Abstract
Cartilage injuries and osteoarthritis are leading causes of disability in developed countries. The regeneration of damaged articular cartilage using cell transplantation or tissue engineering holds much promise but requires the identification of an appropriate cell source with a high proliferative propensity and consistent chondrogenic capacity. Human fetal mesenchymal stem cells (MSCs) have been isolated from a range of perinatal tissues, including first-trimester bone marrow, and have demonstrated enhanced expansion and differentiation potential. However, their ability to form mature chondrocytes for use in cartilage tissue engineering has not been clearly established. Here, we compare the chondrogenic potential of human MSCs isolated from fetal and adult bone marrow and show distinct differences in their responsiveness to specific growth factors. Transforming growth factor beta 3 (TGFβ3) induced chondrogenesis in adult but not fetal MSCs. In contrast, bone morphogenetic protein 2 (BMP2) induced chondrogenesis in fetal but not adult MSCs. When fetal MSCs co-stimulated with BMP2 and TGFβ3 were used for cartilage tissue engineering, they generated tissue with type II collagen and proteoglycan content comparable to adult MSCs treated with TGFβ3 alone. Investigation of the TGFβ/BMP signaling pathway showed that TGFβ3 induced phosphorylation of SMAD3 in adult but not fetal MSCs. These findings demonstrate that the initiation of chondrogenesis is modulated by distinct signaling mechanisms in fetal and adult MSCs. This study establishes the feasibility of using fetal MSCs in cartilage repair applications and proposes their potential as an in vitro system for modeling chondrogenic differentiation and skeletal development studies.
Introduction
Mesenchymal stem cells (MSCs) are readily accessible, easily expandable, and possess the potential to differentiate into mesoderm-derived lineages, making these cells particularly suited for use in skeletal tissue engineering strategies [1,2]. Human bone marrow-derived MSCs have been shown to have the ability to differentiate into chondrocytes in vitro [3,4], and they can be used to tissue engineer cartilage [5]. We have shown their potential in clinical applications by using them for the production of a segment of trachea that was successfully implanted into a patient with bronchomalacia [6,7]. However, MSCs exhibit significant heterogeneity and variation in potency both within and between patients [8]. In addition, adult MSCs undergo replicative senescence, resulting in a decrease in their prevalence, proliferation rate, and differentiation capacity [9,10]. This variability and tendency to senesce is severely limiting and poses significant implications for advancements in the use of MSC-based therapies for cartilage repair.
The ability to tissue engineer cartilage with biomechanical and structural integrity similar to native tissue relies on a suitable model for differentiation studies and the identification of a cell source with consistent chondrogenic capacity. For these reasons, there has been much interest in exploring MSC subpopulations [11] and in the identification of alternative sources of MSCs to provide a cell population optimized for in vitro chondrogenesis even after a high number of population doublings. Fetal MSCs have enhanced plasticity, proliferation propensity, and expansion potential compared with adult MSCs [12]. They appear to form an intermediate cell type between adult MSCs and embryonic stem cells (ESCs), as they have active telomerase and express pluripotency markers, albeit at a considerably lower level than ESCs [12], and, therefore, their use is not limited by the risk of teratoma formation or tumorigenicity associated with ESCs [13,14]. Fetal MSCs also lack intracellular HLA class II and have lower HLA class I expression compared with adult MSCs, which suggests that these cells may be immunologically inert [15]. In addition, MSCs derived from first-trimester fetal bone have greater osteogenic potency [16] and undergo accelerated osteogenesis in vitro and bone formation in vivo [17] compared with adult MSCs, suggesting that the anatomical locus and developmental role of these cells may contribute to lineage commitment and potency associated with skeletal tissue differentiation. For these reasons, fetal stem cells warrant further investigation for use in cartilage regeneration.
Human perinatal fetal stem cells harvested from prenatal and extraembryonic tissues, including fetal bone marrow, blood, liver, amniotic fluid, and umbilical cord blood, have shown trilineage differentiation potential along adipogenic, osteogenic, and chondrogenic lineages [12,17–21]. However, their potential to generate mature chondrocytes suitable for cartilage tissue engineering has not yet been fully established. Transforming growth factor beta 3 (TGFβ3) is the growth factor that is most widely used to initiate in vitro chondrogenic differentiation of MSCs [4,22]. Alternative members of the TGFβ superfamily, the bone morphogenetic proteins (BMPs) have been shown to have the ability to induce de novo ectopic cartilage formation in a system that recapitulates endochondral ossification during skeletogenesis [23,24]. Specifically, BMP2 has been shown to promote condensation of the mesenchymal cells in the developing limb [25,26]. In addition, BMPs have also been used to induce chondrogenesis of MSCs in vitro both alone [27–30] and in combination with TGFβ3 [27,30–32].
Considering the ability of BMPs to stimulate synthesis of cartilage extracellular matrix proteins in human articular chondrocytes in vitro [33] and their important role in cartilage formation and development in vivo [34–36], we hypothesized that they may preferentially stimulate the initiation and regulation of chondrogenic differentiation in fetal MSCs. The objective of this study was to investigate the chondrogenic capacity of human first-trimester fetal MSCs compared with adult bone marrow MSCs using micromass pellet cultures and cartilage tissue engineering.
Materials and Methods
Collection of bone marrow
Adult bone marrow samples were collected from patients with osteoarthritis undergoing total hip replacement surgery, in full accordance with Southmead Hospital Research Ethics Committee guidelines. Human first-trimester bone marrow samples were obtained from patients after elective termination of pregnancy. The Research Ethics Committee of Hammersmith and Queen Charlottes's Hospitals (London, United Kingdom) approved the collection of fetal tissue for research purposes. Patients and pregnant women provided written consent for the clinical procedure and the use of cells for research. Adult bone marrow samples were obtained from a total of 20 patients ranging from 45 to 81 years old. Bone marrow samples were collected from four fetuses with gestational ages of 8+4, 9+4, 11+0, and 11+1 (weeks+days).
Isolation and culture of bone marrow-derived MSCs
Adult bone marrow aspirates were seeded into tissue culture flasks containing expansion medium consisting of Dulbecco's-modified Eagle's Medium (DMEM) supplemented with 1000 mg/L glucose, 10% (v/v) fetal bovine serum (FBS, from a selected batch), 100 units/mL penicillin, 100 μg/mL streptomycin (all from Sigma), and 2 mM glutamax (Invitrogen). Nonadherent cells were washed away with medium changes, and MSCs were isolated by plastic adherence. First-trimester fetal bone marrow derived MSCs were isolated as previously described [19]. Fetal MSCs were cultured in the same expansion medium except DMEM was supplemented with 4500 mg/L glucose, 50 units/mL penicillin, and 50 μg/mL streptomycin. Both fetal and adult MSCs were expanded in the presence of 5 ng/mL fibroblast growth factor (FGF-2; Peprotech) until they reached ∼80% confluency and were then harvested and stored in a liquid nitrogen facility until further use.
Immunophenotyping of MSCs
Fetal and adult MSCs were trypsinised and washed in a buffer consisting of PBS containing 0.5% (w/v) bovine serum albumin (BSA) and stained with the following anti-human antibodies: CD105-fluorescein isothiocyanate (FITC), CD90-phycoerythrin (PE), CD73-PE, CD146-PE, CD271-PE, VCAM-FITC, STRO-1, CD45-PE, (all from R&D Systems), and CD34-FITC (BD Bioscience). The appropriate mouse isotype antibodies were used as controls. Samples were processed using an FACSCanto II flow cytometer (BD Bioscience) and analyzed with FlowJo Software (Treestar).
Adipogenic differentiation
Confluent control MSCs were incubated in α-MEM basal medium containing 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin (all from Sigma), and 2 mM glutamax (Invitrogen). Cells stimulated to undergo differentiation were cultured in basal medium supplemented with Adipogenic Supplement (R&D Systems). After 21 days of differentiation, cells were fixed in 4% paraformaldehyde for 30 min, then washed in 60% isopropanol, and stained with 0.3% oil red O (Sigma) for 30 min to visualize lipid vacuoles.
Osteogenic differentiation
Sub-confluent control MSCs were maintained in α-MEM basal medium supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin (all from Sigma), and 2 mM glutamax (Invitrogen). For osteogenic differentiation, MSCs were cultured in basal medium containing Osteogenic Supplement (R&D Systems). After 14 days of differentiation, cells were fixed in 70% ethanol for 1 h, washed with PBS and the mineralized deposits in the matrix were visualized by 40 mM alizarin red (Sigma) after 5 min of staining.
