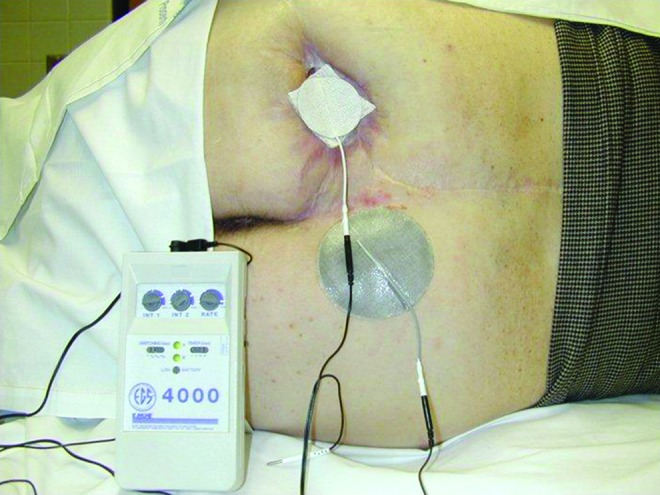
Conductively coupled monopolar electrode placement with treatment electrode placed on a conductive saline, moist gauze or on a wafer hydrogel sheet dressing. The return electrode is applied to intact skin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 7.
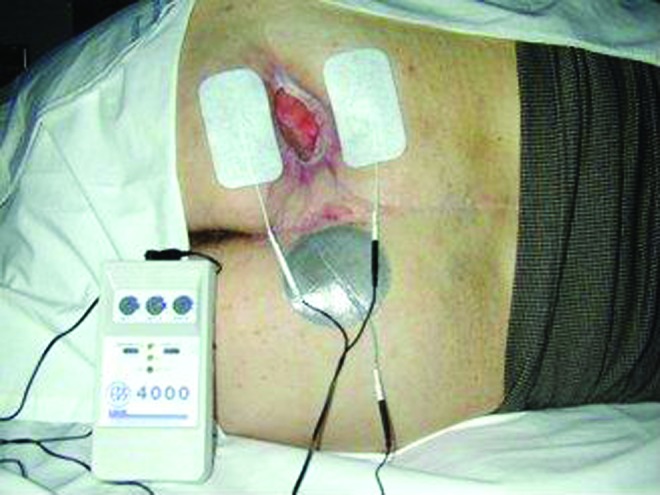
Conductively coupled bipolar treatment electrodes of opposite polarity positioned on opposite sides of a wound. This electrode placement approach has been utilized to deliver LVBPC in six clinical studies.13,14, 34–37 LVBPC, low voltage biphasic pulsed current. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Both DC and PC are delivered to wound tissues via conductive coupling.38 The material make-up of electrodes varies from carbon to hydrogel and polymer. Some carbon electrodes can be disinfected and reused on the same patient. For all wounds, the shallow or deep dead space is filled with hydrogel or saline moist gauze, and the treatment electrode of appropriate polarity is placed in contact with the filler. This ensures that the exogenous EF will be delivered to the wound edge and the interior. Selection of the treatment electrode polarity can be based on findings from cell culture electrotaxis studies or on an anecdotal (alternating polarity) suggestion as follows:
Cathode: Enhances motility of cells—epithelial,4 fibroblast,6 and keratinocyte cells10
Anode: Enhances motility of cells—macrophage,9 neutrophil39
Alternate polarity (+/−) at least every week, based on stage of healing.40
ES devices: low- and high-voltage PC
Conductively coupled ES devices are classified according to the voltage range delivered to treatment electrodes. Low-voltage devices deliver CDC as well as monophasic and biphasic waveforms of longer durations (μs to ms) and, therefore, require lower driving voltages (clinically between 20 and 35 V) (Fig. 4). All commercially available conductively coupled ES devices except HVPC devices fall into this category. HVPC devices have a monophasic waveform with very short phase durations which are usually <50 μs that require a high driving voltage (clinically between 75 and 150 V although the maximum voltage available is 500 V). During wound treatment with HVPC, the no-current interval between successive pulses represents 99% of each second of a treatment period; thus, the total current per second delivered to tissue does not exceed more than 1.2–1.5 mA. If the voltage setting during wound treatment is 100 V, the paired pulse charge is only 3–3.5 micro-coulomb (μC). Thus, for a pulse rate set at 100 pulses/s (pps), the total pulse charge accumulation (dosage) does not exceed 350 μC/s.41
Electric charge (ES wound-treatment dosage)
Electric charge is a physical property of matter (e.g., wound tissue with endogenous EF) that causes it to experience a force when near other electrically charged matter.42 Charge is measured in units called coulombs (C), representing a specific quantity of electrons, that is, electrically energy. The dosage or charge delivered into wound tissues through a treatment electrode to enhance healing is in the μC range. Several independent clinical studies that used the same low-voltage (10–50 V) pulsed current (LVPC) device now known as woundEL™ (Fig. 8) with a monophasic waveform (Fig. 4) have reported wound-healing outcomes.12,17,18,20 The stimulus parameters used in the studies were 132 μs duration, 30 mA and 128, or 64 pps (Fig. 4). The calculated wound-healing dosage per 1 h treatment per day was 250–500 μC/s, which equates to 0.89 C/day at 64 pps and 1.78 C/day at 128 pps.41
Figure 8.
