Abstract
Human-related fertility preservation strategies have enormous potential for helping sustain and protect other species, especially to assist managing or ‘rescuing’ the genomes of genetically valuable individuals, including endangered species. However, wider-scale applications are limited by significant physiological variations among species, as well as a lack of fundamental knowledge of basic reproductive traits and cryosensitivity. Systematic and comparative cryopreservation studies (e.g. on membrane biophysical properties and resilience to freezing temperatures) are required to successfully recover gametes and gonadal tissues after thawing and eventually produce healthy offspring. Such data are currently available for humans and a few laboratory and livestock animals, with virtually all other species, including wildlife, having gone unstudied. Interestingly, there also are commonalities among taxa that allow a protocol developed for one species to provide useful information or guidance for another. However, when a rare animal unexpectedly dies there is no time for a prospective understanding of that species’ biophysical traits. Because the odds of success will be much lower in such instances, it is essential that more fundamental studies be directed at more species. But also worthwhile is thinking beyond these systematic characterisations to consider the potential of a ‘universal preservation protocol’ for animal biomaterials.
Additional keywords: biobanking, endangered species, gametes, gonadal tissues, long term storage, universal protocol
Introduction
There have been significant advances during the past decade in preserving the fertility of humans, particularly for cancer patients, in whom chemical or radiation therapies threaten future reproduction (Waimey et al. 2013). For adults, the usual approach is the pre-emptive cryopreservation of oocytes or spermatozoa before cancer treatment. For the prepubertal patient, most research efforts have been directed at freezing, storing and then post-thaw in vitro culture or grafting of gonadal tissues as a source of gametes (Waimey et al. 2013). Manipulation of the gonads is particularly exciting because both the ovary and testis have enormous supplies of untapped germplasm (largely in the form of immature gametes) that never contribute to reproduction. It is the potential of this vast, unused repository of premature oocytes and spermatozoa trapped in the gonads that is intriguing to a growing number of reproductive biologists (Comizzoli et al. 2010; Jewgenow et al. 2011). For preserving the fecundity potential of their cancer patients or for overcoming infertility issues (Waimey et al. 2013), this source of germplasm also has possibilities for other mammalian species. For example, the ability to culture and/or harvest oocytes and spermatozoa from the gonadal tissues of livestock and then incubate to maturity offers near-unlimited genetic material for improving reproductive efficiency and food security. For rare genotypes or species as well, the capacity to produce viable germplasm from gonadal cells would ensure the reproductive capacity of every genetically valuable individual (Lermen et al. 2009; Comizzoli et al. 2010), thereby contributing to the ultimate goal of achieving population sustainability. This is important for animals that have not yet produced sufficient numbers of descendants, including those that: (1) are living, but have failed to reproduce naturally (perhaps due to sexual incompatibility, a physical defect or lack of partners); (2) die unexpectedly; (3) are nearing reproductive senescence; or (4) have been long dead, but their rescued genes have value when reinfused into the contemporary population.
Although the goals of fertility preservation vary among the human biomedical, livestock production and wildlife management communities, the fundamental tools remain largely the same, and all three groups could benefit from the sharing of knowledge and techniques. Furthermore, there are common alignments; for example, the recognition that banks of frozen biomaterials are invaluable for preserving and enhancing reproductive potential and perpetuating an individual’s genome. All three of these scientific communities have long advocated the need to preserve biomaterials at cold temperatures (Wildt et al. 1997; Mazur et al. 2008; Lermen et al. 2009; Baker 2012). For wildlife species, these ‘genome resource banks’ have been essential for managing selected rare species, as well as characterising the uniqueness of certain taxa (Wildt et al. 1997; Lermen et al. 2009). And although most of the past attention for humans, farm animals and wildlife has been on spermatozoa, oocytes and embryos, activities now are increasingly shifting to the freeze storage of gonadal tissues (Lermen et al. 2009; Jewgenow et al. 2011).
Regardless of the source of the germplasm, the ability to use it to produce offspring remains rigidly linked to having thorough knowledge of the fundamental reproductive traits of the target species. Our research community argued for more species-specific research (interestingly, at the Annual Conference of the International Embryo Transfer Society) more than 25 years ago (Wildt et al. 1986) and have continued this advocacy to the present time (Wildt et al. 2010). What we actually fully understand about reproductive processes is very limited, as well as highly prejudiced to a few eutherian mammals (Wildt et al. 2010). And even among the 5500 existing mammal species, most knowledge about contemporary reproductive mechanisms is derived from a few laboratory and livestock species and humans (Wildt et al. 2003, 2010). However, when detailed studies are conducted to fully comprehend reproductive details about oestrous cyclicity, time of ovulation, sperm production or processing and methods for ensuring gamete interaction, then there are compelling examples of how fertility preservation can contribute to the recovery and management of endangered species. The black-footed ferret (Mustela nigripes; Howard and Wildt 2009) and giant panda (Ailuropoda melanoleuca; Huang et al. 2012) are two examples that illustrate the value of incorporating AI with fresh or frozen–thawed spermatozoa into captive breeding programs. This also represents fertility preservation that not only has contributed to species recovery, but also, in both cases, reintroductions of animals back into their native range. Although embryo technologies have resulted in a few significant milestone births (Saragusty and Arav 2011), IVF and embryo transfer (ET) have not been used for the routine reproductive management of any wildlife species for a variety of reasons, including the lack of basic physiological information (Wildt et al. 2010).
Therefore, there are thousands of species that could benefit from advances in human and livestock reproductive technologies, but are not because the basic knowledge needed to apply the tools does not exist. And although much of the information already available for the whole organism and cellular and molecular biology for humans and livestock is relevant (Comizzoli et al. 2010), much of it is not (Leibo and Songsasen 2002; Lermen et al. 2009). For example, challenges to preserving germplasm are due largely to marked variations in the structure and function of gametes across species, especially those elements that regulate tolerance to osmotic and toxic effects of cryoprotectants and resistance to chilling injuries (Gilmore et al. 1998; Critser et al. 2002; Woods et al. 2004).