Chondrogenic differentiation
For differentiation in monolayer culture, 3×105 MSCs were seeded into a T25 cm2 tissue culture flask. The cells were maintained in DMEM basal medium supplemented with 4500 mg/L glucose, 100 units/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 80 μM ascorbic acid-2-phosphate, 100 nM dexamethasone (all from Sigma), 2 mM glutamax, and 1% insulin transferrin selenium (ITS; both from Invitrogen). MSCs stimulated to undergo differentiation were cultured in chondrogenic medium consisting of basal medium supplemented with 10 ng/mL recombinant human TGF β3 (rhTGFβ3). The medium was changed every 3 days for a total of 14 days. Micromass pellet cultures were prepared by centrifuging 4×105 MSCs into a pellet at 1500 rpm for 5 min. The pellets were cultured in the same basal medium described earlier that was supplemented with either 10 ng/mL rhTGFβ3 or rhTGFβ1 and/or 100 ng/mL recombinant human BMP 2 (rhBMP2) (CHO-derived) or rhBMP6 (NSO-derived) or rhBMP7 (CHO-derived) (all from R&D Systems). Undifferentiated controls were maintained in basal medium. The medium was changed every 3 days for a total of 21 days. Three replicate pellets were prepared for each condition, pooled, and manually homogenized in lysis solution after differentiation.
RNA was isolated from the cells using a Total RNA Purification Kit (Norgen Biotek), and cDNA was synthesized using a PrimeScript RT Reagent Kit (Takara), as per the manufacturer's instructions. The relative expression of chondrogenic and hypertrophic marker genes (Table 1) was measured by a real-time quantitative polymerase chain reaction (qRT-PCR) on a Rotor-Gene 6000 using SYBR Green I (Qiagen). Data were analyzed with REST 2009 gene quantification software (Qiagen) and normalized to beta actin.
Table 1.
Real-Time Quantitative Polymerase Chain Reaction Primer Sequences
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Type II collagen (COL2A1) | GGTCTTGGTGGAAACTTTGCT | GGTCTTGCATTACTCCCAAC |
| Aggrecan (ACAN) | CTGCTTCCGAGGCATTTCAG | CTTGGGTCACGATCCACTCC |
| Type I collagen (COL1A2) | TCTGGATGGATTGAAGGGACA | CCAACACGTCCTCTCTCACC |
| Sox9 (SOX9) | CTTTGGTTTGTGTTCGTGTTTTC | AGAGAAAGAAAAAGGGAAAGGTAAGTTTT |
| Type X collagen (COL10A1) | ATGCTGCCACAAATACCCTTT | GGAATGAAGAACTGTGTCTTGGT |
| Alkaline phosphatase (ALP) | CGGAACTCCTGACCCTTGAC | TGTTCAGCTCGTACTGCATGTC |
| Indian hedgehog (IHH) | CCTCAGTTGATGCTGCTAATTC | AACAGTCTCTGGATGTGTCTTG |
| Matrix metalloproteinase 13 (MMP13) | TCTGAACTGGGTCTTCCAAAA | GCATCTACTTTATCACCAATTCCT |
| Runt-related transcription factor 2 (RUNX2) | GCCTTCAAGGTGGTAGCCC | CGTTACCCGCCATGACAGTA |
| Bone morphogenetic protein receptor type II (BMPR2) | CACTCAGTCCACCTCATTCATTT | TTGTTTACGGTCTCCTGTCAAC |
| Transforming growth factor beta receptor II (TGFBR2) | AAGATGACCGCTCTGACATCA | CTTATAGACCTCAGCAAAGCGAC |
| Beta actin | GACAGGATGCAGAAGGAGATTACT | TGATCCACATCTGCTGGAAGGT |
Cartilage tissue engineering
Cartilage tissue engineering with fetal and adult MSCs was performed as previously described [5]. Briefly, 3×105 MSCs were seeded onto fibronectin (Sigma)-coated sterile polyglycolic acid scaffold discs (5×2 mm) (Biomedical Structures). The constructs were cultured in chondrogenic medium (detailed in Chondrogenic Differentiation), which was further supplemented with 10 μg/mL human pancreatic insulin (Sigma) after 7 days until the end of culture to promote extracellular matrix formation by the differentiated cells [37]. Constructs were incubated at 37°C on a rotating platform for a total of 35 days, which has previously been shown to be the minimum timeframe for effective quantification of the extracellular matrix using our biochemical analyses [5]. The constructs were then harvested, freeze dried, and weighed. The extracellular matrix was solubilized by 2 mg/mL bovine pancreatic trypsin digestion overnight, followed by 15 min of boiling to inhibit further enzyme activity [38]. The remaining undigested scaffold was freeze dried, weighed, and subtracted from the original weight to provide the dry weight of the extracellular matrix. Specific assays were used to measure the amounts of type I and II collagen and proteoglycan. The quantity of type II collagen was determined using inhibition ELISA with a mouse IgG monoclonal antibody to denatured type II collagen [39]. Type I collagen was measured by inhibition ELISA using a rabbit anti-peptide antibody to type I collagen [38]. The proteoglycan content was quantified by using a dimethylmethylene blue (Sigma) colorimetric assay for sulfated glycosaminoglycan (GAG) [40].
Histological staining
Frozen constructs of engineered cartilage prepared from fetal and adult MSCs were cut into 7 μm sections, stained with hematoxylin and eosin (H&E) to assess cellularity and with 0.1% safranin-O (Sigma), and counterstained with 0.02% Fast Green F to indicate the GAG deposition. Additional sections were pretreated with hyaluronidase (Sigma) and pronase (from Streptomyces griseus; Roche Applied Science) and then incubated with antibodies against collagen type I (1:100 dilution) and II (1:20 dilution; both from Cambridge Biosciences) as previously described [41]. Antibodies were detected using biotinylated secondary antibodies and diaminobenzidine substrate (Vector Laboratories). Sections were counterstained with hematoxylin, and normal goat IgG (Santa Cruz Biotechnology) was used as a negative control.
Phosphorylated-SMAD protein detection
Fetal and adult MSCs grown in a monolayer culture were treated with 1 ng/mL rhTGFβ3 or 25 ng/mL rhBMP2 (both from R&D Systems) alone and in combination for 60 min. Cells maintained in basal medium described earlier were used as untreated controls. The cells were lysed in lysis buffer containing 50 nM Tris-HCl pH6.8, 1% sodium dodecyl sulfate, and 10% glycerol to extract protein. Total protein concentration was determined using a bicinchoninic acid colorimetric assay (Pierce). For each sample, 25 μg of protein was loaded onto 4%–12% Novex Tris/Glycine gels (Invitrogen) and transferred onto a 0.2 μM nitrocellulose membrane. Membranes were blocked with 5% BSA in tris-buffered saline (TBS; 20 mM Tris pH7.6, 137 mM NaCl) with 0.1% Tween-20 at 20°C for 1 h followed by an overnight incubation at 4°C with antibodies against SMAD family member 3 (SMAD3), phosphorylated-SMAD3 (pSMAD3), SMAD1, SMAD5, pSMAD1/5 (pSMAD1 and pSMAD5), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:000 dilution; all from Cell Signaling) in TBS supplemented with 5% BSA and 0.1% Tween-20. Bound monoclonal antibodies were detected with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:2000 dilution; Cell Signaling) and enhanced chemiluminescence (Santa Cruz Biotechnology). Densitometry was used to quantify the detected bands using ImageJ software, and the optical density of each band (OD×mm2) was expressed as a fold change of phosphorylation induction.
TGFβ and BMP receptor expression
Fetal and adult MSCs grown in monolayer culture were treated with 10 ng/mL rhTGFβ3 or 100 ng/mL rhBMP2 (both from R&D Systems) alone and in combination for 48 h. Cells maintained in basal medium described earlier were used as untreated controls. RNA was isolated from the cells using a Total RNA Purification Kit (Norgen Biotek), and cDNA was synthesized using a PrimeScript RT Reagent Kit (Takara), as per the manufacturer's instructions. The relative expression of BMP receptor II (BMPR2) and TGF beta receptor II (TGFBR2) (Table 1) was measured by qRT-PCR on a Rotor-Gene 6000 using SYBR Green I (Qiagen). Data were analyzed with REST 2009 gene quantification software (Qiagen) and normalized to beta actin.
