SUMMARY
Reproduction is quintessential to species survival. But what is underappreciated for this discipline is the wondrous array of reproductive mechanisms among species—variations as diverse as the morphology of the species themselves (more than 55,000 vertebrate and 1.1 million invertebrate types). We have investigated only a tiny fraction of these species in reproductive science. Besides the need to fill enormous gaps in a scholarly database, this knowledge has value for recovering and genetically managing rare species as well as addressing certain reproductive issues in humans. This article provides examples, first to advise against oversimplifying reproduction and then to show how such knowledge can have practical use for managing whole animals, populations, or even saving an entire species. We also address the expected challenges and opportunities that could lead to creative shifts in philosophy and effective actions to benefit more species as well as a future generation of reproductive scientists.
INTRODUCTION
Reproductive biology is interesting to scientists, and the public, because almost everyone is fascinated with the topic of sex. Beyond this fundamental appeal are the many practical consequences of our scientific work—the actual ability to “enhance” or “suppress” reproduction that, in turn, confers far-reaching benefits from improved food production and genetic management to combating infertility to better family planning.
Since the 4,000-year-old Kahun Papyrus described the use of vaginal pessaries made of crocodile dung and fermented milk for human contraception (David, 1996), thousands of articles have been written about reproduction in animals. The first contemporary reproductive data were largely descriptive of whole animal sexual behavior and cycles, easily recovered spermatozoa and forays into artifi-cial insemination and gamete cryopreservation (Senger, 2003; Leibo, 2004). Development of radio- and then enzyme immunoassays allowed describing the diversity of reproductive hormone patterns among species. Practical progress then followed in regulating reproduction, from improved contraception to synchronizing female reproductive cycles. Of particular significance was the emergence of an array of technologies from transferring an embryo from one animal to another to dazzling manipulations involving Petri dish culture, micro-injections, surgeries, and freezings (Bavister, 2002). These accomplishments consistently have enhanced reproductive ability and offspring production, largely in humans and food animal species, while clearly demonstrating the practical influence of our discipline. While sig-nificant studies continue in developmental biology, many scientists have moved on from studying the “whole” animal—and its organs, germplasm, and hormones—to focus within the cell, to organelles, cytoplasm, and the genes that regulate reproductive activity. This remarkable shift to the molecular is obvious from perusing any journal on reproductive science.
Although vast new insights have emerged about intracel-lular mechanisms, we seem to have lost an appreciation for studying the animal itself. In fact, many of our younger colleagues have no experience studying in vivo systems. This problem is accentuated by our discipline’s consistent reliance on a handful of “traditional” research species. Most modern scientific publications (and the attendant financial support) are devoted to humans and predominantly laboratory rodents and livestock, although the latter at apparently declining numbers (Ireland et al., 2008). The National Institutes of Health (NIH, 2008) has promoted and supported about 20 animal models to address human health-related issues, including various rodents, nonhuman primates, fishes (zebrafish and xiphophorus), the chicken and invertebrates (Caenorhabditis elegans, Drosophila, among others). However, the overwhelmingly popular research animal for human health-related studies and basic biological research is the ubiquitous laboratory mouse.
Let us consider studying other animals—of which there are more than 55,000 vertebrate (Fig. 1) and more than 1.1 million described invertebrate species (New World Encyclopedia, 2008). Amazingly, almost all of these have gone understudied, or completely ignored, in reproductive science. For the purposes of this article, we defined virtually every one of these species as “nontraditional” (excluding those animals that are commonly investigated in reproductive science, i.e., “traditional”; see Table 1 footnote for explicit definition). In 2003, we reported that only about 100 of the 5,500 mammal species on the planet (~2%) were “well-studied” in reproductive biology (Wildt et al., 2003). Based on objective criteria, there were about 90 wildlife (or other) species that seemed fairly well understood in our discipline. Since reproductive investigations tend to be mammal-centric, we predict that even less information is available for other taxa. In fact, for this article we evaluated the output of 10 well-known scientific journals specializing solely or mostly in the reproductive sciences (Table 1). Data were compiled by reviewing the table of contents (and, if necessary abstracts) and recording the species studied. From January 1999 through September 2009, we discovered that only 9.8% of the 12,118 published manuscripts surveyed involved other than the already well represented (Table 1). Over this almost 11-year interval, we encountered only 714 reproduction-oriented articles devoted to nontradi-tional mammals, 62 for birds, 41 for reptiles, 43 for amphibians, and 338 for fishes. By contrast, there were 5,380 published manuscripts for the common mouse (15.3%), rat (8.6%), and cow (20.5%), collectively these three species comprising 44.4% of the total.
Figure 1.
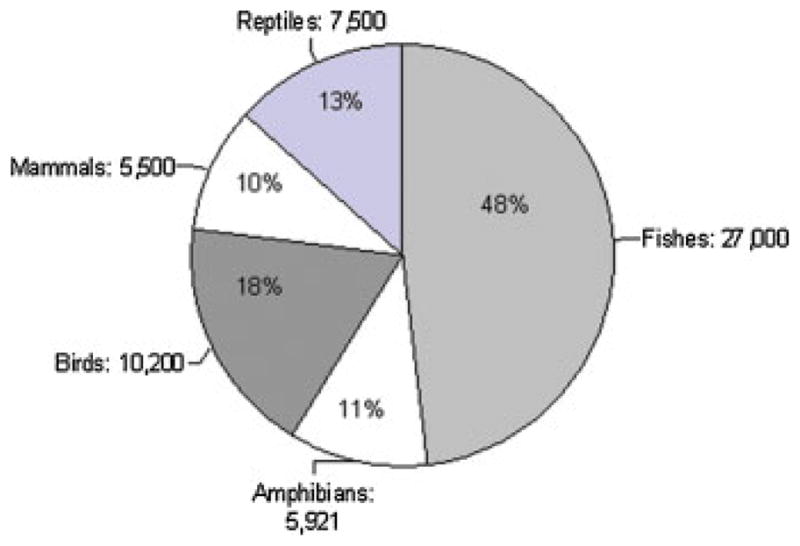
Numbers (and percentages) of described vertebrate species.
TABLE 1.
Number of Publications per Scientific Journals for Nontraditional Species* (January 1999 to September 2009)
| Journals | No. of total reproductive science manuscriptsa | No. of manuscripts for nontraditional species*
|
||||
|---|---|---|---|---|---|---|
| Mammals | Birds | Reptiles | Amphibians | Fishes | ||
| Animal Reproduction Sciences | 1,434 | 88 | 1 | 11 | 1 | 24 |
| Biology of Reproduction | 3,991 | 104 | 14 | 6 | 17 | 145 |
| Cryobiologyb | 197 | 10 | 1 | — | 3 | 44 |
| Journal of Mammalogyb | 36 | 32 | — | — | — | — |
| Molecular Reproduction and Development | 1,598 | 19 | — | 6 | 12 | 16 |
| Reproduction | 1,604 | 117 | 9 | 4 | 3 | 16 |
| Reproduction of Domestic Animals | 680 | 24 | 4 | — | — | 3 |
| Reproduction Fertility and Development | 650 | 121 | 7 | 1 | 5 | 2 |
| Theriogenology | 1,852 | 153 | 13 | 5 | 1 | 87 |
| Zoo Biologyb | 76 | 46 | 13 | 8 | 1 | 1 |
| Total | 12,118 | 714 | 62 | 41 | 43 | 338 |
| Percent of total publications | 5.9 | 0.5 | 0.3 | 0.3 | 2.8 | |
All vertebrate species with the exception of human, cow, pig, sheep, goat, horse, domestic buffalo, dog, cat, rabbit, mouse, rat, hamster, guinea pig, gerbil, macaque monkey, baboon, domestic chicken, turkey, zebrafish.
Exclude review manuscripts and conference proceedings.
Based on only manuscripts in reproductive sciences.
This is disturbing—and naïve. Such a narrow approach gives the impression that we as disciplinary experts thoroughly comprehend how animals reproduce. In reality—and in the absence of publications on Panamanian golden frogs, whooping cranes, desert tortoises, and clouded leopards—we are lulled into believing that reproduction is one-dimensional. Even in our own laboratory (where we have published articles on more than 50 species), we have experienced newly arriving graduate students moaning that “everything already is known in animal reproduction.” Nothing could be more wrong, as species (by definition) have evolved unique reproductive adaptations that need to be learned and appreciated.