This papers serves three purposes. The first is as a reminder that reproductive science and cryobiology have been largely directed to only a few species, and generally those that serve our direct self-interests (human health and a desire for children, food and companion animals). Second, it is important to emphasise that there is great value in studying animal species in a comparative fashion to: (1) discover new and interesting ways about how animals reproduce; and (2) determine those traits that allow preservation of the whole genome to ensure reproduction, species integrity and heterozygosity. Here we focus most of our discussion on gametes and gonadal tissue preservation because a recent review has highlighted variations in embryo freezing success among wild species (Saragusty and Arav 2011). Our third purpose is to look beyond current approaches and our pre-emptive reliance on fully understanding species specificities before being able to store and use biomaterials. Therefore, we speculate briefly on more universal approaches that potentially have broader-scale applicability to preserving fertility while circumventing the complex biological variations among species.
Examples in comparative seminal traits and cryosensitivity
Although semen is relatively simple to recover from many species, so much more is to be learned about taxon-inherent seminal traits and the sensitivity of spermatozoa to freeze–thawing. Although it appears obvious that small species usually produce minute ejaculate volumes (e.g. 10–50 μL for a black-footed ferret; Santymire et al. 2006) and gigantic animals produce prodigious volume (e.g. >100 mL for the African elephant Loxodonta africana; Kiso et al. 2011), it is well established that sperm concentration and total sperm output are unrelated to body mass (Comizzoli et al. 2012). For sperm processing, seminal plasma osmolarity and pH dictate the composition of the required seminal extenders as well as dilution processes to retain sperm structure and function during freezing, storage and thawing (Rossato et al. 2002). Although generally seminal fluid osmolarity remains slightly higher (350 mOsmol L−1) than that of conspecific serum (~300 mOsmol L−1; Comizzoli et al. 2012), there are notable exceptions; for example, the value in black-footed ferret semen can reach 790 mOsmol L−1 (Santymire et al. 2006). In contrast, and based on evaluation of hundreds of species, pH remains the least variable metric, generally remaining near neutral or only slightly alkaline (Comizzoli et al. 2012).
The initial quality of the recovered spermatozoa influences the subsequent ability of these cells to endure freezing and thawing stress. A useful example is the condition of teratospermia, the production of >40% malformed spermatozoa per ejaculate that is common to certain (but not all) species in the Felidae family (Pukazhenthi et al. 2002). These cells start out with a disadvantage by being challenged not only in form, but also in function, and can rarely withstand freeze–thawing or even cooling to 5°C (Pukazhenthi et al. 2002). Interestingly, the presence of pleiomorphisms does not always reflect sperm defects, as illustrated by the koala (Phascolarctos cinereus) ejaculate that routinely contains as many as 10 distinctive sperm morphotypes, all forms believed associated with normal functionality (Wildt et al. 1991; Johnston et al. 1997).
Sperm DNA fragmentation and damage, including in the context of cryopreservation, is a topic of growing interest, especially in better understanding the fertility of individuals. One recent comparative study across 11 mammalian species revealed that the stability of sperm DNA is related to the genomic design of basic proteins in the sperm head (Gosálvez et al. 2011). Specifically, the ratio between Protamine 1 and Protamine 2 is a determinant of sperm nuclear stability after cryopreservation. It is possibly more significant than the number of cysteine groups in Protamine 1, which is related directly to DNA stability during incubation. These findings are especially relevant to developing optimal cryopreservation methods for marsupial spermatozoa (Gosálvez et al. 2011).
Although it is relatively easy to define sperm structure, there are few data on membrane biophysical properties, even in common domestic and laboratory species. Yet this information is what allows understanding species-specific osmotic tolerances and permeability that ultimately allow formulating science-based protocols for cellular freezing and thawing (Leibo and Songsasen 2002; Gosálvez et al. 2011). In the absence of specific biophysical data, the approach for developing sperm cryomethods has been largely empirical; that is, adapting a satisfactory ‘standard’ protocol for the bull, ram, pig or horse (Leibo and Songsasen 2002) to the species of interest. In many cases, a single cryoprotectant can be widely applicable. For example, the use of glycerol has allowed the recovery of spermatozoa after thawing in diverse species and at similar volume-to-volume concentrations (4%–8%), ranging from various felid species (Crosier et al. 2006; Stoops et al. 2007) to marine mammals (Robeck and O’Brien 2004; Robeck et al. 2011) to the Asian elephant (Elephas maximus; Saragusty et al. 2009; Thongtip et al. 2009). More recently, spermatozoa from the Przewalski’s horse (Equus ferus przewalskii; Pukazhenthi et al. 2010), Baird’s tapir (Tapirus bairdii; Pukazhenthi et al. 2011) and Indian rhinoceros (Rhinoceros unicornis; Stoops et al. 2010) have been found to respond well to cryodilution and freezing protocols originally developed for the domestic horse (all members of Perissodactyla). In contrast are the wildebeest (Connochaetes taurunus) and greater kudu (Tragelaphus strepsiceros), which produce more sensitive spermatozoa that can only tolerate 2.0%–2.5% glycerol (Schiewe et al. 1991), generally only half that commonly used in livestock. At the other extreme are some marsupials (i.e. koala, wallaby, possum), where spermatozoa destined for freezing can survive in higher numbers in at least threefold more glycerol than used in more conventional mammals (Zee et al. 2008).
For the actual preservation process itself, spermatozoa from most mammalian species studied to date have been found to withstand slow freezing (−0.5°C min−1), producing at least some good motility recovery after thawing (Comizzoli et al. 2012). When comparisons are made across diverse species, the container element appears to be a critical factor in recovering viable spermatozoa after freezing (Comizzoli et al. 2012). Thus, when developing methods for a new species, the emphasis has usually been on the container approach; for example, the survivability of spermatozoa in plastic straws cooled over liquid nitrogen vapour versus pipetting pellets onto dry ice before immersion in liquid nitrogen. However, there always seem to be other usual, species-specific traits to consider. For example, the giant panda produces comparatively large-sized, round-headed spermatozoa that are amazingly cryoresistant, even surviving placement in ice water without extender or cryoprotectant (Spindler et al. 2004). This phenomenon is not common to the Ursidae family as a whole and is absent in same-sized spermatozoa of the Japanese black bear (Ursus thibetanus japonicus; Okano et al. 2006) or brown bear (Ursus arctos; Anel et al. 2010).