Statistical analysis
A comparison of differences between individual groups was performed using the Mann–Whitney U test, or analysis of variance using the nonparametric Kruskal–Wallis test with a Dunn's post-hoc correction was used for a comparison of multiple groups. In all analyses, P<0.05 was considered significant.
Results
Immunophenotypic comparison of human fetal and adult MSCs
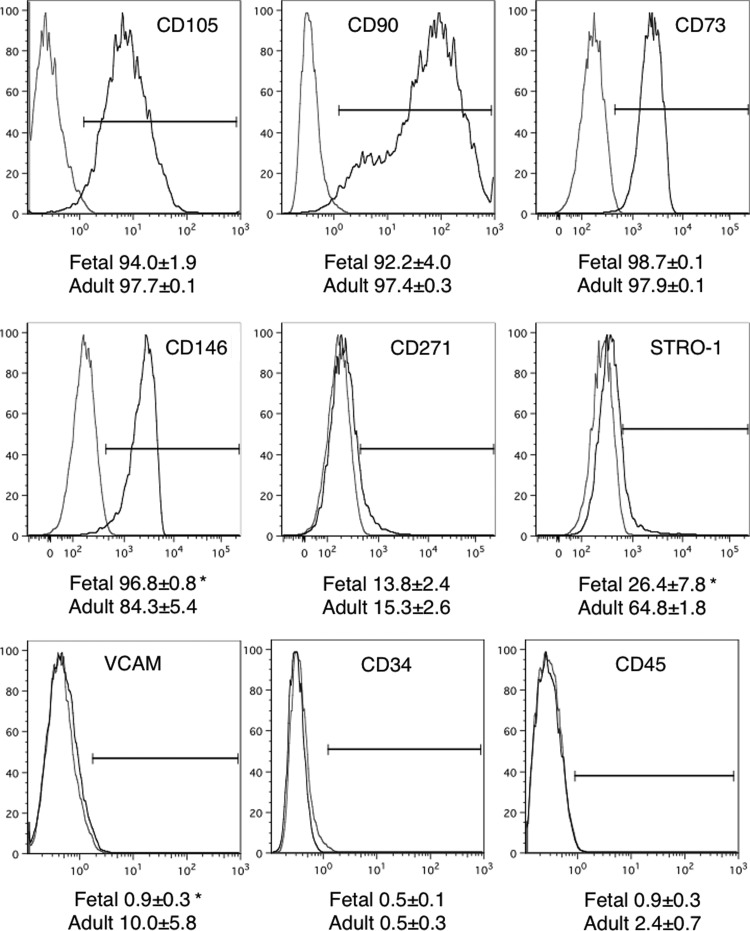
MSCs derived from fetal and adult bone marrow were characterized by flow cytometry for the expression of a range of surface MSC-marker proteins that have been traditionally used to characterize mesenchymal populations (Fig. 1). Fetal MSCs from passages 3 and 4 and adult MSCs from passages 1 to 3 were used for analyses. Both fetal and adult cell populations expressed very high levels (in excess of 90%) of the MSC marker proteins CD105, CD90, and CD73 and relatively low levels (∼15%) of CD271, without any significant differences between the two populations. There was a significantly higher expression of the pericyte marker CD146 on MSCs from fetal MSCs compared with adult bone marrow MSCs; however, they were equally negative for hematopoietic stem cell markers CD34 and CD45. STRO-1 expression was variable and significantly more highly expressed on adult than fetal MSCs while VCAM levels, although low in both populations, showed a significant 10% increase on adult MSCs (Fig. 1). These data indicate that both the fetal and adult bone marrow-derived adherent cell populations share similar cell surface marker protein profiles, which are representative of MSCs.
FIG. 1.
Immunophenotypic analyses of mesenchymal stem cell (MSC) Marker Proteins. The expression of cell surface markers used to characterize MSCs isolated from human first-trimester fetal and adult bone marrow was determined using flow cytometry. Representative plots of a fetal MSC sample is shown, with isotype controls in gray. Population frequencies for each marker are shown on the plots (mean±SEM; n=4).
Multilineage differentiation potential of fetal and adult MSCs
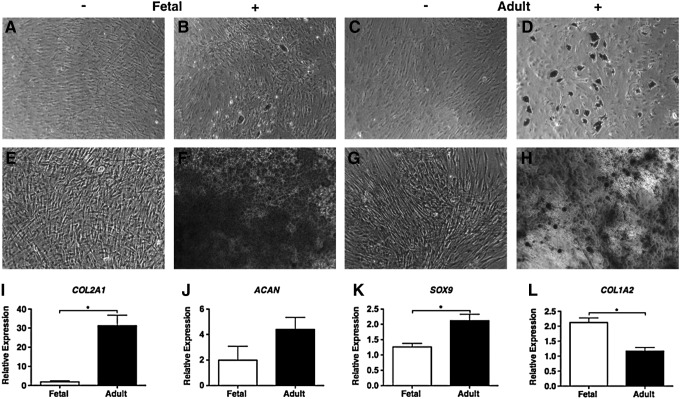
To investigate whether fetal MSCs possess a similar multilineage potential to adult MSCs, their capacity for in vitro adipogenic, osteogenic, and chondrogenic differentiation was determined. Their adipogenic ability was indicated by the presence of lipid vacuoles identified by oil red O stain. After 21 days of differentiation, there was some evidence of lipid droplet accumulation that could only be detected in three out of the four fetal MSC samples tested, compared with the prominent presence of fat vacuoles in all four of the adult MSC samples (Fig. 2A–D). Osteogenic potential was determined by alizarin red stain to identify calcified mineral deposits in the extracellular matrix after 14 days of differentiation. All fetal MSC samples demonstrated clear osteogenic induction, exhibiting a mineralized matrix that was more strongly stained compared with the differentiated adult MSCs (Fig. 2E–H). The chondrogenic capacity of fetal and adult MSCs was investigated by inducing differentiation driven by TGFβ3 in monolayer culture. After 14 days, the expression of cartilage-specific marker genes was determined using real-time qPCR. Fetal and adult MSCs showed distinct differences in their responsiveness to TGFβ3. Type II collagen and Sox 9 mRNA was significantly up-regulated in adult MSCs compared with fetal MSCs (Fig. 2I, K). Adult MSCs stimulated with TGFβ3 also showed higher (four-fold) aggrecan expression compared with fetal MSCs (Fig. 2J). In contrast, the expression of type I collagen expression was significantly greater in fetal than adult MSCs (Fig. 2L).
FIG. 2.
Multilineage differential potential of fetal and adult MSCs. The adipogenic potential of fetal (B) and adult (D) MSCs is shown by oil red O staining. Osteogenesis is shown by alizarin red staining in fetal (F) and adult (H) MSCs. Undifferentiated controls (A, C, E, G). Representative samples are shown at 10×magnification (scale bar is 100 μm). Chondrogenic marker gene types II collagen (I), aggrecan (J), Sox 9 (K), and type I collagen (L) were measured by real-time qPCR analyses in fetal and adult MSCs and are expressed relative to an undifferentiated control and normalized to beta actin. Each bar is the mean±SEM (n=4), *P<0.05 by Mann-Whitney U-test.
Taken together, our data show that the fetal bone marrow-derived cell populations express surface marker proteins related to MSCs and demonstrate potent osteogenic and some adipogenic potential; however, the chondrogenic capacity of these cells in response to TGFβ3 stimulation was markedly reduced compared with adult MSCs.