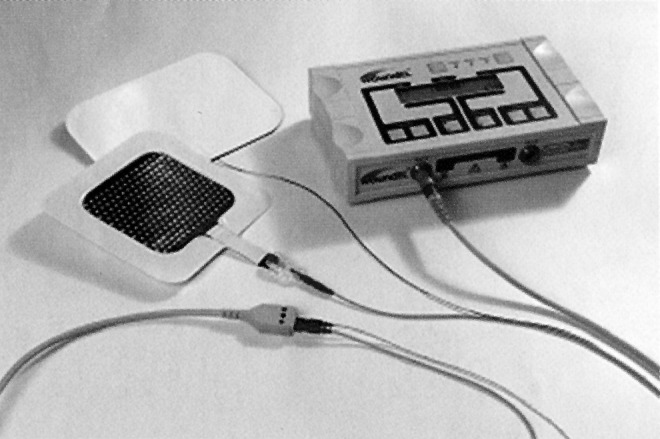
The woundEL® LVMPC device (Göteborg, Sweden) delivers the same signal previously used in clinical wound-healing studies performed in the United States and Germany that reported positive healing outcomes.12,17,18,20 Permission granted by Molnlycke Health Care.
Two other independent clinical studies that used HVPC (clinically 75–150 V) devices and described stimulus parameters (20–60 μs; 75–150 V; and 105 pps) also reported positive wound-healing outcomes.20,21 In these studies, the authors indicated that the charge (dosage) quantities were 342 μC/s. These electrical dosages correspond to the range of dosage values previously reported in the four LVPC studies. The strength of the endogenous wound EFs measured in animals and humans that have been observed to direct cell migration (electrotaxis) after wounding have been measured between 10 and 100 μA/cm2.3,4 When an exogenous EF from ES is imposed on an indolent wound EF, one may presume that the latter would be perturbed and ostensibly restored to normal. Using the parameters described earlier for LVPC and HVPC, additional clinical research is needed to confirm or refute whether the imposition of the charge quantities mentioned on wounds with languid EFs will result in enhanced wound healing.
Results
The dosage range of 250–500 μC/s represents a small window of electrical energy that has been shown to produce very favorable wound-healing results in four studies which used an LVPC signal (Fig. 4)12,17,18,20 and in two studies that used an HVPC signal (Fig. 5).20,21 Despite the fact that the research designs were not comparable in these six studies, it is, nevertheless, most encouraging that the same stimulus parameters used in the different devices and studies yielded reproducible wound-healing results. Clearly, further basic science and clinical research is needed to determine whether there is an optimal exogenous EF dosage that enables the wound healing rate to exceed the outcomes reported here.