Examples in comparative oocyte traits and cryosensitivity
Mammalian oocytes are remarkably different from spermatozoa in their tolerance to cold exposure and protocol requirements to avoid injury from freezing (Songsasen and Comizzoli 2009). Because the volume of a round oocyte (generally ~120 μm in diameter) is larger than that of a spermatozoon, there is a smaller surface-to-volume ratio and a correspondingly higher vulnerability to chilling and intracellular ice formation (Songsasen and Comizzoli 2009). The naturally fragile cytoskeleton of ova also lessens their resistance to volumetric changes (Saragusty and Arav 2011). Adding to the overall challenge is the thick, protective and all-encompassing zona pellucida, as well as the mammalian oocyte’s plasma membrane, which has a low permeability coefficient that impedes movement of cryoprotectant and water (Songsasen and Comizzoli 2009). Interestingly, the oocytes of certain taxonomic groups, namely felids (Comizzoli et al. 2008) and bovids (McEvoy et al. 2000), or species (pig; Sturmey and Leese 2003) produce prodigious amounts of cytoplasmic lipid, thereby markedly increasing cryosensitivity.
In addition to their morphology, oocytes vary markedly among species in terms of their biophysical properties. For example, intraovarian oocytes of felids are more tolerant to cold temperatures and osmotic changes than counterparts from bovids, cervids or equids that are cold shock sensitive (Wolfe and Wildt 1996; Pope et al. 2006; Comizzoli et al. 2012). There are two other confounders of this variability. First, oocyte developmental stage can influence cryopotential. For example, immature oocytes at the germinal vesicle stage are more cold resistant than counterparts at the MII stage, because the former cells do not yet contain a temperature-sensitive meiotic spindle (Comizzoli et al. 2004, 2008; Songsasen and Comizzoli 2009). There also is exciting evidence of ‘markers’ of a given oocyte’s survivability to a freeze–thaw stress. Specifically, those oocytes with a higher level of cytoplasmic homogeneity or meeting a sufficient number of encompassing cumulus cells (Songsasen and Comizzoli 2009) appear to be better cryotargets with ultimately higher survivability. Although the number of these types of studies has grown, similar efforts with wildlife species to predict an oocyte’s tolerance to low temperatures has been limited due to cell unavailability (Leibo and Songsasen 2002; Saragusty and Arav 2011). In addition, despite significant advances in enhancing oocyte preservation in common species like the mouse, rhesus monkey and human, comparative and basic biophysical studies are still needed for laboratory and livestock animals (Songsasen et al. 2002; Critser et al. 2002; Songsasen and Comizzoli 2009). Likewise deserving of attention are alternative oocyte cryopreservation methods, including ultrarapid or vitrification protocols (on electron microscope grids and cryoloops; Saragusty and Arav 2011). Among the most non-traditional mammal models, successful vitrification of immature oocytes followed by IVF, ET and pregnancy has been reported recently in the domestic cat (Tharasanit et al. 2011).
Examples in comparative gonadal tissue cryopreservation
There is powerful potential in having a viable bank of testicular and ovarian tissues. Such repositories could be sources of early stage germ cells that could be grown in culture or the tissues themselves grafted to an appropriate host, both for the purposes of eventually producing mature cells useful for IVF. Such practices could change the way we reproductively manage species or populations. Gametes could be rescued from adult individuals that die unexpectedly or from prepubertal animals. Spermatozoa and ova could also be made available year round from seasonally breeding species. However, substantial basic research is required first, largely due to the complexity of gonadal tissue structure and cell heterogeneity, emphasising the similarities and differences among diverse species. This is no small feat given that there is almost no contemporary information on osmotic tolerance, toxicity and chilling sensitivity for gonadal tissue of any species (Jewgenow et al. 2011; Comizzoli et al. 2012).
Testicular pieces (0.5–1.0 mm3) have been frozen in cryovials using a programmable unit after equilibrating in glycerol (human; Hovatta et al. 1996) or dimethyl sulfoxide (DMSO; mouse, hamster and marmoset; Ehmcke and Schlatt 2008). These studies have been deemed successful based on favourable post-thaw histology or by measuring the resumption of gametogenesis after xenografting (Ehmcke and Schlatt 2008). Interestingly, there appears to be significant species variance in tissue cryosensitivity. For example, we have found that felid testicular tissue survives vitrification better than the same tissue treated the same way from laboratory rodents (based on both structural and functional assays; Comizzoli and Wildt 2012a). Further, it can be anticipated that there are innumerable variables that influence this differing sensitivity, including simple processing of the tissue. For example, factors that are important for preserving carnivore and ungulate testes include: (1) transport temperature of the freshly excised tissue to the laboratory; (2) the need for seminiferous tubule isolation using collagenase and hyaluronidase; and (3) a closed vitrification system (i.e. tissue sealed in a plastic straw to avoid contact with liquid nitrogen; or vitrification in a dry-shipper container; Comizzoli et Wildt 2012a).
Recognising the significance of having capacity to rescue oocytes from ovarian tissue, we have been exploring taxon- or species-specific protocols as well. Partnerships with zoological institutions have been essential for conducting these comparative studies. Zoos ship whole ovaries in a container maintained at 4°C to our laboratory, where the tissue is cut into 1–2 mm3 pieces, equilibrated in cryoprotectant and then preserved using diverse methods. From a management and ‘insurance’ perspective, we commonly preserve individual, small ovarian biopsies rather the whole gonad, which also allows storage at multiple sites. To date, findings have clearly demonstrated the value of vitrification (especially in 15% ethylene glycol + 15% DMSO + 0.5 M sucrose) over ‘slow freezing’ for preserving primordial follicles within the ovarian cortex of felids (adult and prepubertal) as well as several ungulate species (Comizzoli et al. 2010, 2012). This success has been based on measuring post-thaw structural integrity and viability in vitro. Most results have demonstrated that vitrification permits retaining approximately 75% of the original structure of these small pieces. Furthermore, approximately 50% of the original viability and normal proliferation index are retained, including from older and prepubertal donors (Comizzoli et al. 2010). Similar studies are ongoing in other laboratories, also with encouraging results (Jewgenow et al. 2011). For example, high survival of slow frozen–thawed ovarian tissue has been achieved in some felid or marsupial (wombat; Vombatus ursinus) species on the basis of cell integrity as well as grafting success (Paris et al. 2004; Cleary et al. 2004; Jewgenow et al. 2011). Our laboratory has also started to routinely preserve the female genome from species that are priorities in our conservation breeding programs, including the black-footed ferret, cheetah, clouded leopard (Neofelis nebulosa), Eld’s deer (Rucervus eldii), scimitar-horned oryx (Oryx dammah) and tufted deer (Elaphodus cephalophus).