TGFβ superfamily induced chondrogenic differentiation
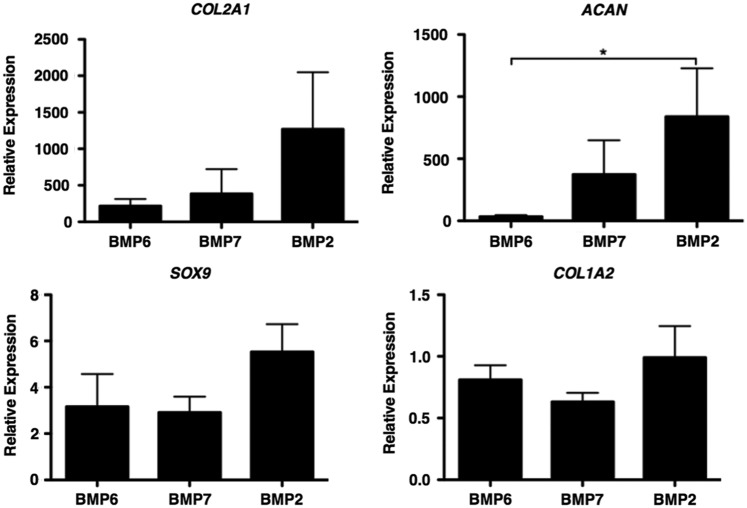
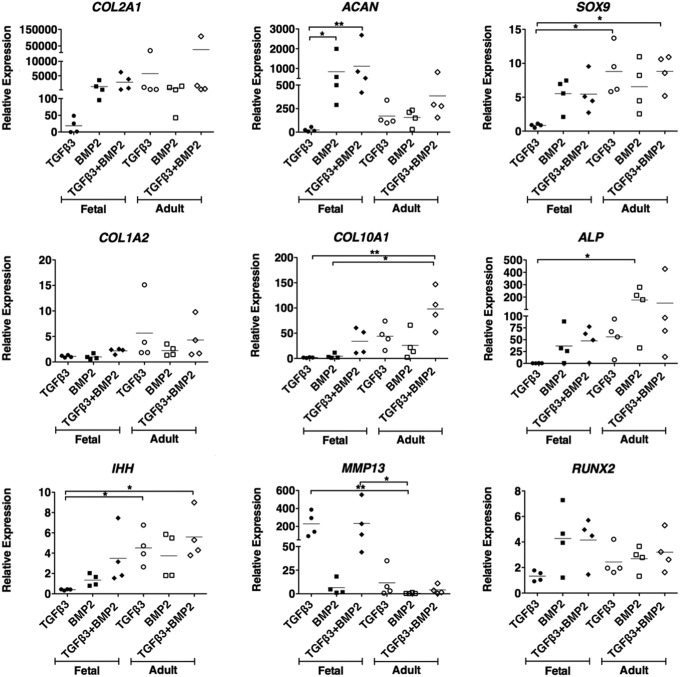
In view of the lack of response of fetal MSCs to TGFβ3 and the knowledge that BMPs are critical for cartilage formation in development in vivo, we went on to determine whether fetal MSCs can be stimulated to undergo chondrogenesis in response to BMPs. As a first screen, micromass pellet cultures were stimulated with TGFβ3 or TGFβ1 and BMP2, BMP6, or BMP7, alone or in combination with each other. After 21 days of differentiation, the expression of cartilage-specific genes as well as a range of markers of hypertrophic terminal differentiation was determined by real-time qPCR. Both TGFβ3 and TGFβ1 had a negligible chondrogenic effect on fetal MSC pellets (data not shown). Fetal MSCs showed significant up-regulation of aggrecan mRNA after stimulation with BMP2 compared with BMP6. Type II collagen and sox 9 were also more highly expressed in fetal MSCs treated with BMP2 than either BMP6 or BMP7 (Fig. 3). The combination of TGFβ3 with BMP2 had no significant advantage compared with BMP2 alone, and both showed similar chondrogenic marker gene expression. Adult MSCs underwent up-regulation of chondrogenic marker genes when stimulated with either TGFβ3 or BMP2 alone or the two in combination. Type I collagen is a marker of the unwanted fibrocartilage phenotype. Interestingly, for fetal cells, there was minimal type I collagen gene expression under all conditions, whereas for adult cells cultured with TGFβ3 (with or without BMP2) there was an approximate five-fold increase in type I collagen gene expression (Fig. 4).
FIG. 3.
Bone morphogenetic protein (BMP)-mediated chondrogenic potential of fetal MSCs. Fetal MSCs were stimulated with BMP2, BMP6, and BMP7 for 21 days. The relative expression of chondrogenic marker genes was determined by real-time qPCR analyses and are expressed relative to an undifferentiated control and normalized to beta actin. *P<0.05, by Kruskal–Wallis with a Dunn's post-hoc correction for multiple comparisons.
FIG. 4.
Chondrogenic capacity of fetal and adult MSCs. Micromass pellet cultures were treated with transforming growth factor beta 3 (TGFβ3) and BMP2, alone and in combination for 21 days. The relative expression of chondrogenic marker genes and hypertrophic marker genes were measured using real-time qPCR analyses and are expressed relative to an undifferentiated control and normalized to beta actin. Each line is the mean±SEM (n=4), *P<0.05, **P<0.01 by Kruskal–Wallis with a Dunn's post-hoc correction for multiple comparisons.
Terminal differentiation of MSCs to the hypertrophic phenotype is undesirable in hyaline cartilage tissue engineering, and the ideal chondrogenic stimulus would avoid activating this pathway. We measured the expression of five markers of hypertrophy, including type X collagen (the archetypal marker) as well as alkaline phosphatase, Indian hedgehog, matrix metalloproteinase 13 (MMP13), and RUNX2. The majority of markers were similarly activated in both fetal and adult MSCs. However, the expression of MMP13 was considerably higher in fetal MSCs treated with TGFβ alone or in combination with BMP2 compared with the significantly negligible expression detected in adult MSCs treated with BMP2. In addition, lower expression levels of type X collagen, alkaline phosphatase, and Indian hedgehog were observed in fetal MSCs differentiated with BMP2 alone compared with adult MSCs under all conditions (Fig. 4).
These data show that fetal MSCs show greater chondrogenic potency when stimulated by BMP2, compared with TGFβ3, whether used on its own or in combination with BMP2. On the other hand, adult MSCs can be induced to undergo chondrogenesis by either TGFβ3 or BMP2.
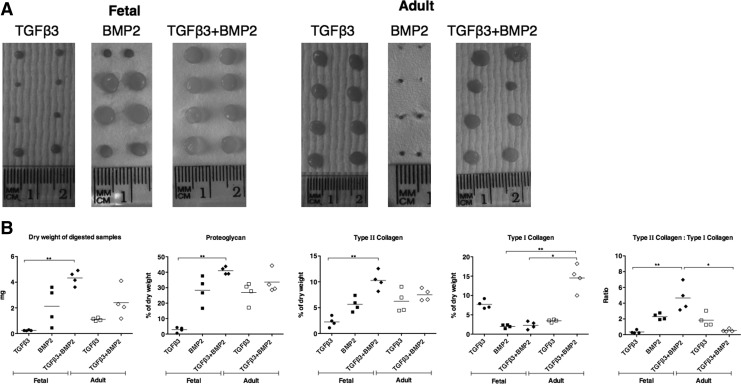
Cartilage tissue engineering
To further investigate the chondrogenic capacity of fetal MSCs, we considered it important to determine whether the observed increase in chondrogenic marker gene expression stimulated by BMP2 could be translated into ECM protein production by tissue engineering three-dimensional cartilage. There was an extensive variation in the macroscopic appearance of tissue engineered constructs depending on the conditions used. For fetal cells, there was limited growth of tissue with TGFβ3 on its own, improved but still variable tissue formation with BMP2, and a more consistent appearance of tissue production with a combination of TGFβ3 and BMP2 (Fig. 5A). These observations correlated well with measurement of the dry weight of each construct for fetal cells (Fig. 5B). For adult cells, there was apparently good tissue formation as judged by macroscopic appearance when using TGFβ3 alone or in combination with BMP2, whereas negligible tissue formation was observed when using BMP2 on its own (Fig. 5A). The dry weight analysis showed more matrix formation using the combination of cytokines compared with TGFβ3 alone (Fig. 5B). The constructs made from adult MSCs stimulated with BMP2 only were not large enough to obtain an accurate dry weight or to perform biochemical measurements, and so, no data are shown in Fig. 4B for this combination.
FIG. 5.
Tissue engineered cartilage. Fetal and adult MSCs stimulated with TGFβ3 and BMP2, alone and in combination, were used to tissue engineer cartilage on polyglycolic acid scaffolds for 35 days. The macroscopic appearance of duplicate constructs of the engineered cartilage is shown (A). The production of cartilage by tissue engineering was measured as dry weight of the extracellular matrix, the biochemical content of type II and type I collagen by specific ELISAs, and proteoglycan measured as glycosaminoglycan by colorimetric assay (B). Each line is the mean±SEM (n=4), *P<0.05, **P<0.01 by Kruskal–Wallis with a Dunn's post-hoc correction for multiple comparisons.