This article is offered to remind budding, and established, reproductive biologists as well as journal editorial boards and funding agencies about the opportunities afforded by investigating nontraditional species—and in diverse ways from whole animals to molecular studies. We focus on three benefits: (1) new knowledge that documents the wondrous abundance of reproductive mechanisms among and within taxa; (2) applying basic data to help save and manage rare species; and (3) contributing in new ways to address human reproductive issues. As a discipline, we have proven we can overcome many human infertility cases, produce more calves from superior cows, and genetically engineer mice. But this is only the beginning. We believe the next triumphs could spring from studying nontraditional species.
AN AMAZING DIVERSITY IN REPRODUCTION
Mechanisms for reproduction are as diverse as animals are in physical appearance, genotype, or geographic origin. Table 2 is a reminder of the remarkable variations in general reproduction for species or broad taxa for a few phenomena related to sexual behavior, gametogenesis, and early embryogenesis. The take home message is that there are no rules in reproductive biology.
TABLE 2.
Marked Variations in Reproductive Mechanisms Among Diverse Species
| Taxon/species | Unique trait | Comment | Refs. |
|---|---|---|---|
| Giant panda | Mono-, short (24–72 hr) estrus per year | <1% of female’s annual lifespan devoted to reproduction | Steinman et al. (2006) |
| Albatross, penguin | Mate fidelity | Rejects suitors and remains with mate for 3–15 years | Jouventin et al. (1999) and Schreiber and Burger (2002) |
| Platypus | Mammal laying eggs | Multiple sex chromosomes (as in birds); soft shelled eggs (as in reptiles); milk secretion (as in mammals) | Daish and Grutzner (2009) |
| Fruit fly, ostracods | Super-giant sperm | Up to 6 cm in length | Pitnick and Markow (1994) |
| Nonhuman primates, mouse | Ejaculate retention in vagina | Sperm plug post-copulation to eliminate male competition | Dunham and Rudolf (2009) |
| Bats, reptiles, some fish, amphibians, birds | Sperm storage in female reproductive tract | Protracted storage for 6 months (bats) to 2 years (sharks) | Racey (1979) and Holt and Lloyd (2009) |
| Sea horse, pipe fish | Male support of fertilization and egg development | Only a few hundred sperm required for fertilization; fertilization and embryo development in a male brooding pouch | Van Look et al. (2007), Dzyuba et al. (2006, 2008), and Ripley (2009) |
| Whiptail lizard | Parthenogenesis | No males required to produce offspring (only females) | Lampert (2008) |
Some seemingly odd features can be inexplicable, such as a single, annual 24–72 hr long estrus in giant pandas or mega-sized sperm in fruit flies. In most cases, unique traits emerge because of natural selection, with idiosyncrasies accumulating that increase an individual’s chances for survival (fitness; Albert and Schluter, 2005). Alternatively, reproductive peculiarities evolve to allow a species to gain a reproductive (rather than survival) advantage. Examples include male-to-male combat or a female choosing one male over another (Anderson, 1994).
But means to reproduce also can differ significantly among species within a related family (Wildt et al., 1992, 1998, 2001). One illustrative example is the Canidae that is comprised of 36 species, one of which already has disappeared (Falkland Island wolf [Dusicyon australis]) with 9 others under threat of extinction (International Union for Conservation of Nature [IUCN], 2009). As a cluster, canids differ from most mammals in having rather unpredictable onsets of reproductive activity and a variable, but protracted proestrus and estrus (each 3–21 days). Full sexual receptivity coincides with elevated (but declining) circulating estrogen and rising progesterone followed by a ~2 month diestrus and then capricious intervals of complete reproductive shutdown (anestrus).
While descriptive of canids generically and the domestic dog specifically, species within the family are extraordinarily variant. Table 3 compares a few reproductive traits for the domestic dog to five other canids with diverse geographic origins. Only the bush dog is similar to the common dog in entering estrus anytime throughout the year. But the very presence of a male bush dog also can reduce the length of the quiet diestrual interval between the female’s cycles (DeMatteo et al., 2006). Most wild canids (whether living in nature or zoos) show strict seasonal reproductive patterns, although the onset of ovarian activity depends on the species or, in the case of the gray wolf, its latitudinal location (Mech, 2002; Table 3). The male of at least one of these species, the maned wolf, produces sperm in the ejaculate for only 6–8 weeks annually. Perhaps most intriguing is the influence of behavioral dominance on reproductive success in some canids. For pack species, such as the gray wolf (Packard et al., 1985) and African wild dog (Creel and Creel, 2002), reproduction is regulated by an alpha male and female within a cooperative, highly complex social organization. Subordinates are suppressed, in the case of African wild dogs by testosterone-fueled aggression by the top-tier male and hyper-estrogenism by the prevailing female. Yet, inferior level females cooperatively care for the alpha bitch’s young (Creel and Creel, 2002). For wild dogs, this probably is an obligate need since the female naturally super-ovulates and gives birth to as many as 20 puppies per litter, despite being only ~25 kg in body weight (Creel and Creel, 2002). Meanwhile, even ovulatory type is inconsistent across canids. At least two species, the maned wolf (Songsasen et al., 2006) and Island fox (Asa et al., 2007) appear to ovulate only in the presence of a male.
TABLE 3.
Marked Variations in Reproductive Mechanisms Among Species in the Canidae Family
| Species | Phenotype | Geographic range | Seasonality | Distinguishing reproductive features |
|---|---|---|---|---|
| Domestic dog (Canis familiaris) |

|
Ubiquitous | None | Spontaneous ovulation Breed-dependent litter size |
| Bush dog (Speothos venaticus) |

|
South America | None | Spontaneous ovulation Presence of a male reduces interestrous interval |
| Maned wolf (Chrysocyon brachyurus) |

|
South America | Strict (October to February) | Sperm production seasonal and short Ovulation only in the presence of a male |
| Gray wolf (Canis lupus) |

|
North America | Strict (January to April; but varies with latitude) | Spontaneous ovulation Behavioral dominance tightly regulates reproductive success to alpha female only Subordinates help raise young |
| African wild dog (Lycaon pictus) |

|
Africa | Strict (April to July) | Spontaneous ovulation Reproduction primarily in alpha female due to behavioral and estrogen suppression of subordinates Litter size up to 20 Subordinates help raise young |
| Island fox (Urocyon littoralis) |

|
North America | Strict (January to March) | Ovulation only in the presence of a male |
That one can easily identify at least one unique reproductive feature for each of six species within the same phylogenetic clade illustrates the vastness in reproductive strategies among animals—even relatives. Yet, the type of rudimentary information found in Table 3 has gone undiscovered for most animals. Newly found species-specific knowledge can begin to fill enormous gaps in our underde-veloped reproductive database, while offering practical ben-efits (as shown below).
APPLYING NEW KNOWLEDGE TO MANAGING AND RECOVERING RARE SPECIES
A by-product to nontraditional species studies can be new information useful to conservation breeding and population recovery. The need is substantial because biodiversity is under assault from habitat loss/degradation, overexploita-tion, pollution, disease, alien species invasions, and human overpopulation (IUCN, 2007). These seemingly overwhelming challenges, combined with media attention on the complexities (and controversies) of climate change, have left people wondering about the actual state of the planet.