Of course, one of the most significant challenges to preserving intragonadal, early stage germplasm is the ability to provoke these cells to achieve full maturation and fertilisation capacity in vitro. Yet the information required to achieve these milestones remains rudimentary at best, even for livestock and common laboratory species (Paris et al. 2004; Cleary et al. 2004; Songsasen et al. 2012). For example, it has been projected that it will be necessary to maintain living follicles in culture for up to 6 months to allow the recovery of viable oocytes in carnivores (Songsasen et al. 2012). Such approaches will require an array of pre-emptive comparative studies within and across species to determine the influence of a host of microenvironmental factors. Among others, these will include the need for external hormonal support and oxygen pressure, the capacity to allow the growing follicle to expand and the ability to eliminate wastes to ensure long-term follicle–oocyte viability (Songsasen et al. 2012).
Working towards new preservation options
Clearly, one of the greatest impediments to applying assisted reproduction and fertility preservation tools more widely is the amazing difference among species in terms of their fundamental biology (Critser et al. 2002; Comizzoli et al. 2010; Wildt et al. 2003, 2010). So, the question must be asked, will we always be encumbered with fully understanding the reproductive fundamentals of every individual species (including the cryointricacies of its cells) before its genome can be saved and later used to produce offspring? We suggest here that the qualified answer is ‘no’, that, on the contrary, there may well be technologies on the horizon that will permit more broad-scale application if not to all members of an animal clade (e.g. mammals), at least to a wide-ranging taxon or family. Achieving such a goal would be one of the most important accomplishments in reproductive biology, exponentially increasing our efficiency at preserving fertility and for producing more valuable offspring. Simultaneously, it would be possible to markedly reduce research costs by eliminating the need to characterise every intricate detail of the reproductive physiology of each species.
Three examples of such options are: (1) harmonised vitrification or ultrarapid freezing approaches; (2) isolated genome preservation; and (3) biostabilisation at room temperature. We continue to be enthusiastic about vitrification (or ultrarapid freezing) because of its comparative simplicity, low cost and ‘field friendliness’ (i.e. the ability to preserve cells in a commercial dry shipper, even in harsh, remote environments, without the need for an on-site source of liquid nitrogen). There have been significant advances in universal ultrafast cooling in the human biomedical field, especially for circulating tumour and other cancer cells that have been effectively preserved and thawed after using a microcapillary device constructed of highly conductive fused silica (Heo et al. 2011). Similarly, a standard vitrification protocol has been demonstrated to support post-thaw survival of coral fragments recovered from a wide-range of species (Hagedorn et al. 2012). Nonetheless, there are challenges. Although isolated cells and tissues can be successfully vitrified (and devitrification avoided during warming), there still are difficult-to-mitigate cryoinjuries to DNA, membranes and cell junctions (Yavin and Arav 2007). In terms of reproductive cells, there has also been noteworthy progress using directional freezing for preserving large volumes of semen from megavertebrates, including the Asian elephant (Saragusty et al. 2009), rhinoceros (Ceratotherium simum simum; Hermes et al. 2009) and several marine mammal species (Robeck et al. 2011). This approach is based on orienting extracellular ice crystals in one direction, thereby permitting more frozen cells to survive by aligning between the crystals to prevent damage. This same technology now is routinely used for preserving human gonadal tissues (Arav and Natan 2009).
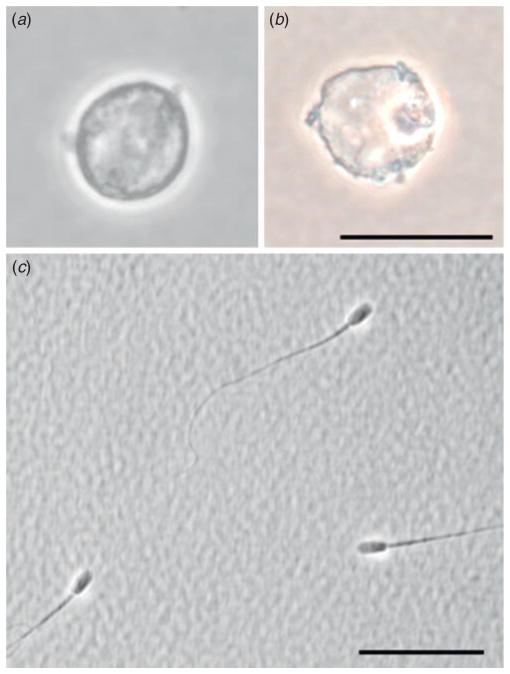
There are also exciting opportunities in preserving the isolated gamete genome via desiccation and storage at suprazero temperatures (Comizzoli and Wildt 2012a; Graves-Herring et al. 2013; Fig. 1). This concept is based on the anhydrobiosis phenomenon that occurs in nature (especially in tardigrades) that allows these organisms to survive remarkably stressful environmental conditions (Crowe et al. 1992). Our laboratory recently has developed a desiccation process for the successful preservation (and storage over several weeks) of the germinal vesicle isolated from the domestic cat oocyte followed by reanimation into a fresh conspecific oocyte (Graves-Herring et al. 2013). Using only the germinal vesicle offers the additional advantage of circumventing the usual challenges of successfully cryopreserving the whole ovum (Songsasen and Comizzoli 2009). These approaches also entail more simplistic sample processing and preservation protocols applicable to many species, all without the requirement for liquid nitrogen.
Fig. 1.
Germplasm preserved with simple approaches in the domestic cat. (a, b) Isolated germinal vesicles after desiccation, storage for 4 weeks and rehydration. (c) Sperm suspension after preservation at room temperature for 2 weeks. Scale bar = 20 μm.