The quality of tissue engineered cartilage was determined by specific assays for proteoglycan and for collagen proteins. Hyaline cartilage is rich in proteoglycans and type II collagen and lacks type I collagen. Therefore, a high ratio of collagen II to collagen I is indicative of good chondrogenic differentiation. There was an additive effect in type II collagen production in fetal MSCs stimulated with BMP2 alone or in combination with TGFβ3, and this was comparable to adult MSCs under all conditions. The type I collagen content was comparably minimal in fetal MSCS stimulated with BMP2 alone or in combination with TGFβ3 and in adult MSCs treated with TGFβ3 alone. The amount of proteoglycan produced comprised 40% of the constructs engineered from fetal MSCs co-stimulated with BMP2 and TGFβ3 compared with 30% in constructs generated by either BMP2-induced fetal MSCs or adult MSCs with TGFβ3 alone or in combination with BMP2.
Therefore, for fetal MSCs, the best cartilage quality was observed when using a combination of TGFβ3 and BMP2, whereas TGFβ3 alone generated the best engineered tissue in adult MSCs (Fig. 5B).
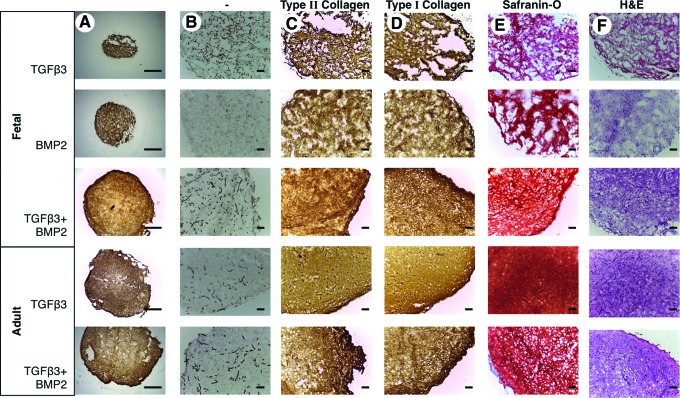
Histological analyses of tissue engineered cartilage
In order to further determine the quality of tissue engineered cartilage, we investigated tissue structure using immunohistochemical analysis. The apparent size of the tissue engineered from fetal MSCs treated with a combination of TGFβ3 and BMP2 was larger and showed more uniform extracellular matrix production across the constructs compared with those incubated with either BMP2 or TGFβ3 alone (Fig. 6A). In line with our biochemical findings, tissue engineered from fetal MSCs stimulated with both TGFβ3 and BMP2 demonstrated an even deposition of proteoglycan, type II and type I collagen across the construct (Fig. 6B–F). In contrast, remnant scaffold fibers can be observed in the unstained control sections and in combination with a reduction in cellularity, abundant hollow voids spanning the circumference and weaker detectable staining are indicative of reduced matrix protein deposition in constructs generated from fetal MSCs stimulated with either TGFβ3 or BMP2 alone (Fig. 6A–F). The tissue engineered from adult MSCs with TGFβ3 alone or in combination with BMP2 was characterized by a substantial and uniform extracellular matrix for all proteins studied, with consistent cellularity (Fig. 6A–F). No histology was possible with adult MSCs stimulated with BMP2 alone because of the lack of tissue formation.
FIG. 6.
Histological analyses of tissue engineered cartilage. The structure and extracellular matrix (ECM) deposition of engineered cartilage tissue is shown at 2.5×magnification (scale bar is 1000 μm) (A). High-magnification images showing the ECM of unstained sections (B), immunostained type II collagen (C) and type I collagen (D), safranin-O staining for proteoglycan (E), and hematoxylin and eosin (H&E) for overall cellularity (F) of representative samples are shown at 10×(scale bar is 100 μm). Color images available online at www.liebertpub.com/scd
TGFβ/BMP SMAD signaling in chondrogenesis of MSCs
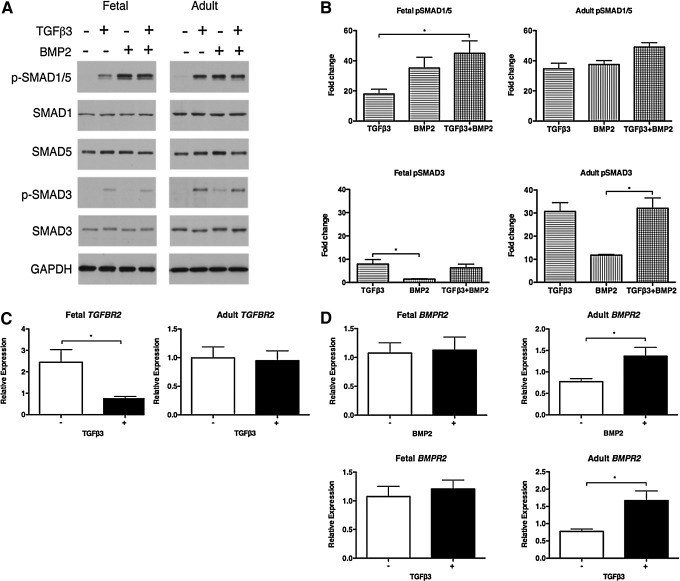
The differences in chondrogenic response of fetal and adult MSCs stimulated with TGFβ3 or BMP2 suggest that these cells may undergo distinct activation of the pathways associated with regulating chondrogenesis. To investigate this possibility, we sought to determine whether we could detect differences in TGFβ/BMP signaling through receptor activation and the phosphorylation of specific SMADs in response to BMP or TGFβ-driven chondrogenesis. The binding of TGFβ3 to TGFβ receptor II (TGFBR2) triggers the phosphorylation of SMAD2 and SMAD3, while the activation of BMP receptor II (BMPR2) by BMP2 induces SMAD1 and SMAD5 phosphorylation.
Fetal MSCs treated with TGFβ3 resulted in negligible phosphorylation of the TGFβ-specific SMAD3 (Fig. 7A), whereas a 30-fold increase in phosphorylated SMAD3 was observed in adult MSCs stimulated with TGFβ3 or in combination with BMP2 (Fig. 7A, B). Total SMAD3 is unchanged in both fetal and adult MSCs under all conditions (Fig. 7A). In addition, fetal MSCs induce significant down-regulation of TGFBR2 expression in response to TGFβ3 treatment, whereas expression levels remain unchanged in adult MSCs stimulated with TGFβ3 (Fig. 7C). We did not observe any significant differences in SMAD2 phosphorylation between fetal and adult MSCs stimulated with TGFβ3 alone (data not shown).
FIG. 7.
Activation of TGFβ/BMP signaling. Fetal and adult MSCs were treated with TGFβ3 and BMP2. The phosphorylation of BMP-specific SMAD1 and SMAD5 and TGFβ-specific SMAD3 was measured by western blotting (A) and quantified using densitometry as fold change normalized to GAPDH (B). TGFβ receptor II (TGFBR2) (C) and BMP receptor II (BMPR2) (D) expression was measured by qPCR. Each line is the mean±SEM (n=4). In (B) *P<0.05 by Kruskal–Wallis with a Dunn's post-hoc correction for multiple comparisons; in (C, D) *P<0.05 by Mann–Whitney U-test.
The BMP-specific SMAD1/5 was strongly phosphorylated in both fetal and adult MSCs treated with either BMP2 alone or in combination with TGFβ3 (Fig. 7A). A significant 45-fold increase in phosphorylated-SMAD1/5 was observed in fetal MSCs co-stimulated with BMP2 and TGFβ3 compared with TGFβ3 alone (Fig. 7B). Fetal MSCs stimulated with BMP2 induced SMAD1/5 phosphorylation that was comparable to the pSMAD1/5 detected in adult MSCs under all conditions, including TGFβ3 alone (Fig. 7A, B). Total SMAD1 and SMAD5 were unchanged in fetal and adult MSCs under all conditions (Fig. 7A). While BMPR2 expression was unchanged in fetal MSCs treated with BMP2, significant up-regulation of BMPR2 was observed in adult MSCs stimulated with either BMP2 or TGFβ3 (Fig. 7D).
These findings correlate with the negligible chondrogenic response observed in fetal MSCs stimulated with TGFβ3 in contrast to the positive induction of chondrogenesis in adult MSCs. Furthermore, the chondrogenic effect of BMP2 alone and in combination with TGFβ3 in fetal MSCs is confirmed by SMAD1/5 phosphorylation. Taken together, these data demonstrate that fetal and adult MSCs have distinct mechanisms controlling the activation of signaling pathways which are required to induce chondrogenesis.