A focus on species can help put the situation in a tangible perspective. For example, in our lifetimes we have witnessed the extermination of the baji river dolphin (Lipotes vexllifer, IUCN, 2009), dusky seaside sparrow (Ammodramus maritimus nigrescens) and, since 1980, more than 120 species of frogs (Stuart et al., 2004), all victims of human indifference, pollution, or disease. Direct killing of animals has caused species extinctions, most notably the extirpation of some gazelles and antelopes from nature, including the scimitar-horned oryx (Oryx dammah; Fig. 2). Thousands of species risk these same fates. There are objective data demonstrating that one in four mammals and one in eight birds are at high risk of extinction, and one out of every three amphibians and half of all tortoises and freshwater turtles are threatened (IUCN, 2009). When actual numbers are counted, many species are at perilously low numbers, for example, fewer than 500 total Philippine eagles (Pithecophaga jefferyi; Bueser et al., 2003), Iberian lynx (Lynx pardinus; von Arx and Breitenmoser-Wursten, 2008), Ethiopian wolves (Canis simensis; Sillero-Zubiri and Marino, 2008), addax (Addax nasomaculatus; Newby and Wacher, 2008), and Sumatran rhinoceroses (Dicerorhinos sumatrensis; van Strien et al., 2008)—far fewer than the legendary conservation icon, the giant panda (Ailuropoda melanoleuca, ~2,000 total individuals). It is now recognized that incomplete biological information on species is a major contributor to extinction vulnerability (Purvis et al., 2000).
Figure 2.

Scimitar-horned oryx driven to extinction in nature due to human hunting.
Historically, approaches for preserving biodiversity have centered on saving habitat and, by default, protecting species living in these native environments (in situ). However, the magnitude of the species crisis now means that all options deserve consideration, including intensive management in captive (ex situ) conditions that includes zoos, breeding centers, or fenced protected areas (many of which are “zoo-like”). Ex situ programs are complex and demand space, accessibility to rare animals, and specialized knowledge and human resources (Wildt et al., in press). But these options also offer incredible opportunities to conduct animal research under controlled conditions unavailable under harsh and unpredictable situations in nature.
There now are impressive models of how reproductive science (always in partnership with other life science disciplines) has contributed to recovering rare species (Table 4). Success has occurred by spurring on controlled, natural breeding or, in some cases, in combination with assisted reproductive technologies (ART). These programs are expensive and complicated (Wildt et al., in press). Scientists and animal managers work together to learn the best conditions (enclosure size, social system, lighting, diet, enrichment needs, disease prevention) to stimulate mating, establish a pregnancy, and promote offspring survival to the next breeding generation. If ART is to be integrated, then there is an exponential need for more basic knowledge, including on the fundamentals of reproductive anatomy, gametes, hormones, and often embryogenesis and cryo-preservation (Wildt et al., 2003, in press). Given the large physiological differences among species, ART as developed in humans and livestock provides only an elementary blueprint and little direct help in producing offspring in nontraditional animals (Wildt et al., 1992, 2001, 2003). And although the enormous potential of many modern ART approaches has been articulated (Pukazhenthi and Wildt, 2004; Pukazhenthi et al., 2006b; Durrant, 2009), in reality only artificial insemination (with fresh or thawed spermatozoa) has contributed to genetic management and species recovery (Pukazhenthi and Wildt, 2004; Howard and Wildt, 2009).
TABLE 4.
Examples Illustrating the Benefits of Reproductive Science for Species Recovery
| Species | Status and comments | Outcome |
|---|---|---|
| Natural reproduction | ||
| California condor (Gymnopyps californianus) | Critically endangered (IUCN, 2009). Only 23 individuals in the wild in 1982. Population plan, ex situ breeding program (with husbandry, reproductive behavior studies) begun in 1985 | Population growth by natural breeding to 362 condors. Reintroduction has contributed to 189 birds now living in nature |
| Golden lion tamarin (Leontopithecus rosalia) | Endangered (IUCN, 2009). All wild individuals in Brazilian reserves (<600 in 1977). Population plan, ex situ breeding program (with husbandry, reproductive behavior, nutrition studies) begun in 1981 | Wild population now comprised ~1,600 in nature and ~480 in captivity. Recovery program has contributed 147 animals for reintroduction |
| Iberian lynx (Lynx pardinus) | Critically endangered (IUCN, 2009). Approximately 200 individuals now in the wild. Population plan, ex situ breeding program established in 2003 after selective, periodic extractions of founders from nature | Studies of reproduction, behavior, genetics, and veterinary medicine are producing a biological database. Ex situ population has grown from 5 to 76 individuals (53% from ex situ breeding). Reintroductions scheduled for 2010 |
| Sumatran rhinoceros (Dicerorhinos sumatrensis) | Critically endangered (IUCN, 2009). Only ~275 animals in the wild and 11 animals living ex situ worldwide | First births (n =3) in captivity beginning in 2001 after intensive reproductive monitoring via ultrasound for timed matings and progesterone therapy to sustain pregnancy (Roth et al., 2001) |
| Natural reproduction with assisted reproductive technologies | ||
| Wyoming toad (Bufo baxteri) | Extinct in the wild (IUCN, 2009). Species recovery team formed in 1987 with breeding facility developed in 1993. Research into husbandry, reproduction, and captive-rearing | More than 50,000 tadpoles released into the wild. More than 2,000 tadpoles produced by IVF also released (Browne et al., 2006) |
| Whooping crane (Grus americana) | Endangered (IUCN, 2009). Only 15 birds remained in 1941, with ex situ recovery beginning in 1966. Three breeding centers and intensive husbandry/reproductive research | First chick produced via AI in 1975 and now a routine procedure. More than 350 free-living birds and 148 ex situ. One center contributed 96 birds produced by AI for reintroduction |
| Black-footed ferret (Mustela nigripes) | Endangered (IUCN, 2009). Only 18 animals remained in 1985. Ex situ recovery plan and collaboration among federal and state agencies and zoos. Husbandry, reproduction, genetics, and nutritional research | Integrated database for genetic management and breeding. More than 6,200 individuals derived from founders, including >140 kits by AI with fresh or thawed sperm. Reintroduction with 800 animals now in the wild |
| Koala (Phascolarctos cinereus) | Least concern (IUCN, 2009). But populations experiencing habitat fragmentation, genetic isolation, some disease. Ex situ collections used in reproductive research and public education | Ex situ genetic management program in place with >30 offspring produced by AI with fresh or chilled sperm (Allen et al., 2008) |
| Scimitar horned oryx (Oryx dammah) | Extinct in the wild (IUCN, 2009). About 10,000 oryx remain in ex situ collections globally. Husbandry, reproduction, health research, and public education | Coordinated research and management have allowed routine reproduction. Reintroduction of 74 oryx into range countries into fenced, naturalistic areas. Genetically valuable offspring (n =14) produced by AI with thawed sperm (Morrow et al., 2009) |
| Bottlenose dolphin (Tursiops truncatus) | Least concern (IUCN, 2009). Wild populations abundant. Ex situ management and ART to avoid wild extractions, support public education, and produce new knowledge | Ten calves produced by AI with fresh or thawed sperm, including use of sex sorting to favor production of females |
| Giant panda (Ailuropoda melanoleuca) | Endangered (IUCN, 2009). Fewer than 2,000 individuals. Static/declining ex situ populations until 1998. Extensive biomedical research in reproduction, behavior, nutrition, genetics, and health | Ex situ population more than doubled from ~120 to ~290 animals in 11 years. Cubs routinely produced by AI with fresh/thawed sperm |
Good basic reproductive information has been essential to genetically managing rare species. Unlike objectives associated with humans (overcoming infertility) or livestock (selecting for genetic combinations that increase milk production or growth rates), conservationists ensure persistence of as many different genes as possible. There is strong evidence for the need to maintain acceptable heterogeneity to maintain species integrity, an ability to adapt, and to avoid the insidious effects of inbreeding (Ballou and Lacy, 1995; Ryan et al., 2003; Taylor, 2003). The challenge for zoos (and in nature) is to resolve the complications associated with managing small populations, many of which are related to different reproductive mechanisms among species.