A third area of potential involves biostabilising cells or entire tissues in a liquid environment to allow preservation at ambient or a suprazero temperature. The concept is inspired from natural phenomena, such as multimonth storage of spermatozoa in the female reproductive tract, as in bats (Holt 2011). Recently, we effectively preserved cooled (4°C) cat spermatozoa for up to 8 weeks in trehalose-based solutions (Fig. 1). Success was assessed as the ability to retain significant DNA integrity and centrosomal structure (presence of centrin) as well as gamete function in the form of sperm aster formation (Comizzoli and Wildt 2012a, 2012b). Encouraging results have also been reported for porcine oocytes that were treated in buffer solution, stored at ambient temperature for multiple days and then found to retain developmental competence on the basis of meiotic resumption (Yang et al. 2010). We suspect that these types of approaches could be harmonised to produce protocols that reduce the need to predetermine species-specific cryosensitivities.
Regardless of whatever technique is tested for its universality, it will remain essential to thoroughly verify the integrity of the DNA sequence and the many epigenetic factors that are influencing genome functionality. Certainly, other rapidly emerging tools, including next generation sequencing (as well as other ‘omics’), in association with bioinformatics will assist in providing the assurance that any new universal approach is preserving genomic integrity and functionality.
Conclusions and final thoughts
Fertility preservation strategies now used to ensure human reproductive health have significant application to the management of biomedical models, livestock species and conserving biodiversity, especially those animal species managed in ex situ collections that are used for ‘insurance’, research, public awareness and, on occasion, re-establishment or reinforcement of wild populations. Among the many approaches available, the most important is the capacity to preserve the genome and genetic diversity via long-term storage of whole (or parts of) the germplasm. With traditional cryopreservation technologies, the processing, storage and ultimate use of spermatozoa and oocytes is impeded at multiple levels, mostly by the simple lack of knowledge about inherent species specificities in reproductive physiology. For certain endangered animals, our laboratory and other groups have learned how to deal with and circumvent this obstacle by first conducting tedious, longitudinal, fundamental studies. This often includes comparative studies, adapting techniques and knowledge about a related species to the target. And although successes have occurred and fascinating new scholarly information has been secured, this approach is time consuming and costly and fails to provide quick assistance in cases of dire need. Thus, we see enormous benefits to looking beyond traditional cryoapproaches to explore methods that can offer more widespread applications without the mandate of knowing every reproductive detail. Based on preliminary evidence cited above, we believe there is substantial opportunity in preserving reproductive cells as well as tissues using novel, ‘more forgiving’ technologies, including genome preservation or membrane biostabilisation at ambient temperatures. Even when (1) more universal approaches become practical and (2) it becomes possible to routinely use immature gametes recovered from within the gonads, it will remain necessary to generate basic physiological data to successfully use the germplasm. Therefore, there will be a shift in the timing of the need for information, from the point of initial recovery, processing and storage to becoming highly efficient at (for example) properly preparing the female for AI or ET. Such information on male and female fertility periods, control of ovarian activities, appropriate deposition of germplasm (or embryos; Fig. 2), and monitoring of normal pregnancy is virtually non-existent for most wildlife species and remains a high priority research activity.
Fig. 2.
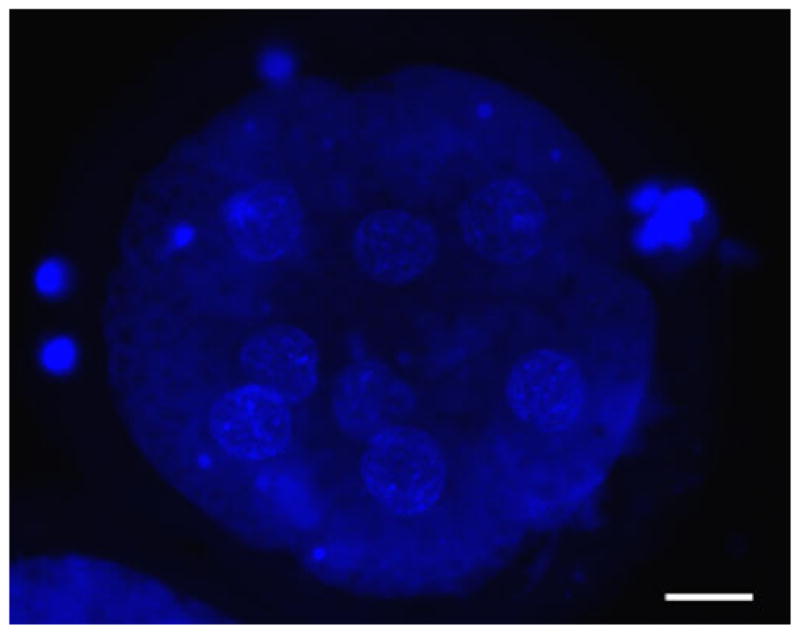
Early cat embryo (fixed and stained with Hoechst) obtained after fertilisation with spermatozoa preserved at room temperature for 2 weeks. Scale bar = 20 μm.
We have argued previously for the need to expand basic research, including into fertility preservation, far beyond mammals (Wildt et al. 2003, 2010; Comizzoli et al. 2012). There are enormous opportunities for studying the fundamental reproductive physiology and then applying the results to conserving mammals, as well as birds, amphibians, reptiles, fish and invertebrates. There is great satisfaction in generating new, often wondrous, data for previously unstudied species, which are then compounded more by applying the findings to ensuring that species populations are stabilised or even saved from extinction. One does not need to be trained as a traditional wildlife biologist; the basic skills and tools that all of us were taught during our training years with laboratory animals or livestock equally apply to tigers, whooping cranes and golden frogs. However, what is guaranteed to be different is the fascinating diversity in reproductive mechanisms that will be discovered. Certainly, major advances being made in human fertility preservation are directly applicable to the big challenges faced by those tasked with managing rare ex situ populations. One of the ultimate priorities, for humans as well as all animal types, becomes either customising or finding universal solutions for preserving biomaterials, with an emphasis on achieving post-storage viability while using practical (user-friendly) technologies that are also low cost. Because all stakeholders (from the biomedical, livestock and wildlife communities) could benefit from more interaction, there could be value in establishing a ‘fertility preservation network’; for example, to share information, tools and to even promote active collaborations and translational approaches. Finally, it is clear that the intersection between preserving fertility and the genome is linked inextricably to conventional cryobiology, or to whatever process eventually evolves that may be non-dependent on extreme low temperatures. But it is also obvious that many opportunities will be lost to exploit germ cells, tissue and DNA if we fail to initially store these biomaterials. Therefore, we close by urging colleagues to ‘save everything’, or at least those tissues and cells that may or may not be valuable now, but will be inevitably due to rapidly emerging stem cell and ‘omics’ technologies. These advances will have far-reaching implications in the practical management and regeneration of living populations.