Discussion
This study has demonstrated that chondrogenesis is modulated through distinct signaling mechanisms in human fetal and adult MSCs. While the initiation of chondrogenic differentiation is preferentially regulated by BMP2 in fetal MSCs, co-stimulation with TGFβ3 generates tissue engineered cartilage of comparable quality to adult MSCs stimulated with TGFβ3 alone. These findings establish the feasibility of using fetal MSCs in cartilage tissue engineering both as a therapy and as a three-dimensional model for studying chondrogenic differentiation.
Fetal and adult bone marrow-derived MSCs share a similar immunophenotype. Our findings are consistent with other reports of MSCs isolated from fetal liver, blood, bone marrow, lung, and spleen [12,18,19,42]. Interestingly, the expression of a pericyte marker CD146 is significantly higher in fetal than adult MSCs. Pericytes have similar expression profiles and functional characteristics to MSCs and are thought to be the ancestral precursor of MSCs [43,44]. This suggests that fetal MSCs may be an immature MSC population which is more closely related to the pericyte. In line with our observations, Maijenburg et al. (2012) reported a decrease in the proportion of CD271-positive MSCs expressing CD146 with an increase in donor age. Furthermore, they identified a unique subset of CD146-positive and CD271-negative MSCs that was only present within fetal cell populations and capable of trilineage differentiation potential. These findings indicate that the mesenchymal cell compartment comprises distinct subpopulations during different phases of life, and the changes in its constituency may reflect specific functions associated with bone marrow development and maintenance [45].
We identified differences in the multilineage potential of fetal and adult MSCs. Although both demonstrated adipogenic ability, fewer lipid vacuoles were observed in fetal MSCs. Interestingly, the osteogenic differentiation potential of fetal MSCs was greater than adult MSCs, which agrees with the findings of Guillot et al. (2008). However, they also report that fibroblast growth factor 2 (FGF2) induced spontaneous osteogenesis in fetal bone marrow-derived MSCs [12,46], whereas we did not observe this phenotype in proliferating fetal MSCs, even though FGF2 was used during the MSC expansion phase to enhance chondrogenic potential [47]. Fetal MSCs have previously been stimulated to undergo adipogenic and osteogenic differentiation [19,31]; however, variations in the differentiation potential of MSCs isolated from second-trimester fetal bone marrow, liver, lung, and spleen were observed [42]. This may suggest that the plasticity of fetal MSCs is, in part, determined by or dependent on the tissue of origin or the gestational age of the fetus.
We have shown here that fetal and adult MSCs have distinct differences in their ability to undergo chondrogenic differentiation. Fetal MSCs demonstrated negligible chondrogenic induction when treated with TGFβ3, a widely used stimulant to induce chondrogenesis in adult MSCs [3,4,22]. However, BMP2 caused significant up-regulation of chondrogenic marker genes type II collagen and aggrecan in fetal MSCs. The expression levels of these genes were similar, whether fetal MSCs were incubated with BMP2 alone or in combination with TGFβ3, and comparable to adult MSCs treated with either TGFβ3 or BMP2. Therefore, chondrogenesis in fetal MSCs is dependent on BMP2 induction, whereas in adult MSCs, either BMP2 or TGFβ3 can be used as the initiating stimulus. The use of BMP2 to enhance TGFβ3-stimulated chondrogenesis in adult MSCs has been previously reported [27,48]. Similarly, in a comparative study of chondrogenic potential, TGFβ3 was unable to stimulate chondrogenesis in MSCs isolated from adipose tissue, whereas the addition of BMP6 to TGFβ3 up-regulated the expression of the TGFβ receptor I gene in adipose tissue and thereby induced a chondrogenic phenotype [49].
Cartilage tissue engineering is a more complex process than chondrogenic induction, as it requires the differentiated cells to synthesize and organize an extracellular matrix that is rich in type II collagen and aggrecan. We show for the first time that human first-trimester fetal bone marrow-derived MSCs can be used to tissue engineer cartilage which is similar in biochemical content to constructs generated from adult MSCs. Chondrogenesis in fetal MSCs measured as up-regulation of specific genes was greatest when using BMP2 alone, with no additional benefit from TGFβ3, whereas in our three-dimensional tissue engineering model, the greatest cartilage formation was seen when using a combination of BMP2 and TGFβ3. This observation suggests that BMP2 alone is sufficient for the initiation of fetal MSC differentiation along the chondrogenic lineage, whereas TGFβ3 supplementation is required in addition to BMP2 for optimal synthesis of extracellular matrix by the fetal MSC-derived chondrocytes. This contrasts with adult MSCs for which either TGFβ3 or BMP2 alone can be used to up-regulate chondrogenic genes, but only TGFβ3 can produce tissue engineered cartilage. Similar to our findings with fetal MSCs, human ESCs stimulated with TGFβ1 failed to demonstrate cartilage formation [50]; however, when co-stimulated with TGFβ3 and BMP2, these cells engineered cartilage-like tissue with a ECM rich in aggrecan and type II collagen with evident lacunae [51]. This suggests that immature MSCs associated with cartilage formation during development may require a BMP-mediated initiation of chondrogenesis, while TGFβ3 is necessary for optimal synthesis and maintenance of cartilage ECM by both fetal and adult MSCs.
The distinct differences in the initiation of chondrogenesis between fetal and adult MSCs are reflected by differences in the TGFβ signaling pathway, which depends on ligand-induced activation of serine/threonine kinase receptors to modulate chondrogenic differentiation. TGFβ ligands bind to TGFBR2 and BMP ligands bind to BMPR2 to induce activation of type I receptors, which stimulate phosphorylation of SMAD2/3 and SMAD1/5/8, respectively. The heteromeric receptor complex then associates with SMAD4, which facilitates translocation to the nucleus in order to regulate the transcription of downstream genes that are involved in chondrogenesis (reviewed in [52,53]).
The TGFβ-specific SMAD3 was phosphorylated by TGFβ3 treatment in adult MSCs, while only negligible induction was observed in fetal MSCs. Furamatsu and colleagues showed that overexpression of SMAD3 resulted in up-regulation of type II collagen gene expression and induced proteoglycan synthesis, indicating its critical involvement in chondrogenesis. While SMAD2 is able to interact with SOX9, SMAD3 appears to dominate the regulation of SOX9 transcription and its association with the COL2A1 enhancer, which collectively encourages the onset of chondrogenesis [54,55]. This suggests that SMAD2 is not directly implicated in chondrogenesis and may explain why we did not observe any differences in SMAD2 phosphorylation between fetal and adult MSCs stimulated with TGFβ3. In addition, SMAD3-mediated TGFβ signals are important in ECM maintenance and preventing terminal differentiation of chondrocytes [56], which supports our hypothesis that TGFβ3 is essential for optimal synthesis and homeostasis of matrix molecules during chondrogenesis of fetal and adult MSCs.
Both fetal and adult MSCs induced strong phosphorylation of BMP-specific SMAD1 and SMAD5 with BMP2 treatment alone or in combination with TGFβ3. However, strikingly, we detected TGFβ3-mediated phosphorylation of SMAD1/5 in adult MSCs as well as a marginal level in fetal MSCs. TGFβ-induced signaling is predominantly activated through TGFBR1 and TGFBR2 to phosphorylate SMAD2/3. However, TGFβ has also been shown to activate SMAD1/5 phosphorylation, traditionally activated by BMP signals in numerous cell types [57]. By signaling through TGFBR1, activin A receptor type II-like 1 (ALK1) is recruited and in combination with TGFBR2, it activates SMAD1/5 phosphorylation [58,59]. This dual signaling results in the formation of mixed-receptor SMAD complexes, which may bind to BMP promoters and influence BMP-mediated transcriptional responses [60]. The expression of TGFBR2 and up-regulation of BMPR2 in adult MSCs stimulated with TGFβ3 indicates that TGFβ-mediated SMAD1/5 phosphorylation may signal through a receptor complex, incorporating TGFBR2-BMPR2 receptors to mediate the initiation of chondrogenesis. Hellingman et al. (2011) showed that phosphorylation of both SMAD2/3 and SMAD1/5/8 is essential at the onset of chondrogenic differentiation and these SMADs remain in an active state in differentiated MSCs, while only SMAD2/3 is present in native articular cartilage [61]. These data indicate that the initiation of chondrogenic differentiation of MSCs may be mediated by either TGFβ or BMP, but can only be maintained through signaling which is associated with SMAD3. This may explain the need for TGFβ3 in addition to BMP2 for optimal cartilage tissue engineering by fetal MSCs, even though BMP2 alone is sufficient for the initiation of chondrogenic differentiation.