Overcoming this complexity is no small feat, so the number of successes has been modest. A few examples are highlighted in Table 4 with four salient points made here. First, it is amazing that some species (California condor, whooping crane, black-footed ferret) were permitted to decline to such low numbers (<25 individuals) before action was taken. Yet in each case and despite few genetic founders, at least some recovery has been possible. Secondly, progress occurred in all cases because of collaborations among people, institutions, and across scientific disciplines. The giant panda is a useful example as its dismal record of ex situ breeding was reversed due to intensive, coordinated research in six life science disciplines with a focus on reproduction (Wildt et al., 2006). Thirdly, the preferred way for animals to reproduce always is by natural breeding between genetically appropriate mates. However, when this approach is inadequate and reproductive knowledge is sufficient, then ART, especially artificial insemination (AI), has been a useful supplementary tool. Illustrations are offered in Table 4 as well as in various citations for the whooping crane (Canadian Wildlife Service and U.S. Fish & Wildlife Service, USFWS, 2005), black-footed ferret (Howard et al., 2003), koala (Allen et al., 2008), dolphin (Robeck et al., 2005) and giant panda (Howard et al., 2006). But only in the case of the whooping crane and black-footed ferret (Fig. 3) has ART helped return an endangered species to nature, which leads to our fourth point—the need for long-term commitment. For example, although more than 6,200 descendants have been produced over two decades from the original 18 surviving black-footed ferrets, there has been no respite. There continues to be an insatiable demand for more animals for reintroduction. And, there are growing threats, primarily disease epidemics in situ (Gober, 2009) and emerging morphological abnormalities, perhaps as a result of restricted heterozygosity (Bronson et al., 2007). Thus, applying fundamental information to species recovery requires scientific inquiry endurance, with species “safety” perhaps never being assured. The difficulty of such last ditch heroics alone justifies the need for more attention now to nontraditional species before population numbers decline (and often with it, gene diversity). Rather than crisis-based reactions, it would be far more prudent and economical to preemptively build comprehensive, scholarly databases. This would ensure readily available information that inevitably will be needed to better manage and conserve all animal resources in the future.
Figure 3.

Black-footed ferrets have been returned to the wild after natural breeding and the use of artificial insemination.
NEW KNOWLEDGE FROM NONTRADITIONAL SPECIES FOR ADDRESSING HUMAN HEALTH
The NIH invests at least $197.26 million annually in basic and applied research directed towards bio-resource development and animal models—genotypes or species that display a biological trait (normally or as a spontaneous or induced pathology or condition) that resembles the same phenomenon in humans or another animal (NIH, 2009). However, ~98% of all NIH awards underwrite rodent models (mostly mice) with declining support of domestic animal proposals (Ireland et al., 2008). Some grants are made to specialized species or taxa (NIH, 2008), but even reliance on nonhuman primates for new reproductive insights has decreased markedly since the 1970s (Hendrickx and Dukelow, 1995).
Ireland et al. (2008) recently have questioned the wisdom of relying on a single animal model and have strongly advocated for more cattle, pig, sheep, goat, poultry, horse, and aquatic species studies for biomedical research. But why stop there? Even these customarily “farmed” and widely investigated animals are not always the best alternative for what might be discovered in nontraditional domestic and wildlife species. For example, our laboratory has found highly conserved reproductive mechanisms between humans and felid species. We determined that certain cats serve important purposes for addressing: (1) teratospermia, the unusual production of malformed spermatozoa, also common in men; (2) infertility syndromes in women, including asynchronous oocyte cytoplasmic and nuclear maturation, ovarian hypersensitivity and luteal dysfunction after gonadotropin therapy; and (3) fertility preservation, such as in vitro maturation or cryopreservation of oocytes or ovarian tissue after intensive medical treatments, such as chemotherapy in girls (Comizzoli et al., in press). In a one-on-one comparison based on overall morphology and four oocyte elements significant to in vitro maturational capacity, the domestic cat was closest to the human (Table 5).
TABLE 5.
Comparative Morphology and In Vitro Maturational Biology of Immature Oocytes in Five Mammalian Species
| Mouse | Cow | Pig | Cat | Human | |
|---|---|---|---|---|---|
| Morphology of an immature oocytea |
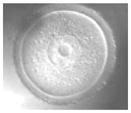
|
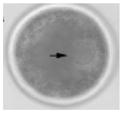
|
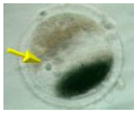
|
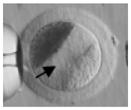
|
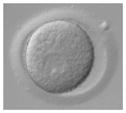
|
| Oocyte diameter (μm, excluding zona pellucida) | 80 | 110 | 125 | 110 | 110 |
| Germinal vesicle diameter (μm) | 30 | 35 | 35 | 45 | 45 |
| Nuclear configuration | Large nucleolus; clumped chromatin | Small nucleolus; clumped chromatin | Small nucleolus; filamentous chromatin | Small nucleolus; fibrillar chromatin | Small nucleolus; fibrillar chromatin |
| Meiosis achieved in vitro (hr) | <24 | ~24 | ~44 | ~24 | ~24 |
| Reference | Liu and Aoki (2002) | Lodde et al. (2007) | Sun et al. (2004) | Comizzoli et al. (2008) | Combelles et al. (2002) |
Pig and cat oocytes were centrifuged to polarize cytoplasmic droplets to visualize the germinal vesicle (arrow).
The point here is less about campaigning for more NIH support for cat research and more about demonstrating that a different species may well be a better animal model than almost-always-used traditional counterparts. An open-minded, comparative approach that delves into the details of alternative models is bound to provide the best scientific perspective and the greatest opportunities to improve human reproductive health. A far-from-inclusive list of how certain wildlife species or broad taxa could contribute to this need is offered in Table 6.
TABLE 6.
Potential Value of Nontraditional Wild Animals as Models for Human Biomedical Reproduction
| Interest | Refs. | |
|---|---|---|
| Prairie vole (Microtus ochrogaster) | Sexual monogamy and pair bonding | Wang and Aragona (2004) |
| Mole rat (Heterocephalus glaber) | Stress-related infertility in a social group | Burland et al. (2004) |
| Little brown bat (Myotis lucifugus) | Menopause and reproductive senescence | Austad (2009) |
| Cheetah (Acinonyx jubatus), clouded leopard (Neofelis nebulosa) | Significance and etiology of teratospermia; influence of stress sensitivity on reproductive success | Pukazhenthi et al. (2006a) and Wielebnowski et al. (2002a,b) |
| African elephant (Loxodonta africana), Asian elephant (Elephas maximus) | Uterine pathologies in aging females; stress-related infertility in a social group; impact of obesity on reproductive function | Aupperle et al. (2008), Freeman et al. (2009), and Clubb et al. (2008) |
| Giant panda (Ailuropoda melanoleuca), roe deer (Capreolus capreolus), mink (Mustela vison) | Capacity to suspend embryo development (diapause) (new insights into embryo preservation at physiological temperatures) | Lopes et al. (2004) |
| Reptiles, fish, amphibians, birds, bats | Protracted sperm storage in the female tract (new insights into somatic cell and gamete preservation) | Holt and Lloyd (2009) |
| Killifish | Producing embryos that can survive desiccation (new insights into embryo preservation) | Tingaud-Sequeira et al. (2009) |
| Amphibians and birds | Species highly sensitive to environmental perturbations, especially endocrine disruptors, environmental toxins, and pollutants | Touart (2004) and Bernanke and Kohler (2009) |
| Caenorrhabditis elegans, Drosophilia sp., Oikopleura sp. | Well-described invertebrates for fertilization, developmental biology, and organogenesis | Koop and Holland (2008) and Leung et al. (2008) |
| White-tail deer (Odocoileus virginianus) (other overly abundant species) | Approaches for broad-scale contraceptive delivery | Miller et al. (2008) |
OPPORTUNITIES AND NEXT STEPS
For all the above reasons, we believe that there is great opportunity for modern reproductive science to extend its mission to more studies of the world’s biodiversity. Expanding our scope in this way has enormous potential for new biological insights, for protecting natural resources and even for advancing human health. We also suspect that studies of nontraditional animals will excite and perhaps result in more financial support for our discipline. The tax-paying public—and our institutional and government overseers—are looking for practical examples of how science is relevant to daily life and personal interests. The average person has little understanding (or interest) in the complexities of science but certainly recognizes the importance of sex and reproduction for ensuring species survival. The images of newborns that we scientists help create evoke potent responses and verify that most people indeed are “biophilic,” having an innate emotional attachment to other living things (Wilson, 1992). This also explains why the public cares intensely about species at risk of extinction (Czech et al., 2001) and animal welfare issues (Blokhuis et al., 2003). However, rarely do we rationalize the value of our scientific discipline for protecting, recovering, or improving the status or well-being of species. Yet, as we have illustrated here, there are models of success, and the need to save species will (unfortunately) continue to be a growth industry. Imagine how reproductive science would be perceived if it was seen as the major contributing discipline for ensuring the vitality and survival of biological diversity.