Acknowledgments
Some of the authors’ studies described herein were funded by the National Center for Research Resources (R01 RR026064), a component of the National Institutes of Health (NIH), and are currently supported by the Office of Research Infrastructure Programs/Office of the Director (R01 OD 010948).
References
- Anel L, Gomes-Alves S, Alvarez M, Borragan S, Anel E, Nicolas M, Martinez-Pastor F, de Paz P. Effect of basic factors of extender composition on post-thawing quality of brown bear electro-ejaculated spermatozoa. Theriogenology. 2010;74:643–651. doi: 10.1016/J.THERIOGENOLOGY.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Arav A, Natan Y. Directional freezing: a solution to the methodological challenges to preserve large organs. Semin Reprod Med. 2009;27:438–442. doi: 10.1055/S-0029-1241052. [DOI] [PubMed] [Google Scholar]
- Baker M. Biorepositories: building better biobanks. Nature. 2012;486:141–146. doi: 10.1038/486141A. [DOI] [PubMed] [Google Scholar]
- Cleary M, Shaw JM, Jenkin G, Trounson AO. Influence of hormone environment and donor age on cryopreserved common wombat (Vombatus ursinus) ovarian tissue xenografted into nude mice. Reprod Fertil Dev. 2004;16:699–707. doi: 10.1071/RD04054. [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE. On the horizon for fertility preservation in domestic and wild carnivores. Reprod Domest Anim. 2012a;47(Suppl 6):261–265. doi: 10.1111/RDA.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE. Centrosomal functions and dysfunctions in cat spermatozoa. In: Schatten H, editor. The Centrosome. Humana Press; New York: 2012b. pp. 49–58. [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Effect of 1,2-propanediol versus 1,2-ethanediol on subsequent oocyte maturation, spindle integrity, fertilization and embryo development in vitro in the domestic cat. Biol Reprod. 2004;71:598–604. doi: 10.1095/BIOLREPROD.104.027920. [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Impact of anisosmotic conditions on structural and functional integrity of cumulus-oocyte complexes at the germinal vesicle stage in the domestic cat. Mol Reprod Dev. 2008;75:345–354. doi: 10.1002/MRD.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Songsasen N, Wildt DE. Protecting and extending fertility for females of wild and endangered mammals. Cancer Treat Res. 2010;156:87–100. doi: 10.1007/978-1-4419-6518-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Songsasen N, Hagedorn M, Wildt DE. Comparative cryobiological traits and requirements for gametes and gonadal tissues collected from wildlife species. Theriogenology. 2012;78:1666–1681. doi: 10.1016/J.THERIOGENOLOGY.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Critser JK, Agca Y, Woods E. Cryopreservation of immature and mature gametes. In: De Jonge CJ, Barratt CLR, editors. Assisted Reproductive Technology: Accomplishment and New Horizons. Cambridge University Press; Cambridge, UK: 2002. pp. 144–166. [Google Scholar]
- Crosier AE, Pukazhenthi BS, Henghali JN, Howard J, Dickman AJ, Marker L, Wildt DE. Cryopreservation of spermatozoa from wild-born Namibian cheetahs (Acinonyx jubatus) and influence of glycerol on cryosurvival. Cryobiology. 2006;52:169–181. doi: 10.1016/J.CRYOBIOL.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/ANNUREV.PH.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S. Animal models for fertility preservation in the male. Reproduction. 2008;136:717–723. doi: 10.1530/REP-08-0093. [DOI] [PubMed] [Google Scholar]
- Gilmore JA, McGann LE, Ashworth E, Acker JP, Raath JP, Bush M, Critser JK. Fundamental cryobiology of selected African mammalian spermatozoa and its role in biodiversity preservation through the development of genome resource banking. Anim Reprod Sci. 1998;53:277–297. doi: 10.1016/S0378-4320(98)00118-3. [DOI] [PubMed] [Google Scholar]
- Gosálvez J, López-Fernández C, Fernández JL, Gouraud A, Holt WV. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol Reprod Dev. 2011;78:951–961. doi: 10.1002/MRD.21394. [DOI] [PubMed] [Google Scholar]
- Graves-Herring JE, Wildt DE, Comizzoli P. Retention of structure and function of the cat germinal vesicle after air-drying and storage at suprazero temperature. Biol Reprod. 2013;88:139. doi: 10.1095/BIOLREPROD.113.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M, Carter V, Martorana K, Paresa MK, Acker J, Baums IB, Borneman EH, Brittsan M, Byers M, Henley M, Laterveer M, Leong JC, McCarthy M, Meyers S, Nelson BD, Petersen D, Tiersch T, Cuevas Uribe R, Wildt DE. Preserving and using germplasm and dissociated embryonic cells for conserving Caribbean and Pacific coral. PLoS One. 2012;7:e33354. doi: 10.1371/JOURNAL.PONE.0033354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YS, Nagrath S, Moore AL, Malinowska I, Zenali M, Kwiatkowski D, Toner M. Universal vitrification of rare cancer cells from different origins by ultra-fast cooling. Cryobiology. 2011;63:326. doi: 10.1016/J.CRYOBIOL.2011.09.076. [DOI] [Google Scholar]
- Hermes R, Göritz F, Saragusty J, Sós E, Molnar V, Reid CE, Schwarzenberger F, Hildebrandt TB. First successful artificial insemination with frozen-thawed semen in rhinoceros. Theriogenology. 2009;71:393–399. doi: 10.1016/J.THERIOGENOLOGY.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Holt WV. Mechanisms of sperm storage in the female reproductive tract: an interspecies comparison. Reprod Domest Anim. 2011;46(Suppl 2):68–74. doi: 10.1111/J.1439-0531.2011.01862.X. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Foudila T, Siegberg R, Johansson K, von Smitten K, Reima I. Pregnancy resulting from intracytoplasmic injection of spermatozoa from a frozen-thawed testicular biopsy specimen. Hum Reprod. 1996;11:2472–2473. doi: 10.1093/OXFORDJOURNALS.HUMREP.A019140. [DOI] [PubMed] [Google Scholar]