Discussion
Although the terminology and technology related to the use of ES for wound healing may seem somewhat complex, one should realize that the therapeutic energy described here is a part of the intricate electromagnetic spectrum (EMS) that represents a vast continuum of energy levels employed by clinicians in several medical fields for treatment and diagnosis of numerous diseases or their symptoms. The energy levels represented in the EMS range from very high intensity, ionizing gamma rays, x-rays, and ultraviolet radiation to the descending intensities of non-ionizing infrared, microwave, radio waves, and EF energies. As previously mentioned, endogenous EFs measured between 10 and 100 μA/cm2,3,4 in animals and humans have been observed to direct cell migration (electrotaxis) after wounding. While there is a dearth of in vivo evidence of directed cell migration by exogenous EFs, the evidence from clinical trials supports the use of ES for the treatment of chronic wounds as recommended by the Centers for Medicare and Medicaid Services (CMMS).43 However, CMMS reimbursement at approximately $13 USD discourages many healthcare practitioners from using ES in wound management. ES for wound healing is typically performed by physical therapists, as along with several other biophysical energies, it is used in wound healing, for example, ultrasound, low-level laser, pulsed lavage with vacuum, positive pneumatic compression, and pulsed radio frequency energy comprise one of the pillars of physical therapy practice. Unlike the energies just mentioned, ES for wound healing is not a new intervention, as positive clinical research outcomes began appearing in the literature more than 40 years ago.23 With steady advances in ES technologies, combined with a plethora of compelling clinical research11–21,23–29,33–37 and the strong pressure ulcer treatment guideline recommendations of the European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel,44 the Registered Nurse Association of Canada,45 and the Paralyzed Veterans of America,46 it seems likely that wound-care practitioners will significantly expand the clinical use of this treatment in chronic wound management. Further strong support for this intervention was presented by Koel47 in 2009 at the 19th Conference of the European Wound Management Association, where he reported on the preliminary phase of a Cochrane review of the effects of ES on wound healing. Twenty randomized controlled trials (RCTs) published between 1985 and 2008 were included in his analysis. ES in these studies was used to treat several wound types, including venous and arterial leg ulcers, diabetic foot ulcers, pressure ulcers, surgical wounds, and wounds of mixed etiology. ES devices used in these studies delivered DC as well as low-voltage monophasic or asymmetric biphasic PC and high-voltage PC (recall that in the Results section, using the parameters of pulse amplitude, frequency, and duration, the charge dosage for studies that used LVPC and HVPC signals in the range of 250–500 μC/s has been calculated). This means that different ES devices can produce the same electrical pulse charge energy needed for healing. This fact is supported by Koel, who presented two summary plots from 13 of the 20 RCTs comparing the effects of ES: one on all applied ES types, the other on monophasic ES. In 13 studies in which all ES types or placebo ES were delivered, 44.4% (187/421) of those who received active ES healed, whereas only 25.9% (87/335) of wounds in the placebo ES group healed. An odds ratio of 2.12 (95% confidence interval [CI], 1.55–2.90) suggests a higher rate of healing in subjects who received ES. Koel also found that of eight studies that compared monophasic active ES or placebo ES, 47.8% (77/161) of wounds healed after active monophasic ES; whereas only 17.1% (25/146) healed with placebo ES with an odds ratio of 4.54 (95% CI, 2.65–7.79). Additional reviews of RCT statistics from human wound-healing ES studies reveal that there is considerable evidence supporting this intervention.11,48 Despite the supporting evidence, physical therapists and other healthcare practitioners use ES “off label,” because the United States Food and Drug Administration has never cleared or labeled any ES device specifically for wound-healing purposes.
Innovation
In recent years, newer technologies are miniaturized, disposable bioelectric dressing-like devices with imbedded electrical circuitry that have been cleared by healthcare regulatory agencies for wound healing in the European Union (EU) (Fig. 3) and for an antibacterial effect on wounds (Fig. 9) in the United States. The former battery-powered dressing device, Posifect™ (Biofisica LLC, Atlanta, GA) delivers micro-amperage CDC. The latter bioelectric dressing device, Procellera™ (Vomaris Innovations, Chandler, AZ) is powered by 25 micro-batteries that when activated by wound moisture deliver 0.6–0.7 V at 10 μA to the wound surface.
Figure 9.
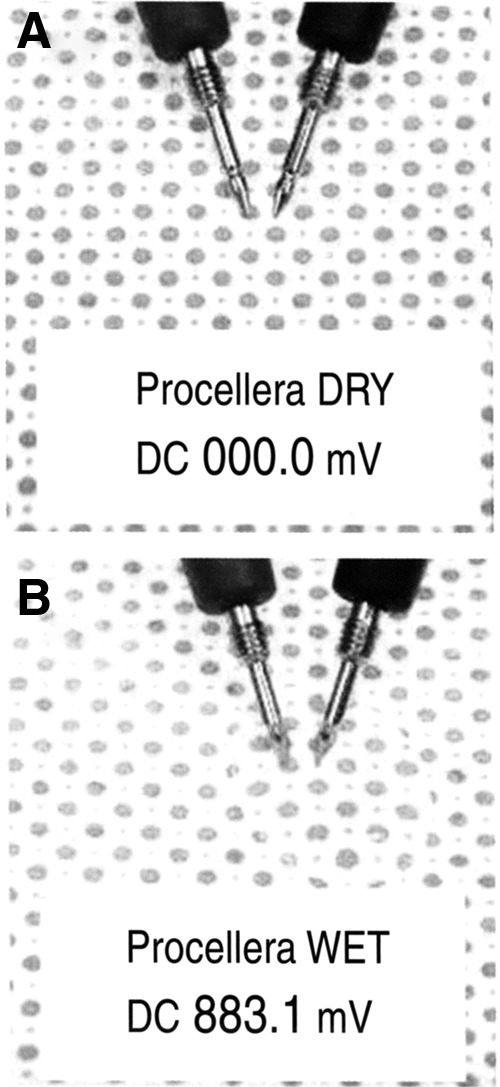
The antimicrobial Procellera™ DC device (Vomaris Innovations, Chandler, AZ) contains 25 micro-batteries and a silver formulary (permission granted by Vomaris Innovations): (A) Dry inactive dressing with zero mV measured. (B) Moist active dressing showing mV measured.