We are not suggesting that reproductive practitioners be re-branded as conservation biologists. However, there are opportunities to look creatively beyond the laboratory mouse to other species to understand reproductive mechanisms that have both scholarly and applied merit. What might be some of the next steps? We offer the following four recommendations as a start:
-
Learn more about what species already are (a) available as research models or (b) identified as high priorities for reproductive science: The NIH recently has begun developing a web-based project “Linking Animal Models to Human Diseases” to assist scientists in identifying best, alternative species for investigating diverse phenomena (National Center for Research Resources, 2009). Many of these substitutes simply can be acquired to explore a new perspective in one’s current area of interest. Often such species are common, readily obtained and can be sustained under standard NIH Animal Care and Use Guidelines.
Accessing wildlife models is more complicated, but possible by investigating and becoming involved in three networks. One is accredited zoos that have become a key force in supporting species conservation globally (contributing millions of dollars annually). Major zoos also employ in-house scientific staff who independently (and with others) study species living ex situ or in situ. As rare animals are no longer extracted from nature for exhibition, a primary purpose of zoos is creating and caring for self-sustaining populations under genetic management arrangements (called Species Survival Plans in the North America) administered through the Association of Zoos and Aquariums (AZA, 2009). These taxon-based, cooperative programs are staffed with volunteer research advisors and committees that coordinate basic and applied research. An analogous network is available in Europe (European Endangered Species Program of the European Association of Zoos and Aquariums [EAZA, 2009]). These working groups are always under-resourced, especially with experienced scientists, a gap that can be filled by academic colleagues. Because zoos want to be involved in science-based successes (e.g., Table 4), there is incentive for these organizations to form partnerships and to provide access to unique specimens for study. More information on how to become involved in the North American and European zoo networks is available (AZA, 2009; EAZA, 2009, respectively).
Scientists also become linked to wildlife programs through the IUCN, the oldest and largest global environmental network that boasts 11,000 volunteer scientists from >160 countries (IUCN, 2009). Within the IUCN, is the Species Survival Commission, an association of 7,500 volunteer experts. The Commission provides scientific advice and information on biodiversity conservation and the value and functioning of species to governments, nongovernmental organizations and local communities—anyone needing assistance in preventing animal (or plant) extinctions. This is largely accomplished through networking within 100 different taxon specialist groups and task forces that develop conservation action plans, always containing species priorities and, often, first-order research needs (see IUCN web-site, about Species Survival Commission specialist groups).
Another option worth examining is the formal government-managed species conservation and recovery programs. For example, the USFWS (2009), a bureau of the Department of Interior, has recognized >1,100 species globally as threatened or endangered. There are 467 animal species covered by recovery plans (mammals, birds, reptiles, amphibians, fishes, snails, clams, crustaceans, insects, arachnids, and coral) (USFWS, 2009). Although some of these plans are outdated or in development, most have scientific inquiry needs, some in reproductive science. The USFWS has limited within-bureau research capacity and consistently relies on extramural partnerships. Examples have included the California condor, whooping crane, and black-footed ferret (highlighted in Table 4), among others. Publicly accessible versions of recovery plans are available at http://ecos.fws.gov/tess_public/TESSWebpageRecovery?sort=1.
Thus, for those interested in contributing to wildlife reproductive science, there are resources to checkout, and an urgent need. We are in the midst of a species crisis. For example, frogs worldwide are dying from an epidemic of chytrid fungus (Batrachochytrium dendrobatidis) that is causing massive extinctions (Skerratt et al., 2007; Kouba and Vance, 2009). Wild frogs are being rescued into ex situ collections, but generally with no information on fundamental reproduction or breeding management. Bats likewise are being exterminated, by the novel fungus Geomyces destruc-tans that causes nearly 100% mortality (Blehert et al., 2009). Like for amphibians, until this disease is mitigated, the only recourse for some bats is to establish captive security populations, but with no information on reproduction or, in most cases, even how to keep these species alive. Such pressing challenges extend to cryptic creatures as well. For example, the saola or Vu Quang ox (Pseudoryx nghetinhensis) is a bovid with an antelope-type lineage that was unknown to the scientific world until 1992 (Fig. 4). This species may still exist in disappearing forest pockets of Vietnam and Laos, but only 11 animals have ever been recorded alive (William Robichaud, personal communication). As a last preventative against extinction, an ongoing field search hopes to recover a few founder specimens for ex situ recovery that, of course, must be linked to quickly learning its reproductive biology (Comizzoli, unpublished observations). This and many species also may benefit from ART and cryopreservation of genomic material. Such tools have been postulated, for instance, to be helpful for conserving the ocean’s coral—badly damaged and being lost due to climate change and pollution (Harvel et al., 2002; Graham et al., 2006). But envision the sheer complexities of investigating these never-before-studied taxa—from sperm that are released only one night per year (during a full moon) to unique gamete cryo-sensitivities from species to species (Hagedorn et al., 2006).
Conduct a “Species Summit”: An ability to seriously shift to broader scale species studies in reproductive science could benefit from group discussions and endorsements that, in turn, could lead to action, including incentive funding. A formal workshop that builds agreement and a policy document on the value of nontraditional animals to scholarly knowledge, conservation, and human health would be particularly useful. Perhaps the topic should be addressed in the context of all of the life sciences with subsequent spin-off discussions and focused actions for disciplines where dividends will be high (i.e., reproductive science for one). A stakeholder-based, facilitated workshop comprised of experts and decision-makers is a proven way to identify priorities and advance topics by consensus. Natural, potential host and participant organizations could include the Society for the Study of Reproduction, NIH, National Science Foundation, USFWS, and AZA. However, given the importance of nontraditional species to food production/security, environmental/climate change, and natural resource protection/conservation, we also would expect participatory interest by the U.S. Department of Agriculture, the Environmental Protection Agency, the National Oceanic and Atmospheric Administration, and the U.S. Geological Survey, among others. Outcomes would be disseminated through our disciplinary and institutional websites, at major conferences via presentations, within the editorial meetings of our scientific journals and even through public service announcements. Among the hoped for effects, would be more expansive thinking and conceivably more innovative funding for diverse species. At the least would emerge a greater appreciation for the scope of potential impact of reproductive science and perhaps a passionate leadership group to drive a network devoted to advocacy and research action.
Encourage nontraditional species articles in our scientific journals: As reflected in Table 1, our reproductive journals have a poor record of publishing manuscripts on other than common species. Clearly, part of this predicament is related to the comparatively few studies conducted in nontraditional species. Nonetheless, as evident from author guidelines, journals tend to prefer submissions on cutting edge mechanisms (especially molecular) while bluntly discouraging “descriptive” studies. Ironically, the latter type information (e.g., hormonal patterns, sperm traits, seasonality, among many others) is exactly what is needed most to fill valuable life history gaps for species and “whole” animals. Therefore, with the given that highest scientific quality must be retained, it would be helpful for journals to give serious consideration to topics without species, animal, organ, cellular, or molecular bias. Further support would be encouraging by submission of articles on whole animals that, in the end, must be one of the key beneficiaries of our work.
Ensure that we (and especially our students) have an appreciation for species and whole animal studies: It is imperative that we preserve the art of studying the complete animal, not just its “parts.” Optimally, every trainee should have the opportunity to conduct high-quality, humane in vivo investigations (such experience is mandatory for fellows joining our laboratory). At the very least, every biology student should be made aware of what is required to perform living animal studies through lectures, literature reviews, and journal clubs.
Figure 4.

Saola, one of the world’s most endangered ungulates.