- Howard JG, Wildt DE. Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology. 2009;71:130–148. doi: 10.1016/J.THERIOGENOLOGY.2008.09.046. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhang H, Li D, Zhang G, Wei R, Huang Z, Zhou Y, Zhou Q, Liu Y, Wildt DE, Hull V. Relationship of the estrogen surge and multiple mates to cub paternity in the giant panda (Ailuropoda melanoleuca): implications for optimal timing of copulation or artificial insemination. Biol Reprod. 2012;87:112. doi: 10.1095/BIOLREPROD.112.102970. [DOI] [PubMed] [Google Scholar]
- Jewgenow K, Wiedemann C, Bertelsen MF, Ringleb J. Cryopreservation of mammalian ovaries and oocytes. Intl Zoo Yrbk. 2011;45:124–132. doi: 10.1111/J.1748-1090.2010.00124.X. [DOI] [Google Scholar]
- Johnston SD, O’Callaghan P, McGowan MR, Phillips NJ. Characteristics of koala (Phascolarctos cinereus adustus) semen collected by artificial vagina. J Reprod Fertil. 1997;109:319–323. doi: 10.1530/JRF.0.1090319. [DOI] [PubMed] [Google Scholar]
- Kiso WK, Brown JL, Siewerdt F, Schmitt DL, Olson D, Crichton EG, Pukazhenthi BS. Liquid semen storage in elephants (Elephas maximus and Loxodonta africana): species differences and storage optimization. J Androl. 2011;32:420–431. doi: 10.2164/JANDROL.110.011460. [DOI] [PubMed] [Google Scholar]
- Leibo SP, Songsasen N. Cryopreservation of gametes and embryos of nondomestic species. Theriogenology. 2002;57:303–326. doi: 10.1016/S0093-691X(01)00673-2. [DOI] [PubMed] [Google Scholar]
- Lermen D, Blömeke B, Browne R, Clarke A, Dyce PW, Fixemer T, Müller P, Fuhr GR, Holt WV, Jewgenow K, Lloyd RE, Lötters S, Paulus M, Reid GM, Rapoport DH, Rawson D, Ringleb J, Ryder OA, Spörl G, Schmitt T, Veith M, Mueller P. Cryobanking of viable biomaterials: implementation of new strategies for conservation purposes. Mol Ecol. 2009;18:1030–1033. doi: 10.1111/J.1365-294X.2008.04062.X. [DOI] [PubMed] [Google Scholar]
- Mazur P, Leibo SP, Seidel GE. Cryopreservation of the germplasm of animals used in biological and medical research: importance, impact, status, and future directions. Biol Reprod. 2008;78:2–12. doi: 10.1095/BIOLREPROD.107.064113. [DOI] [PubMed] [Google Scholar]
- McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil. 2000;118:163–170. [PubMed] [Google Scholar]
- Okano T, Nakamura S, Komatsu T, Murase T, Miyazawa K, Asano M, Tsubota T. Characteristics of frozen–thawed spermatozoa cryopreserved with different concentrations of glycerol in captive Japanese black bears (Ursus thibetanus japonicus) J Vet Med Sci. 2006;68:1101–1104. doi: 10.1292/JVMS.68.1101. [DOI] [PubMed] [Google Scholar]
- Paris MC, Snow M, Cox SL, Shaw JM. Xenotransplantation: a tool for reproductive biology and animal conservation? Theriogenology. 2004;61:277–291. doi: 10.1016/S0093-691X(03)00234-6. [DOI] [PubMed] [Google Scholar]
- Pope CE, Gómez MC, Dresser BL. In vitro production and transfer of cat embryos in the 21st century. Theriogenology. 2006;66:59–71. doi: 10.1016/J.THERIOGENOLOGY.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi B, Spindler R, Wildt D, Bush LM, Howard J. Osmotic properties of spermatozoa from felids producing different proportions of pleiomorphisms: influence of adding and removing cryoprotectant. Cryobiology. 2002;44:288–300. doi: 10.1016/S0011-2240(02)00035-4. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi BS, Lapiana F, Padilla L, Santiestevan J, Coutinho da Silva M, Alvarenga M, Wildt DE. Improved sperm cryopreservation in the critically endangered Przewalski’s horse (Equus ferus przewalskii) using different cryoprotectants. Biol Reprod. 2010;83:675. [Google Scholar]
- Pukazhenthi BS, Togna GD, Padilla L, Smith D, Sanchez C, Pelican K, Sanjur OI. Ejaculate traits and sperm cryopreservation in the endangered Baird’s tapir (Tapirus bairdii) J Androl. 2011;32:260–270. doi: 10.2164/JANDROL.110.011833. [DOI] [PubMed] [Google Scholar]
- Robeck TR, O’Brien JK. Effect of cryopreservation methods and precryopreservation storage on bottlenose dolphin (Tursiops truncatus) spermatozoa. Biol Reprod. 2004;70:1340–1348. doi: 10.1095/BIOLREPROD.103.025304. [DOI] [PubMed] [Google Scholar]
- Robeck TR, Gearhart SA, Steinman KJ, Katsumata E, Loureiro JD, O’Brien JK. In vitro sperm characterization and development of a sperm cryopreservation method using directional solidification in the killer whale (Orcinus orca) Theriogenology. 2011;76:267–279. doi: 10.1016/J.THERIOGENOLOGY.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Rossato M, Balercia G, Lucarelli G, Foresta C, Mantero F. Role of seminal osmolarity in the reduction of human sperm motility. Int J Androl. 2002;25:230–235. doi: 10.1046/J.1365-2605.2002.00353.X. [DOI] [PubMed] [Google Scholar]
- Santymire RM, Marinari PE, Kreeger JS, Wildt DE, Howard J. Sperm viability in the black-footed ferret (Mustela nigripes) is influenced by seminal and medium osmolality. Cryobiology. 2006;53:37–50. doi: 10.1016/J.CRYOBIOL.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141:1–19. doi: 10.1530/REP-10-0236. [DOI] [PubMed] [Google Scholar]
- Saragusty J, Hildebrandt TB, Behr B, Knieriem A, Kruse J, Hermes R. Successful cryopreservation of Asian elephant (Elephas maximus) spermatozoa. Anim Reprod Sci. 2009;115:255–266. doi: 10.1016/J.ANIREPROSCI.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Schiewe MC, Bush M, de Vos V, Wildt DE. Semen characteristics, sperm freezing and endocrine profiles in free-ranging wildebeest (Connochaetes taurinus) and greater kudu (Tragelaphus strepsiceros) J Zoo Wildl Med. 1991;22:58–72. [Google Scholar]