Key Findings.
• ES devices that produce DC and mono and biphasic PC waveforms represent the primary ES technologies that are reported to enhance wound healing.
• The electric charge or electric energy dosage that has resulted in human wound-healing outcomes from LVPC and HVPC devices falls between 250 and 500 μC/s.
• Miniaturized and disposable bioelectric dressing devices have been cleared by healthcare regulatory agencies for wound healing and antibacterial uses in the EU and United States respectively.
Abbreviations and Acronyms
- AC
alternating current
- CDC
continuous direct current
- DC
direct current
- EF
electric field
- EMF
electromagnetic field or electromotive force
- EMS
electromagnetic spectrum
- ES
electrical stimulation
- HVPC
high voltage pulsed current
- Hz
cycles per second (for alternating current)
- LVPC
low voltage pulsed current
- MPC
monophasic pulsed current
- PC
pulsed current
- pps
pulses per second (for pulsed current)
- SWC
standard wound care
Acknowledgments and Funding Sources
The author thanks Nick Schroeder, Senior Graphic Designer at Marquette University, for his assistance with the figures shown in this article. No funding sources are acknowledged.
Author Disclosure and Ghostwriting
The author has no competing interests. No ghostwriters were involved in the writing of this work.
About the Author
Professor Luther Kloth is a Catherine Worthingham Fellow of the American Physical Therapy Association and Emeritus Professor of Physical Therapy at Marquette University, Milwaukee, Wisconsin. He is a Board Certified Wound Specialist through the American Board of Wound Management and Fellow of the American College of Clinical Wound Specialists. He also serves on the Editorial Advisory Boards of Advances in Skin and Wound Care and The Journal of the American College of Clinical Wound Specialists. He is a founding member of the Association for the Advancement of Wound Care and is co-editor and author of the textbook Wound Healing: Evidence-based Management, 4th ed., published in 2010 by the F.A. Davis Company, Philadelphia. His research and numerous publications have focused on studying the effects of biophysical technologies such as electrical stimulation, low-frequency ultrasound, and normothermia on wound healing.
References
- 1.Branski LK, Gauglitz GG, Herndon DN, and Jeschke MG: A review of gene and stem cell therapy in cutaneous wound healing. Burns 2009; 35: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, and Longaker MT: Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009; 17: 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao M: Electric fields in wound healing—an overriding signal that directs cell migration. Semin Cell Dev Biol 2009; 20: 674. [DOI] [PubMed] [Google Scholar]
- 4.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester J, Bourne H, Devreotes P, McCaig C, and Penninger J: Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 2006; 442: 457. [DOI] [PubMed] [Google Scholar]
- 5.Lin F, Baldessari F, Gyenge CC, Sato T, Chambers RD, Santiago JG, and Butcher EC: Lymphocyte electrotaxis in vitro and in vivo. J Immunol 2008; 181: 2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugimoto M, Maeshige N, Honda H, Yoshikawa Y, Uemura M, Yamamoto M, and Terashi H: Optimum microcurrent stimulation intensity for galvanotaxis in human fibroblasts. J Wound Care 2012; 21: 5. [DOI] [PubMed] [Google Scholar]
- 7.Bourguignon GJ. and Bourguignon LYW: Electrical stimulation of protein and DNA synthesis in human fibroblasts. FASEB J 1987; 1: 398. [DOI] [PubMed] [Google Scholar]
- 8.Bourguignon GJ, Jy W, and Bourguignon LYW: Electric stimulation of human fibroblasts causes an increase of Ca2+ influx and the exposure of additional insulin receptors. J Cell Physiol 1989; 140: 379. [DOI] [PubMed] [Google Scholar]