Finally, every biologist needs to keep in mind the planet’s wondrous, uninvestigated biodiversity and how working with whole animals (populations or entire species) is both fulfilling and fun. It is what motivated us, as co-authors, to become involved in scientific discovery. The early articles of Roger Short and John Hanks on free-living elephants, Gerald Lincoln on roe deer, Rodney Meade on western spotted skunks, Marilyn Renfree on tammar wallabys, M.C. Chang on ferrets, W. Richard Dukelow on squirrel monkeys, and Bill Breed on wild hopping mice were among the unpretentious, but elegant publications that inspired us to become reproductive biologists. Let us strive to carry on the legacy of these pioneers through the study of diverse species.
Acknowledgments
The authors thank JoGayle Howard and Janine Brown for helpful comments, Megan Brown and Kristen Donohue for assisting with surveying the paucity of publications in reproductive science on nontraditional species, and Kimberly Terrell and Rebecca Hobbs for sharing insights into diverse reproductive mechanisms among taxa. Photograph credit goes to: Table 3: David Kersey (domestic dog), Adriano Gambarini (bush dog), Jessica Kordell (maned wolf), Micaela Szykman Gunther (African wild dog), Tim Coonan (island fox); Table 5: Francoise Urner (mouse and human oocytes), Alberto Luciano (bovine oocyte), Chung Hsing (porcine oocyte); Figure 2: Jessie Cohen; Figure 3: Mehgan Murphy; Figure 4: William Robichaud.
Abbreviations
- ART
assisted reproductive technologies
- AZA
Association of Zoos and Aquariums
- EAZA
European Association of Zoos and Aquariums
- IUCN
International Union for Conservation of Nature
- NIH
National Institutes of Health
- USFWS
U.S. Fish & Wildlife Service
References
- Albert AYK, Schluter D. Selection and the origin of species. Curr Biol. 2005;15:R283–R288. doi: 10.1016/j.cub.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Allen CD, Burridge M, Mulhall S, Chafer ML, Nicolson VN, Pyne M, Zee YP, Jago SC, Lundie-Jenkins G, Holt WV, Carrick FN, Curlew JD, Lisle AT, Johnston SD. Successful artificial insemination in the koala (Phascolarctos cinereus) using extended and extended-chilled semen collected by electrojaculation. Biol Reprod. 2008;78:661–666. doi: 10.1095/biolreprod.107.064824. [DOI] [PubMed] [Google Scholar]
- Anderson M. Sexual selection. Princeton: Princeton University Press; 1994. p. 624. [Google Scholar]
- Asa CS, Bauman JE, Coonan TJ, Gray MM. Evidence for induced estrus or ovulation in a canid, the island fox (Urocyon littoralis) J Mammal. 2007;88:436–440. [Google Scholar]
- Aupperle H, Reischauer A, Bach F, Hildebrandt T, Goritz F, Jager K, Scheller R, Klaue HJ, Schoon HA. Chronic endometritis in an Asian elephant (Elephas maximus) J Zoo Wildl Med. 2008;39:107–110. doi: 10.1638/2006-0045.1. [DOI] [PubMed] [Google Scholar]
- Austad SN. Comparative biology of aging. J Gerontol A Biol Sci Med Sci. 2009;64:199–201. doi: 10.1093/gerona/gln060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZA. 2009 http://www.aza.org/species-survival-plan-program. Downloaded on September 9, 2009.
- Ballou J, Lacy RC. Identifying genetically important individuals for management and genetic diversity in captive populations. In: Ballou JD, Gilpin M, Foose T, editors. Population management for survival and recovery. Cambridge: Cambridge University Press; 1995. pp. 76–111. [Google Scholar]
- Bavister BD. Early history of in vitro fertilization. Biol Reprod. 2002;124:181–196. doi: 10.1530/rep.0.1240181. [DOI] [PubMed] [Google Scholar]
- Bernanke J, Kohler HR. The impact of environmental chemicals on wildlife vertebrates. Rev Environ Contam Toxicol. 2009;198:1–47. doi: 10.1007/978-0-387-09647-6_1. [DOI] [PubMed] [Google Scholar]
- Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, Coleman JTH, Darling SR, Gargas A, Nivern R, Okoniewski JC, Rudd RJ, Stone WB. Bat white-nose syndrome: An emerging fungal pathogen? Science. 2009;323:227. doi: 10.1126/science.1163874. [DOI] [PubMed] [Google Scholar]
- Blokhuis HJ, Jones RB, Geers R, Miele M, Veissier I. Measuring and monitoring animal welfare: Transparency in the food product quality chain. Anim Welf. 2003;12:445–455. [Google Scholar]
- Bronson E, Bush M, Viner T, Murray S, Wisely S, Deem SL. Mortality of captive black-footed ferrets (Mustela nigripes) at the Smithsonian’s National Zoological Park, 1989–2004. J Zoo Wildl Med. 2007;38:169–176. doi: 10.1638/1042-7260(2007)038[0169:MOCBFM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Browne RK, Seratt J, Vance C, Kouba A. Hormonal priming, induction of ovulation and in vitro fertilization of the endangered Wyoming toad (Bufo baxteri) Biol Reprod Endocrinol. 2006;4:34. doi: 10.1186/1477-7827-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueser GLL, Bueser KG, Afan DS, Salvador DI, Grier JW, Kennedy RS, Miranda HC. Distribution and nesting density of Philippine eagles (Pithecophaga jefferyi) on Mindanao Island, Philippines: What do we know after 100 years? Ibis. 2003;145:130–135. [Google Scholar]
- Burland TM, Bennett NC, Jarvis JU, Faulkes CG. Colony structure and parentage in wild colonies of co-operatively breeding Damaraland mole-rats suggest incest avoidance alone may not maintain reproductive skew. Mol Ecol. 2004;13:2371–2379. doi: 10.1111/j.1365-294X.2004.02233.x. [DOI] [PubMed] [Google Scholar]
- Canadian Wildlife Service and U.S. Fish & Wildlife Service. International recovery plan for the whooping crane. Ottawa: Recovery of Nationally Endangered Wildlife. (RENEW); Albu-querque: U.S. Fish & Wildlife Service; 2005. p. 162. [Google Scholar]
- Clubb R, Rowcliffe M, Lee P, Mar KU, Moss C, Mason GJ. Compromised survivorship in zoo elephants. Science. 2008;322:1649. doi: 10.1126/science.1164298. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in vitro matured human oocytes. Hum Reprod. 2002;17:1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Impact of anisomotic conditions on structural and functional integrity of cumulus-oocyte complexes at the germinal vesicle stage in the domestic cat. Mol Reprod Dev. 2008;75:345–354. doi: 10.1002/mrd.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Songsasen N, Wildt DE. Protecting and extending fertility options for females of wild and endangered species. In: Woodruff TK, Zoloth L, Campo-Engelstein L, Rodri-guez S, editors. Oncofertility: Reflections from the humanities and social sciences. New York: Springer Press; (in press) [Google Scholar]
- Creel S, Creel NM. The African wild dog: Behavior, ecology and conservation. Princeton: Princeton University Press; 2002. p. 341. [Google Scholar]
- Czech B, Devers PK, Krausman PR. The relationship of gender to species conservation attitudes. Wildl Soc Bull. 2001;29:187–194. [Google Scholar]
- Daish T, Grutzner F. Location, location, location! Monotremes provide unique insights into the evolution of sex chromosome silencing in mammals. DNA Cell Biol. 2009;28:91–100. doi: 10.1089/dna.2008.0818. [DOI] [PubMed] [Google Scholar]
- David AR. The pyramid builders of ancient Egypt: A modern investigation of the Pharoah’s workforce. London: Routledge; 1996. p. 264. [Google Scholar]
- DeMatteo KE, Porton IJ, Kleiman DG, Asa CS. The effect of the male bush dog (Speothos venaticus) on the female reproductive cycle. J Mammal. 2006;87:723–732. [Google Scholar]
- Dunham AE, Rudolf VH. Evolution of sexual size monomorphism: The influence of passive mate guarding. J Evol Biol. 2009;22:1376–1386. doi: 10.1111/j.1420-9101.2009.01768.x. [DOI] [PubMed] [Google Scholar]
- Durrant BS. The importance and potential of artificial insemination in CANDES (companion animals, non-domestics, endangered species) Theriogenology. 2009;71:113–122. doi: 10.1016/j.theriogenology.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Dzyuba BB, Van Look KJW, Cliffe A, Koldewey HJ, Holt WV. Effect of parental age and associated size on fecundity, growth and survival in the yellow seahorse, Hippocampus kuda. J Exp Biol. 2006;209:3055–3061. doi: 10.1242/jeb.02336. [DOI] [PubMed] [Google Scholar]
- Dzyuba BB, Van Look KJW, Kholodnyy VS, Satake N, Cheung S, Holt WV. Variable sperm size and motility activation in the pipefish, Syngnathus abaster: Adaptations to paternal care or environmental plasticity? Reprod Fertil Dev. 2008;20:474–482. doi: 10.1071/rd07221. [DOI] [PubMed] [Google Scholar]
- EAZA. 2009 www.eaza.net. Downloaded on September 9, 2009.
