Abstract
We provide arguments to the debate question and update a previous meta-analysis with recently published studies on effects of sugar-sweetened beverages (SSBs) on body weight/composition indices (BWIs). We abstracted data from randomized controlled trials examining effects of consumption of SSBs on BWIs. Six new studies met these criteria: 1) human trials, 2) 3 weeks duration, 3) random assignment to conditions differing only in consumption of SSBs, and 4) including a BWI outcome. Updated meta-analysis of a total of seven studies that added SSBs to persons’ diets showed dose-dependent increases in weight. Updated meta-analysis of eight studies attempting to reduce SSB consumption showed an equivocal effect on BWIs in all randomized subjects. When limited to subjects overweight at baseline, meta-analysis showed a significant effect of roughly 0.25 standard deviations (more weight loss/less weight gain) relative to controls. Evidence to date is equivocal in showing that decreasing SSB consumption will reduce the prevalence of obesity. Although new evidence suggests that an effect may yet be demonstrable in some populations, the integrated effect size estimate remains very small and of equivocal statistical significance. Problems in this research area and suggestions for future research are highlighted.
Keywords: Randomized controlled trials, soda, beverages, soft drinks, obesity, weight loss, bias
INTRODUCTION
The proposition we have been asked to address and for which we evaluate the available evidence is as follows:
“There is sufficient scientific evidence that decreasing sugar-sweetened beverage (SSB) consumption will reduce the prevalence of obesity and obesity-related diseases.”
What we are debating
In examining the proposition, it is useful to carefully consider several of its components as follows:
Sufficient Evidence
The word sufficient invites the question, sufficient for what? As the remainder of the proposition indicates, the answer is for drawing a conclusion that decreasing SSB consumption will reduce the prevalence of obesity and obesity-related diseases. This must be distinguished from the question of sufficiency for taking public health action or guiding public health policy. What constitutes sufficiency for actions (as opposed to drawing conclusions) is not a purely scientific question that can be answered objectively. Such decisions depend only in part on scientific evidence of the likely effects of those actions and also depend on other inputs including but not limited to legal authority, moral values, and personal tastes, none of which are determined by empirical evidence. The question “Is there sufficient evidence for action” is inherently subjective and depends on which action, in which regulatory context, and according to whose tastes and moral values? As Sir Austin Bradford Hill wrote, “The evidence is there to be judged on its merits and the judgment …should be utterly independent of what hangs upon it – or who hangs because of it.” (1)
Scientific Evidence
We are not asked for conjecture, but rather whether empirical evidence exists showing that decreasing SSBs has the effects stated. We therefore examine the highest quality evidence available in the form of randomized controlled trials (RCTs). Because such trials are ethically possible and have been performed, we assert that this type of scientific evidence supersedes correlation or cohort studies (2). When RCTs are not possible, other evidence must be amassed to attempt to inform causation. However, RCTs are possible to address this question and data are available. Hence, we rely on these results in the present case as they are probative (by probative we mean studies which can generate evidence which settles questions by proving or disproving propositions, as opposed to simply influencing the strength of speculation) with respect to causation (3).
Decreasing
We cannot assume that the effects of decreasing consumption are the opposite (direction and magnitude) of the effects of increasing consumption. Therefore, we provide examinations of available experimental reports that evaluate both interventions so as to quantify the observed effects in each case.
Reduce the prevalence of obesity and obesity-related diseases
As to “obesity-related diseases,” one must first demonstrate an effect on obesity to suggest an effect on obesity-related diseases. Else in what way can the diseases be said to be obesity-related? We therefore focus our present meta-analysis on studies of the effect on body weight or body composition.
What we are not debating
Just as we have clarified the proposition being debated, it is equally important to not be distracted by questions that we have not been asked to address. For example, we have not been asked to address whether obesity is a crisis, if fructose is toxic, are some sugars worse than others, are food company marketing budgets too large, have portion sizes increased to absurd levels, do SSBs affect dental caries, are pictures of an average American’s sugar consumption dramatic, is liberty better than paternalism (or vice versa), is food marketing like tobacco marketing, or do we sometimes need to take public health actions in the absence of strong evidence. Although these are provocative questions, they are not germane to the necessary evaluation of evidence regarding the question we have been asked to debate. Yet we mention them because they and similar questions are often introduced into such discussions and serve as emotion-raising distractions to an evaluation of the pertinent evidence.
There is Evidence to Support Conjecture
We freely concede that there is evidence to support the conjecture that reducing SSB consumption might reduce obesity and obesity-related diseases. However, many of these data are not probative in terms of causation. Specifically, there are three forms of human evidence supporting this conjecture.