- 9.Orida N. and Feldman JD: Directional protrusive pseudopodial activity and motility in macrophages induced by extracellular electric fields. Cell Motil 1982; 2: 243. [DOI] [PubMed] [Google Scholar]
- 10.Nishmura KY, Isseroff RR, and Nuccitelli R: Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J Cell Sci 1996; 109 (Pt 1): 199. [DOI] [PubMed] [Google Scholar]
- 11.Kloth LC: Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005; 4: 23. [DOI] [PubMed] [Google Scholar]
- 12.Feedar JA, Kloth LC, and Gentzkow GD: Chronic dermal ulcer healing enhance with monophasic pulsed electrical stimulation. Phys Ther 1991; 71: 639. [DOI] [PubMed] [Google Scholar]
- 13.Baker LL, Rubayi S, Villar F, and Demuth SK: Effect of electrical stimulation waveform on healing of ulcers in human beings with spinal cord injury. Wound Rep Regen 1996; 4: 72. [DOI] [PubMed] [Google Scholar]
- 14.Baker LL, Chambers R, Demuth SK, and Villar F: Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care 1997; 20: 405. [DOI] [PubMed] [Google Scholar]
- 15.Franek A, Taradaj J, Polak A, Cierpka L, and Blaszczak E: Efficacy of high voltaje stimulation for healing of venous leg ulcers in surgically and conservatively treated patients. Phlebologie 2006; 35: 127 [Google Scholar]
- 16.Franek A, Kostur R, Polak A, Taradaj J, Szlachta Z, Blaszczak E, Dolibog P, Dolibog P, Koczy B, and Kucio C: Using high voltage electrical stimulation in the treatment of recalcitrant pressure ulcers: results of a randomized, controlled clinical study. Ostomy Wound Manage 2012; 58: 30. [PubMed] [Google Scholar]
- 17.Gentzkow GD, Pollack SV, Kloth LC, and Stubbs HA: Improved healing of pressure ulcers using dermapulse, a new electrical stimulation device. Wounds 1991; 3: 158 [Google Scholar]
- 18.Gentzkow GD, Alon G, Taler GA, Eltorai IM, and Montroy RE: Healing of refractory stage III and IV pressure ulcers by a new electrical stimulation device. Wounds 1993; 5: 160 [Google Scholar]
- 19.Akers TK. and Gabrielson AL: The effect of high voltage galvanic stimulation on the rate of healing of decubitus ulcers. Biomed Sci Instrum 1984; 20: 99. [PubMed] [Google Scholar]
- 20.Kloth LC. and Feedar JA: Acceleration of wound healing with high voltage, monophasic, pulsed current. Phys Ther 1988; 71: 503. [DOI] [PubMed] [Google Scholar]
- 21.Griffin JW, Tooms RE, Mendlus RA, Clifft JK, Vander Zwaag R, and el-Zeky F: Efficacy of high voltage pulsed current for healing of pressure ulcers in patients with spinal cord injury. Phys Ther 1991; 71: 433. [DOI] [PubMed] [Google Scholar]
- 22.American Physical Therapy Association: Electrotherapeutic Terminology in Physical Therapy: Section on Clinical Electrophysiology. Alexandria, VA: American Physical Therapy Association, 2001 [Google Scholar]
- 23.Assimacopoulos D: Low intensity negative electric current in treatment of ulcers of leg due to chronic venous insufficiency: preliminary report of three cases. Am J Surg 1968; 115: 683. [DOI] [PubMed] [Google Scholar]
- 24.Wolcott LE, Wheeler PC, Hardwicke HM, and Rowley BA: Accelerated healing of skin ulcers by electrotherapy: preliminary clinical results. South Med J 1969; 62: 795. [DOI] [PubMed] [Google Scholar]
- 25.Gault WR. and Gatens PF: Use of low intensity direct current in management of ischemic skin ulcers. Phys Ther 1976; 56: 265. [DOI] [PubMed] [Google Scholar]
- 26.Carley PJ. and Wainapel SF: Electrotherapy for acceleration of wound healing: low intensity direct current. Arch Phys Med Rehabil 1985; 66: 443. [PubMed] [Google Scholar]
- 27.Wood JM. and Evans PE: A multi-center study on the use of pulsed low intensity direct current for healing chronic stage II and III decubitus ulcers. Arch Dermatol 1993; 129: 999. [PubMed] [Google Scholar]