- Freeman EW, Guagnano G, Olson D, Keele M, Brown JL. Social factors influence ovarian acyclicity in captive African elephants (Loxodonta africana) Zoo Biol. 2009;28:1–15. doi: 10.1002/zoo.20187. [DOI] [PubMed] [Google Scholar]
- Gober P. Endang Sp Bull. 2009. Spring. A challenging future for the black-footed ferret; pp. 38–39. [Google Scholar]
- Graham NA, Wilson SK, Jennings S, Polunin NV, Bijoux JP, Robinson J. Dynamic fragility of oceanic coral reef ecosystems. Proc Natl Acad Sci USA. 2006;103:8425–8429. doi: 10.1073/pnas.0600693103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M, Carter VL, Stevn RA, Krupp D, Leong JC, Lang RP, Tiersch TR. Preliminary studies of sperm cryopreservation in the mushroom coral, Fungia scutaria. Cryobiology. 2006;52:454–458. doi: 10.1016/j.cryobiol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Harvel CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hendrickx AG, Dukelow WR. Reproductive biology. In: Taylor BT, Abee CR, Henrickson R, editors. Nonhuman primates in biomedical research. Amsterdam: Elsevier, Inc; 1995. pp. 147–191. [Google Scholar]
- Holt WV, Lloyd RE. Sperm storage in the vertebrate female reproductive tract: How does it work so well? Theriogenology. 2009 doi: 10.1016/j.theriogenology.2009.07.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Howard JG, Wildt DE. Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology. 2009;71:130–148. doi: 10.1016/j.theriogenology.2008.09.046. [DOI] [PubMed] [Google Scholar]
- Howard JG, Marinari PE, Wildt DE. Black-footed ferret: Model for assisted reproductive technologies contributing to in situ conservation. In: Holt WV, Pickard A, Rodger JC, Wildt DE, editors. Reproductive sciences and integrated conservation. Cambridge: Cambridge University Press; 2003. pp. 249–266. [Google Scholar]
- Howard JG, Zhang Z, Li D, Huang Y, Zhang M, Hou R, Ye Z, Li G, Zhang J, Huang S, Zhang H, Zhang J, Spindler RE, Wildt DE. Male reproductive biology in giant pandas. In: Wildt DE, Zhang A, Zhang H, Janssen DL, Ellis S, editors. Giant pandas: Biology, veterinary medicine and management. Cambridge: Cambridge University Press; 2006. pp. 159–197. [Google Scholar]
- Ireland JJ, Roberts RM, Palmer GH, Bauman DE, Bazer FW. A commentary on domestic animals as dual-purpose models that benefit agricultural and biomedical research. J Anim Sci. 2008;86:2797–2805. doi: 10.2527/jas.2008-1088. [DOI] [PubMed] [Google Scholar]
- International Union for Conservation of Nature (IUCN) Facts. 2007 doi: 10.1098/rsta.2023.0053. http://www.iucn.org/media/materials/fact_sheets/?324/Species-extinction. Downloaded on August 17, 2009. [DOI] [PubMed]
- International Union for Conservation of Nature (IUCN) IUCN Red List of Threatened Species. 2009 www.iucn.org/. Downloaded on September 6, 2009. www.iucnredlist.org. Downloaded on August 17, 2009SSC Specialist Groups www.iucn.org/work/programmes/species/about_SSC/specialist_groups/. Downloaded on September 6, 2009.
- Jouventin P, Lequette B, Dobson FS. Age-related mate choice in the wandering albatross. Anim Behav. 1999;57:1099–1106. doi: 10.1006/anbe.1999.1083. [DOI] [PubMed] [Google Scholar]
- Koop D, Holland LZ. The basal chordate amphioxus as a simple model for elucidating developmental mechanisms in vertebrates. Birth Defects Res C Embryo Today. 2008;84:175–187. doi: 10.1002/bdrc.20128. [DOI] [PubMed] [Google Scholar]
- Kouba A, Vance CK. Applied reproductive technologies and genetic resource banking for amphibian conservation. Reprod Fertil Dev. 2009;21:719–737. doi: 10.1071/RD09038. [DOI] [PubMed] [Google Scholar]
- Lampert KP. Facultative parthenogenesis in vertebrates: Reproductive error or chance? Sex Dev. 2008;2:290–301. doi: 10.1159/000195678. [DOI] [PubMed] [Google Scholar]
- Leibo S. The early history of gamete cryobiology. In: Fuller BJ, Lane N, Benson EE, editors. Life in the frozen state. New York: CRC Press; 2004. pp. 347–370. [Google Scholar]
- Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Aoki E. Transcriptional activity associated with meiotic competence in fully grown mouse GV oocytes. Zygote. 2002;10:327–332. doi: 10.1017/s0967199402004069. [DOI] [PubMed] [Google Scholar]
- Lodde V, Modina S, Galbusera C, Franciosi F, Luciano AM. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: Interplay with gap junction functionality and developmental competence. Mol Reprod Dev. 2007;74:740–749. doi: 10.1002/mrd.20639. [DOI] [PubMed] [Google Scholar]
- Lopes FL, Desmarais JA, Murphy BD. Embryonic diapause and its regulation. Reproduction. 2004;128:669–678. doi: 10.1530/rep.1.00444. [DOI] [PubMed] [Google Scholar]
- Mech LD. Breeding season of wolves, Canis lupus, in relation to latitude. Can Field Nat. 2002;116:139–140. [Google Scholar]
- Miller LA, Gionfriddo JP, Fagerstone KA, Rhyan JC, Killian GJ. The single-shot GnRH immunocontraceptive vaccine (GonaCon) in white-tailed deer: Comparison of several GnRH preparations. Am J Reprod Immunol. 2008;60:214–223. doi: 10.1111/j.1600-0897.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- Morrow CJ, Penfold LM, Wolfe BA. Artificial insemination in deer and non-domestic bovids. Theriogenology. 2009;71:149–165. doi: 10.1016/j.theriogenology.2008.09.001. [DOI] [PubMed] [Google Scholar]
- National Center for Research Resources. New animal model resource planned. Reporter. 2009;33:16. [Google Scholar]
- New World Encyclopedia. 2008 http:///www.newworldencyclopedia.org/entry/Vertebrate?oldid=795299. Downloaded August 17, 2009.
- Newby J, Wacher T. Addax nasomaculutus. IUCN 2009. IUCN Red List of Threatened Species. 2008 Version 1 2009.1.. www.iucnredlist.org. Downloaded on September 6, 2009.
- NIH. 2008 http://www.ncrr.nih.gov/comparative_medicine. Downloaded on September 14, 2009.
- NIH. 2009 http:/www.ncrr.nih.gov/about_us/budget/2010/congres-sional_justification/content/justification.htm.