Successful cartilage repair strategies rely on the production of a stable tissue engineered construct that will not undergo hypertrophy or mineralize and be replaced by bone when implanted in vivo. Fetal MSCs co-stimulated with both TGFβ3 and BMP2 and adult MSCs stimulated with TGFβ3 alone show similar expression of hypertrophy markers with the exception of MMP13, which is more highly expressed in fetal MSCs. In line with our findings, BMP2 has been reported to promote cartilage formation in first-trimester fetal chondrocytes by enhancing type II collagen expression, while the expression of MMP13 was increased by TGFβ [62]. Inhibition of SMAD1/5/8 phosphorylation during chondrogenesis of MSCs in vitro has been shown to repress expression of type X collagen and MMP13 and to prevent terminal differentiation, whereas inhibition of SMAD2/3 had no effect on MMP13 expression [61]. In addition, Blaney Davidson et al. (2009) show that MMP13 expression is up-regulated through activin receptor-like kinase 1 and SMAD1/5/8 phosphorylation [63]. These studies suggest that the increase in MMP13 expression in fetal MSCs could be mediated by TGFβ3 through the SMAD1/5/8 signaling pathway and may influence its transcriptional activation by a dosage-specific ligand response, as BMP2 initiates chondrogenesis in these cells. Fetal MSCs showed negligible expression of hypertrophic markers COL10A1, IHH and MMP13 in response to BMP2 alone. Similarly, BMP2 has been shown to have a minimal effect on the expression levels of these genes in chondrocytes [64]. This suggests that TGFβ3 may have a role in enhancing ECM synthesis as well as in influencing terminal differentiation in fetal MSCs.
Chondrogenic differentiation of MSCs stimulated with TGFβ has been shown to be associated with premature induction of terminal differentiation and increased expression of hypertrophy marker genes in vitro [65]. MSCs transfected with BMP2 induce up-regulation of hypertrophic markers in vitro [66] and initiate osteoblast differentiation and new bone synthesis in vivo [67]. Tissue engineered constructs produced by chondrocytes on BMP2-crosslinked scaffolds showed increased expression of hypertrophy genes, including MMP13 and type X collagen, although this was not followed by chondrocyte maturation and bone matrix formation after in vivo implantation. It is speculated that the in vitro culture period reduces the concentration of BMP2 within the scaffold and prevents subsequent osteogenic differentiation after implantation [68]. This may, in part, explain the hypertrophic phenotype of MSCs transfected with BMP2. The cartilage tissue engineering system detailed in this report relies solely on exogenous BMP2 stimulation during in vitro culture, which suggests that fetal MSCs may be able to avoid accelerated terminal maturation which is mediated by BMP2 compared with adult MSCs stimulated with TGFβ3 alone.
We have demonstrated that fetal MSCs are able to tissue engineer cartilage of comparable quality to adult MSCs and established the feasibility of their use in cartilage repair applications. Fetal MSCs have an enhanced expansion potential and proliferative propensity [12]; therefore, the quantity of cells that can be harvested from a single fetal tissue donation would generate a substantial stock of allogeneic cells for use in the treatment of many patients [69]. This provides the opportunity for developing a new therapeutic approach for the treatment of osteoarthritis and other diseases of cartilage. Furthermore, we have indicated that fetal and adult MSCs show distinct regulation of TGFβ/BMP signaling which is associated with the initiation of chondrogenesis. We propose that fetal MSCs may represent an intermediary cell type and a suitable model for differentiation studies to further our understanding of the signaling pathways and mechanisms associated with differentiation and cartilage development with implications for tissue engineering strategies.
Acknowledgments
This work was funded by a grant from the Rosetree Trust and the James Tudor Foundation. APH is funded, in part, by an endowed chair from Arthritis Research UK.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M. and Kon E. (2013). Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc 21:1717–1729 [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. (2005). Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 11:1198–1211 [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S. and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 4.Johnstone B, Hering TM, Caplan AI, Goldberg VM. and Yoo JU. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238:265–272 [DOI] [PubMed] [Google Scholar]
- 5.Kafienah W, Mistry S, Dickinson SC, Sims TJ, Learmonth I. and Hollander AP. (2007). Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum 56:177–187 [DOI] [PubMed] [Google Scholar]
- 6.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, et al. (2008). Clinical transplantation of a tissue-engineered airway. Lancet 372:2023–2030 [DOI] [PubMed] [Google Scholar]
- 7.Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, Crowley C, McLaren C, Fierens A, et al. (2012). Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 380:994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pevsner-Fischer M, Levin S. and Zipori D. (2011). The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev 7:560–568 [DOI] [PubMed] [Google Scholar]
- 9.Bernardo ME, Emons JA, Karperien M, Nauta AJ, Willemze R, Roelofs H, Romeo S, Marchini A, Rappold GA, et al. (2007). Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res 48:132–140 [DOI] [PubMed] [Google Scholar]
- 10.Fehrer C. and Lepperdinger G. (2005). Mesenchymal stem cell aging. Exp Gerontol 40:926–930 [DOI] [PubMed] [Google Scholar]
- 11.Arufe MC, De la Fuente A, Fuentes I, de Toro FJ. and Blanco FJ. (2010). Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem 111:834–845 [DOI] [PubMed] [Google Scholar]
- 12.Guillot PV, Gotherstrom C, Chan J, Kurata H. and Fisk NM. (2007). Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 25:646–654 [DOI] [PubMed] [Google Scholar]
- 13.Fong CY, Gauthaman K. and Bongso A. (2010). Teratomas from pluripotent stem cells: a clinical hurdle. J Cell Biochem 111:769–781 [DOI] [PubMed] [Google Scholar]
- 14.Blum B. and Benvenisty N. (2008). The tumorigenicity of human embryonic stem cells. Adv Cancer Res 100:133–158 [DOI] [PubMed] [Google Scholar]
- 15.Gotherstrom C, West A, Liden J, Uzunel M, Lahesmaa R. and Le Blanc K. (2005). Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica 90:1017–1026 [PubMed] [Google Scholar]
- 16.Guillot PV, De Bari C, Dell'Accio F, Kurata H, Polak J. and Fisk NM. (2008). Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation 76:946–957 [DOI] [PubMed] [Google Scholar]
- 17.Mirmalek-Sani SH, Tare RS, Morgan SM, Roach HI, Wilson DI, Hanley NA. and Oreffo RO. (2006). Characterization and multipotentiality of human fetal femur-derived cells: implications for skeletal tissue regeneration. Stem Cells 24:1042–1053 [DOI] [PubMed] [Google Scholar]
- 18.Gotherstrom C, Ringden O, Westgren M, Tammik C. and Le Blanc K. (2003). Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant 32:265–272 [DOI] [PubMed] [Google Scholar]
- 19.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I. and Fisk NM. (2001). Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98:2396–2402 [DOI] [PubMed] [Google Scholar]
- 20.Kolambkar YM, Peister A, Soker S, Atala A. and Guldberg RE. (2007). Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol 38:405–413 [DOI] [PubMed] [Google Scholar]
- 21.de Mara CS, Duarte AS, Sartori-Cintra AR, Luzo AC, Saad ST. and Coimbra IB. (2012). Chondrogenesis from umbilical cord blood cells stimulated with BMP-2 and BMP-6. Rheumatol Int 33:121–128 [DOI] [PubMed] [Google Scholar]
- 22.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO. and Pittenger MF. (1998). Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4:415–428 [DOI] [PubMed] [Google Scholar]
- 23.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM. and Wang EA. (1988). Novel regulators of bone formation: molecular clones and activities. Science 242:1528–1534 [DOI] [PubMed] [Google Scholar]
- 24.Wang EA, Rosen V, D'Alessandro JS, Bauduy M, Cordes P, Harada T, Israel DI, Hewick RM, Kerns KM, LaPan P, et al. (1990). Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A 87:2220–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duprez DM, Coltey M, Amthor H, Brickell PM. and Tickle C. (1996). Bone morphogenetic protein-2 (BMP-2) inhibits muscle development and promotes cartilage formation in chick limb bud cultures. Dev Biol 174:448–452 [DOI] [PubMed] [Google Scholar]