- 28.Hampton S. and King L: Healing an intractable wound using bio-electric stimulation therapy. Br J Nurs 2005; 14: 30. [PubMed] [Google Scholar]
- 29.Hampton S. and Collins F: Treating a pressure ulcer with bio-electric stimulation therapy. Br J Nurs 2006; 15: S14. [DOI] [PubMed] [Google Scholar]
- 30.Nuccitelli R, Nuccitelli P, Ramlatchan S, Sanger R, and Smith PJ: Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen 2008; 16: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson RM. and Currier DP: Clinical Electrotherapy, Norwalk CT: Appleton and Lange, 1987, p. 63 [Google Scholar]
- 32.Newton RA. and Karselis TC: Skin pH following high voltage pulsed galvanic stimulation. Phys Ther 1983; 63: 1593. [DOI] [PubMed] [Google Scholar]
- 33.Debreceni L, Gyulai M, Debreceni A, and Szabo K: Results of transcutaneous electrical stimulation (TES) in cure of lower extremity arterial disease. Angiology 1995; 46: 613. [DOI] [PubMed] [Google Scholar]
- 34.Kaada B: Promoted healing of chronic ulceration by transcutaneous nerve stimulation (TNS). VASA 1983; 12: 262. [PubMed] [Google Scholar]
- 35.Karba B. and Vodovnik L: Promoted healing of chronic wounds due to electrical stimulation. Wounds 1991; 3: 16 [Google Scholar]
- 36.Stefanovska A, Vodovnik L, Benko H, and Turk R: Treatment of chronic wounds by means of electric and electromagnetic fields. Value of FES parameters for pressure sore treatment. Med Biol Eng Comput 1993; 31 (Pt 2): 231. [DOI] [PubMed] [Google Scholar]
- 37.Cukjati D, Robnik-Sikonja M, Rebersek S, Kononenko I, and Miklavcic D: Prognostic factors in the prediction of chronic wound healing by electrical stimulation. Med Biol End Comput 2001; 39: 542. [DOI] [PubMed] [Google Scholar]
- 38.Conductive Coupling http://en.wikipedia.org/wiki/Conductive_coupling (accessed January19, 2013)
- 39.Fukushima K, Senda N, Inui H, Miura H, Tamai Y, and Murakami Y: Studies on galvanotaxis of leukocytes. 1. Galvanotaxis of human neutrophilic leukocytes and methods of its measurements. Med J Osaka Univ 1953; 4: 195 [Google Scholar]
- 40.Houghton PE: Electrical Stimulation Therapy Application Techniques. Continuing education course. London, Canada: School of Physical Therapy, University of Western Ontario, January17–18, 2009 [Google Scholar]
- 41.Kloth LC: Wound healing with conductive electrical stimulation—it's the dosage that counts. J Wound Technol 2009; 6: 30 [Google Scholar]
- 42.Electric Charge http://en.wikipedia.org/wiki/Electric_charge (accessed January19, 2013)
- 43.Department of Health and Human Services (DHHS) Centers for Medicare and Medicaid Services CMS Manual System. Pub. 100-03 Medicare National Coverage Determinations. 1/270.1/Electrical Stimulation (ES) and Electromagnetic Therapy for the Treatment of Wounds. Bethesda, MD, 2004 [Google Scholar]
- 44.National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel: Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Washington, DC: National Pressure Ulcer Advisory Panel, 2009 [Google Scholar]
- 45.Registered Nurse Association of Ontario Nursing Best Practice Guidelines Program: Assessment & Management of Stage I to IV Pressure Ulcers. Toronto, Canada, 2007 [Google Scholar]
- 46.Consortium for Spinal Cord Medicine: Clinical Practice Guideline for Health Care Professionals. Pressure Ulcer Prevention and Treatment Following Spinal Cord Injury. Washington, DC: Paralyzed Veterans of America, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Koel G: Review: Electrotherapy for Stimulation of Wound Healing. Poster Presentation 19th Conference of the European Wound Management Association Helsinki, Finland, May20–22, 2009 [Google Scholar]
- 48.Kloth LC. and Zhao M: Endogenous and exogenous electrical fields for wound healing. In: Wound Healing: Evidence Based Management, edited by McCulloch JM. and Kloth LC. Philadelphia, PA: FA Davis, 2010 [Google Scholar]