- Packard JM, Seal US, Mech DL, Plotka ED. Causes of reproductive failure in two family groups of wolves (Canis lupus) Z Tierpsychol. 1985;69:24–40. [Google Scholar]
- Pitnick S, Markow TA. Large-male advantages associated with costs of sperm production in Drosophila hydei, a species with giant sperm. Proc Natl Acad Sci USA. 1994;91:9277–9281. doi: 10.1073/pnas.91.20.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukazhenthi B, Wildt DE. Which reproductive technologies are most relevant to studying, managing and conserving wildlife? Reprod Fertil Dev. 2004;16:33–46. doi: 10.10371/RD03076. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi BS, Neubauer K, Jewgenow K, Howard JG, Wildt DE. The impact and potential etiology of teratospermia in the domestic cat and its wild relatives. Theriogenology. 2006a;66:112–121. doi: 10.1016/j.theriogenology.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi BS, Comizzoli P, Travis AL, Wildt DE. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fertil Dev. 2006b;18:77–90. doi: 10.1071/rd05117. [DOI] [PubMed] [Google Scholar]
- Purvis A, Gittleman JL, Cowlishaw G, Mace GM. Predicting extinction risk in declining species. Proc R Soc Lond B. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racey PA. The prolonged storage and survival of spermatozoa in Chiroptera. J Reprod Fertil. 1979;56:391–402. doi: 10.1530/jrf.0.0560391. [DOI] [PubMed] [Google Scholar]
- Ripley JL. Osmoregulatory role of the paternal brood pouch for two Syngnathus species. Comp Biochem Physiol A Mol Integr Physiol. 2009;154:98–104. doi: 10.1016/j.cbpa.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Robeck TR, Steinman KJ, Yoshioka M, Jensen E, O’Brien JK, Katsumata E, Gili C, McBain JF, Sweeny J, Monfort SL. Estrous cycle characterization and artificial insemination using frozen-thawed spermatozoa in the bottlenose dolphin (Tursiops truncatus) Reproduction. 2005;129:659–674. doi: 10.1530/rep.1.00516. [DOI] [PubMed] [Google Scholar]
- Roth TL, O’Brien JK, McRae MA, Bellem AC, Romo SJ, Kroll JL, Brown JL. Ultrasound and endocrine evaluation of the ovarian cycle and early pregnancy in the Sumatran rhinoceros, Dicerorhinos sumatrensis. Reproduction. 2001;121:139–149. doi: 10.1530/rep.0.1210139. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Lacy RC, Margulis SW. Impacts of inbreeding on components of reproductive success. In: Holt WV, Pickard A, Rodger JC, Wildt DE, editors. Reproductive sciences and integrated conservation. Cambridge: Cambridge University Press; 2003. pp. 82–96. [Google Scholar]
- Schreiber EA, Burger J. Biology of marine birds. New York: CRC Press; 2002. p. 722. [Google Scholar]
- Senger PL. Pathways to pregnancy and parturition. Pullman, WA: Current Conceptions, Inc; 2003. pp. 6–8. [Google Scholar]
- Sillero-Zubiri C, Marino J. Canis simensis. IUCN 2009 Red List of Threatened Species, Version 2009.1. 2008 www.iucnredlist.org. Downloaded on August 17, 2009.
- Skerratt L, Berger L, Speare R, Cashins S, McDonald K, Phillott A, Hines H, Kenyon N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- Songsasen N, Brown JL, Rodden M, Wildt DE. Patterns of gonadal hormone metabolites in the maned wolf (Chrysocyon brachyurus) Theriogenology. 2006;66:1743–1750. doi: 10.1016/j.theriogenology.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Steinman KJ, Monfort SL, McGeehan L, Kersey DC, Gual-Sil F, Snyder RJ, Wang P, Nakao T, Czekala NM. Giant pandas: Biology, veterinary medicine and management. In: Wildt DE, Zhang A, Zhang H, Janssen DL, Ellis S, editors. Endocrinology of the giant panda and application of hormone technology to species management. Cambridge: Cambridge University Press; 2006. pp. 198–230. [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Sun XS, Liu Y, Yue KZ, Ma SF, Tan JH. Changes in germinal vesicle (GV) chromatin configurations during growth and maturation of porcine oocytes. Mol Reprod Dev. 2004;69:228–234. doi: 10.1002/mrd.20123. [DOI] [PubMed] [Google Scholar]
- Taylor AC. Assessing the consequences of inbreeding for population fitness: Past challenges and future prospects. In: Holt WV, Pickard A, Rodger JC, Wildt DE, editors. Reproductive sciences and integrated conservation. Cambridge: Cambridge University Press; 2003. pp. 67–81. [Google Scholar]
- Tingaud-Sequeira A, Zapater C, Chauvigné F, Otero D, Cerdá J. Adaptive plasticity of killifish (Fundulus heteroclitus) embryos: Dehydration-stimulated development and differential aquaporin-3 expression. Am J Physiol Reg Integr Comp Physiol. 2009;296:R1041–R1052. doi: 10.1152/ajpregu.91002.2008. [DOI] [PubMed] [Google Scholar]
- Touart LW. Factors considered in using birds for evaluating endocrine-disrupting chemicals. ILAR J. 2004;45:462–468. doi: 10.1093/ilar.45.4.462. [DOI] [PubMed] [Google Scholar]
- USFWS. Endang Sp Bull, Spring Issue. 2009;64 http://www.fws.gov/Endangered/bulletin.html. Downloaded on September 8, 2009. [Google Scholar]
- Van Look KJW, Dzyuba B, Cliffe A, Koldewey HJ, Holt WV. Dimorphic sperm and the unlikely route to fertilization in the yellow seahorse. J Exp Biol. 2007;210:432–437. doi: 10.1242/jeb.02673. [DOI] [PubMed] [Google Scholar]
- van Strien NJ, Manullang B, Sectionov, Isnan W, Khan MKM, Sumardja E, Ellis S, Han KH, Boeadi KH, Payne J, Bradley ME. Dicerorhinos sumatrensis. IUCN 2009 Red List of Threatened Species, Version 2009.1. 2008 www.iucnredlist.org. Downloaded on August 17, 2009.
- von Arx M, Breitenmoser-Wursten C. Lynx pardinus. IUCN 2009 Red List of Threatened Species, Version 2009.1. 2008 www.iucnredlist.org. Downloaded on August 17, 2009.
- Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Wielebnowski NC, Fletchall N, Carlstead K, Busso JM, Brown JL. Non-invasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zoo Biol. 2002a;21:77–98. [Google Scholar]
- Wielebnowski NC, Ziegler K, Wildt DE, Lukas J, Brown JL. Impact of social management on reproductive, adrenal and behavioral activity in the cheetah (Acinonyx jubatus) Anim Conserv. 2002b;5:291–301. [Google Scholar]
- Wildt DE, Donoghue AM, Johnston LA, Schmidt PM, Howard JG. Species and genetic effects on the utility of biotechnology for conservation. In: Moore HDM, Holt WV, Mace GM, editors. Biotechnology and the conservation of genetic diversity. Oxford: Clarendon Press; 1992. pp. 45–61. [Google Scholar]
- Wildt DE, Brown JL, Swanson WF. Reproduction in cats. In: Knobil E, Neill J, editors. Encyclopedia of reproduction. New York: Academic Press, Inc; 1998. pp. 497–510. [Google Scholar]
- Wildt DE, Ellis S, Howard JG. Linkage of reproductive sciences: From ‘quick fix’ to ‘integrated’ conservation. In: Con-cannon PW, England GCW, Farstad W, Linde-Forsberg C, Verstegen JP, Doberska C, editors. Advances in reproduction in dogs, cats and exotic carnivores. Colchester: Journals of Reproduction and Fertility Ltd; 2001. pp. 295–307. [PubMed] [Google Scholar]
- Wildt DE, Ellis E, Janssen D, Buff J. Toward more effective reproductive science in conservation. In: Holt WV, Pickard A, Rodger JC, Wildt DE, editors. Reproductive sciences and integrated conservation. Cambridge: Cambridge University Press; 2003. pp. 2–20. [Google Scholar]
- Wildt DE, Zhang A, Zhang H, Janssen D, Ellis S. Giant pandas: Biology, veterinary medicine and management. Cambridge: Cambridge University Press; 2006. p. 586. [Google Scholar]
- Wildt DE, Swanson WF, Brown J, Sliwa A, Vargas A. Felids ex situ: Managed programs, research and species recovery. In: Macdonald D, Loveridge AJ, editors. The biology and conservation of wild felids. Oxford: Oxford University Press; (in press) [Google Scholar]
- Wilson EO. The diversity of life. Cambridge: Harvard University Press; 1992. pp. 349–351. [Google Scholar]