- Songsasen N, Comizzoli P. A historic overview of embryos and oocyte preservation in the world of mammalian in vitro fertilization and biotechnology. In: Borini A, Coticchio G, editors. Preservation of Human Oocytes. Informa Healthcare; London: 2009. pp. 1–11. [Google Scholar]
- Songsasen N, Ratterree MS, VandeVoort CA, Pegg DE, Leibo SP. Permeability characteristics and osmotic sensitivity of rhesus monkey (Macaca mulatta) oocytes. Hum Reprod. 2002;17:1875–1884. doi: 10.1093/HUMREP/17.7.1875. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Comizzoli P, Nagashima J, Fujihara M, Wildt DE. The domestic dog and cat as models for understanding the regulation of ovarian follicle development in vitro. Reprod Domest Anim. 2012;47(Suppl 6):13–18. doi: 10.1111/RDA.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler RE, Huang Y, Howard JG, Wang P, Zhang H, Zhang G, Wildt DE. Acrosomal integrity and capacitation are not influenced by sperm cryopreservation in the giant panda. Reproduction. 2004;127:547–556. doi: 10.1530/REP.1.00034. [DOI] [PubMed] [Google Scholar]
- Stoops MA, Bond JB, Bateman HL, Campbell MK, Levens GP, Bowsher TR, Ferrell ST, Swanson WF. Comparison of different sperm cryopreservation procedures on post-thaw quality and heterologous in vitro fertilisation success in the ocelot (Leopardus pardalis) Reprod Fertil Dev. 2007;19:685–694. doi: 10.1071/RD06078. [DOI] [PubMed] [Google Scholar]
- Stoops MA, Atkinson MW, Blumer ES, Campbell MK, Roth TL. Semen cryopreservation in the Indian rhinoceros (Rhinoceros unicornis) Theriogenology. 2010;73:1104–1115. doi: 10.1016/J.THERIOGENOLOGY.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Leese HJ. Energy metabolism in pig oocytes and early embryos. Reproduction. 2003;126:197–204. doi: 10.1530/REP.0.1260197. [DOI] [PubMed] [Google Scholar]
- Tharasanit T, Manee-In S, Buarpung S, Chatdarong K, Lohachit C, Techakumphu M. Successful pregnancy following transfer of feline embryos derived from vitrified immature cat oocytes using ‘stepwise’ cryoprotectant exposure technique. Theriogenology. 2011;76:1442–1449. doi: 10.1016/J.THERIOGENOLOGY.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Thongtip N, Mahasawangkul S, Thitaram C, Pongsopavijitr P, Korn-kaewrat K, Pinyopummin A, Angkawanish T, Jansittiwate S, Rungsri R, Boonprasert K, Wongkalasin W, Homkong P, Dejchaisri S, Wajjwalku W, Saikhun K. Successful artificial insemination in the Asian elephant (Elephas maximus) using chilled and frozen–thawed semen. Reprod Biol Endocrinol. 2009;7:75. doi: 10.1186/1477-7827-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waimey KE, Duncan FE, Su HI, Smith K, Wallach H, Jona K, Coutifaris C, Gracia CR, Shea LD, Brannigan RE, Chang J, Zelinski MB, Stouffer RL, Taylor RL, Woodruff TK. Future Directions in Oncofertility and Fertility Preservation: a report from the 2011 Oncofertility Consortium Conference. J Adolesc Young Adult Oncol. 2013;2:25–30. doi: 10.1089/JAYAO.2012.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt DE, Schiewe MC, Schmidt PM, Goodrowe KL, Howard JG, Phillips LG, O’Brien SJ, Bush M. Developing animal model systems for embryo technologies in rare and endangered wildlife. Theriogenology. 1986;25:33–51. doi: 10.1016/0093-691X(86)90182-2. [DOI] [Google Scholar]
- Wildt DE, Bush M, O’Brien SJ, Murray ND, Taylor A, Graves JA. Semen characteristics in free-living koalas (Phascolarctos cinereus) J Reprod Fertil. 1991;92:99–107. doi: 10.1530/JRF.0.0920099. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Rall WF, Critser JK, Monfort SL, Seal US. Genome resource banks: ‘living collections’ for biodiversity conservation. Bioscience. 1997;47:689–698. doi: 10.2307/1313209. [DOI] [Google Scholar]
- Wildt DE, Ellis SE, Janssen D, Buff J. Toward more effective reproductive science in conservation. In: Holt WV, Pickard A, Rodger JC, Wildt DE, editors. Reproductive Sciences and Integrated Conservation. Cambridge University Press; Cambridge: 2003. pp. 2–20. [Google Scholar]
- Wildt DE, Comizzoli P, Pukazhenthi B, Songsasen N. Lessons from biodiversity: the value of nontraditional species to advance reproductive science, conservation, and human health. Mol Reprod Dev. 2010;77:397–409. doi: 10.1002/MRD.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BA, Wildt DE. Development to blastocysts of domestic cat oocytes matured and fertilized in vitro after prolonged cold storage. J Reprod Fertil. 1996;106:135–141. doi: 10.1530/JRF.0.1060135. [DOI] [PubMed] [Google Scholar]
- Woods EJ, Benson JD, Agca Y, Critser JK. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004;48:146–156. doi: 10.1016/J.CRYOBIOL.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Yang CR, Miao DQ, Zhang QH, Guo L, Tong JS, Wei Y, Huang X, Hou Y, Schatten H, Liu Z, Sun QY. Short-term preservation of porcine oocytes in ambient temperature: novel approaches. PLoS One. 2010;5:e14242. doi: 10.1371/JOURNAL.PONE.0014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology. 2007;67:81–89. doi: 10.1016/J.THERIOGENOLOGY.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Zee YP, Holt WV, Gosalvez J, Allen CD, Nicolson V, Pyne M, Burridge M, Carrick FN, Johnston SD. Dimethylacetamide can be used as an alternative to glycerol for the successful cryopreservation of koala (Phascolarctos cinereus) spermatozoa. Reprod Fertil Dev. 2008;20:724–733. doi: 10.1071/RD08036. [DOI] [PubMed] [Google Scholar]