- 26.Lyons KM, Pelton RW. and Hogan BL. (1990). Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development 109:833–844 [DOI] [PubMed] [Google Scholar]
- 27.Sekiya I, Larson BL, Vuoristo JT, Reger RL. and Prockop DJ. (2005). Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res 320:269–276 [DOI] [PubMed] [Google Scholar]
- 28.Majumdar MK, Wang E. and Morris EA. (2001). BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol 189:275–284 [DOI] [PubMed] [Google Scholar]
- 29.Carlberg AL, Pucci B, Rallapalli R, Tuan RS. and Hall DJ. (2001). Efficient chondrogenic differentiation of mesenchymal cells in micromass culture by retroviral gene transfer of BMP-2. Differentiation 67:128–138 [DOI] [PubMed] [Google Scholar]
- 30.Schmitt B, Ringe J, Haupl T, Notter M, Manz R, Burmester GR, Sittinger M. and Kaps C. (2003). BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation 71:567–577 [DOI] [PubMed] [Google Scholar]
- 31.Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J. and Bunyaratvej A. (2004). Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun 320:914–919 [DOI] [PubMed] [Google Scholar]
- 32.Ronziere MC, Perrier E, Mallein-Gerin F. and Freyria AM. (2010). Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed Mater Eng 20:145–158 [DOI] [PubMed] [Google Scholar]
- 33.Sailor LZ, Hewick RM. and Morris EA. (1996). Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. J Orthop Res 14:937–945 [DOI] [PubMed] [Google Scholar]
- 34.Wozney JM. (1989). Bone morphogenetic proteins. Prog Growth Factor Res 1:267–280 [DOI] [PubMed] [Google Scholar]
- 35.Pizette S. and Niswander L. (2000). BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol 219:237–249 [DOI] [PubMed] [Google Scholar]
- 36.Oshin AO. and Stewart MC. (2007). The role of bone morphogenetic proteins in articular cartilage development, homeostasis and repair. Vet Comp Orthop Traumatol 20:151–158 [DOI] [PubMed] [Google Scholar]
- 37.Blunk T, Sieminski AL, Gooch KJ, Courter DL, Hollander AP, Nahir AM, Langer R, Vunjak-Novakovic G. and Freed LE. (2002). Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng 8:73–84 [DOI] [PubMed] [Google Scholar]
- 38.Dickinson SC, Sims TJ, Pittarello L, Soranzo C, Pavesio A. and Hollander AP. (2005). Quantitative outcome measures of cartilage repair in patients treated by tissue engineering. Tissue Eng 11:277–287 [DOI] [PubMed] [Google Scholar]
- 39.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C. and Poole AR. (1994). Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest 93:1722–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handley CJ. and Buttle DJ. (1995). Assay of proteoglycan degradation. Methods Enzymol 248:47–58 [DOI] [PubMed] [Google Scholar]
- 41.Kafienah W, Jakob M, Demarteau O, Frazer A, Barker MD, Martin I. and Hollander AP. (2002). Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng 8:817–826 [DOI] [PubMed] [Google Scholar]
- 42.in ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH. and Fibbe WE. (2003). Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88:845–852 [PubMed] [Google Scholar]
- 43.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr., Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC. and Zago MA. (2008). Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 36:642–654 [DOI] [PubMed] [Google Scholar]
- 44.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313 [DOI] [PubMed] [Google Scholar]
- 45.Maijenburg MW, Kleijer M, Vermeul K, Mul EP, van Alphen FP, van der Schoot CE. and Voermans C. (2012). The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica 97:179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillot PV, Abass O, Bassett JH, Shefelbine SJ, Bou-Gharios G, Chan J, Kurata H, Williams GR, Polak J. and Fisk NM. (2008). Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood 111:1717–1725 [DOI] [PubMed] [Google Scholar]
- 47.Solchaga LA, Penick K, Goldberg VM, Caplan AI. and Welter JF. (2010). Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A 16:1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen B, Wei A, Tao H, Diwan AD. and Ma DD. (2009). BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A 15:1311–1320 [DOI] [PubMed] [Google Scholar]
- 49.Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F. and Richter W. (2007). Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol 211:682–691 [DOI] [PubMed] [Google Scholar]
- 50.Ng KK, Thatte HS. and Spector M. (2011). Chondrogenic differentiation of adult mesenchymal stem cells and embryonic cells in collagen scaffolds. J Biomed Mater Res A 99:275–282 [DOI] [PubMed] [Google Scholar]
- 51.Bai HY, Chen GA, Mao GH, Song TR. and Wang YX. (2010). Three step derivation of cartilage like tissue from human embryonic stem cells by 2D-3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. J Biomed Mater Res A 94:539–546 [DOI] [PubMed] [Google Scholar]
- 52.Massague J, Seoane J. and Wotton D. (2005). Smad transcription factors. Genes Dev 19:2783–2810 [DOI] [PubMed] [Google Scholar]
- 53.Itoh S, Itoh F, Goumans MJ. and Ten Dijke P. (2000). Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem 267:6954–6967 [DOI] [PubMed] [Google Scholar]
- 54.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y. and Asahara H. (2005). Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem 280:8343–8350 [DOI] [PubMed] [Google Scholar]
- 55.Furumatsu T, Tsuda M, Yoshida K, Taniguchi N, Ito T, Hashimoto M. and Asahara H. (2005). Sox9 and p300 cooperatively regulate chromatin-mediated transcription. J Biol Chem 280:35203–35208 [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Chen L, Xu X, Li C, Huang C. and Deng CX. (2001). TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol 153:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daly AC, Randall RA. and Hill CS. (2008). Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol 28:6889–6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P. and ten Dijke P. (2002). Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S. and ten Dijke P. (2003). Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell 12:817–828 [DOI] [PubMed] [Google Scholar]
- 60.Gronroos E, Kingston IJ, Ramachandran A, Randall RA, Vizan P. and Hill CS. (2012). Transforming growth factor beta inhibits bone morphogenetic protein-induced transcription through novel phosphorylated Smad1/5-Smad3 complexes. Mol Cell Biol 32:2904–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hellingman CA, Davidson EN, Koevoet W, Vitters EL, van den Berg WB, van Osch GJ. and van der Kraan PM. (2011). Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A 17:1157–1167 [DOI] [PubMed] [Google Scholar]
- 62.Johansson N, Saarialho-Kere U, Airola K, Herva R, Nissinen L, Westermarck J, Vuorio E, Heino J. and Kahari VM. (1997). Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn 208:387–397 [DOI] [PubMed] [Google Scholar]
- 63.Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, van den Berg WB. and van der Kraan PM. (2009). Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol 182:7937–7945 [DOI] [PubMed] [Google Scholar]
- 64.Chen-An P, Andreassen KV, Henriksen K, Karsdal MA. and Bay-Jensen AC. (2013). Investigation of chondrocyte hypertrophy and cartilage calcification in a full-depth articular cartilage explants model. Rheumatol Int 33:401–411 [DOI] [PubMed] [Google Scholar]
- 65.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T. and Richter W. (2006). Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54:3254–3266 [DOI] [PubMed] [Google Scholar]
- 66.Steinert AF, Proffen B, Kunz M, Hendrich C, Ghivizzani SC, Noth U, Rethwilm A, Eulert J. and Evans CH. (2009). Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis Res Ther 11:R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Partridge K, Yang X, Clarke NM, Okubo Y, Bessho K, Sebald W, Howdle SM, Shakesheff KM. and Oreffo RO. (2002). Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun 292:144–152 [DOI] [PubMed] [Google Scholar]
- 68.Jeong CG, Zhang H. and Hollister SJ. (2012). Three-dimensional polycaprolactone scaffold-conjugated bone morphogenetic protein-2 promotes cartilage regeneration from primary chondrocytes in vitro and in vivo without accelerated endochondral ossification. J Biomed Mater Res A 100:2088–2096 [DOI] [PubMed] [Google Scholar]
- 69.Applegate LA, Scaletta C, Hirt-Burri N, Raffoul W. and Pioletti D. (2009). Whole-cell bioprocessing of human fetal cells for tissue engineering of skin. Skin Pharmacol Physiol 22:63–73 [DOI] [PubMed] [Google Scholar]