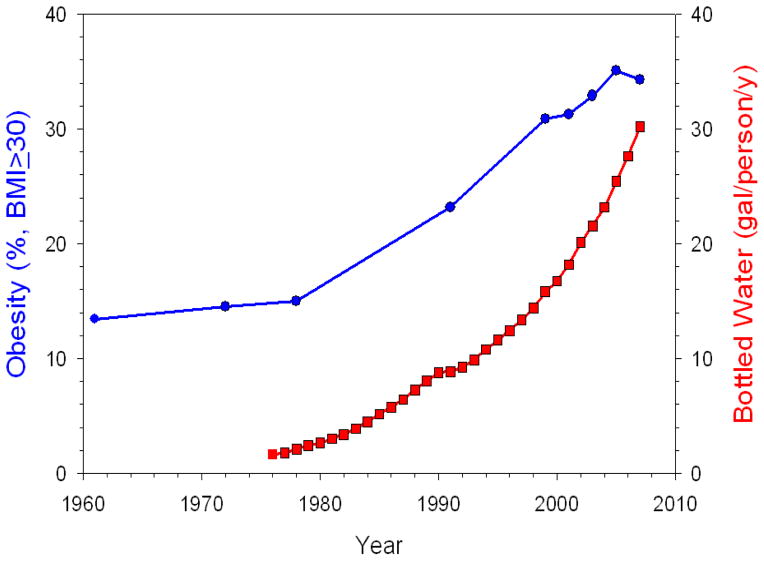
First, we address ecological correlation. SSB consumption has risen just as obesity rates have risen (4). This is the weakest form of evidence available. Other beverage consumption patterns (e.g., bottled water (5) depicted in Figure 1) have also demonstrated a strong correlation with the obesity epidemic in the United States (5–7).
Figure 1.
Rise in obesity rates (62) (round markers) and bottled water consumption (5) (square markers), United States.
BMI = body mass index, kg/m2
Second, we note an association in some observational studies (8–10). Whereas there is an ever-growing body of epidemiologic studies, some of which demonstrate statistically significant associations, it is well known that association does not establish causation. Moreover, the association is weak (11), inconsistent (12, 13), and biased (14), as we will discuss later. Again, as Dr. Hu (our debate opponent) wrote, “Although the overall results were not entirely consistent, the weight of epidemiologic and experimental evidence indicates greater consumption of sugar-sweetened beverages is associated with weight gain and obesity in children and adults. However, the existing studies suffer from many methodological limitations, including cross-sectional design, small sample size, short follow-up, inadequate dietary assessment, and a lack of repeated measures of diet and lifestyle….any single dietary factor is unlikely to have a large effect on body weight.” (13)
For the third and final point which supports conjecture, we acknowledge that lesser compensation with liquid versus solid calories has been found in some in short-term feeding studies (15–17). By compensation, we refer to the definition provided by Mattes (18) whereby later energy intake may be reduced to compensate for preloads or added calories from some other intervention. It must also be acknowledged that compensation for added intake may also take the form of altered energy expenditure, which can offset the intake component of energy balance. Few feeding studies examine this component. Additionally, short-term feeding effects are by no means equivalent to long-term weight effects (19). Moreover, the short-term effects are inconsistent, with some studies showing near perfect compensation for liquid calories (11, 20, 21) and others showing imperfect but equivalent (between forms) compensation to solid calories (22). Finally, there is far more than zero compensation as implied by common and exaggerated public statements such as, “When we drink sugary beverages, we simply do not compensate by eating less food” (23) or “Liquid calories don’t register with our appetite controls” (24).
We agree with Dr. Pan and Dr. Hu’s statement in 2011 that “… the isolated tests in the laboratory may not be directly reproduced in real life because the effect of any food or food component on satiety could be influenced by other dietary factors. Thus, results from short-term, well-controlled interventions may not be representative of a real-life setting, and long-term clinical trials on different physical forms of carbohydrates on energy intake and weight management are still lacking”(25). Later in this article, we provide even more compelling evidence from longer-term trials on weight that some compensation for added liquid calories indeed occurs.
Evaluation of Evidence to Draw Scientifically Supported Conclusions
When randomized trials can be performed ethically and safely (which they have been), these study results are the strongest level of evidence of independent effects. Many scientists who have gone on record on the question we now debate have acknowledged the limitations of association studies and the need for well-designed randomized trials (13, 26–28). If these scientists are also not convinced without such trials, it is curious that strong statements are then made about weaker forms of evidence. Use of Hill’s guidelines (1) is irrelevant in the instance of the effects of SSBs on weight because randomized trials can be done (and have been done). In such situations, the ‘totality’ of the evidence, including evidence that is not probative, should not be relied upon for drawing conclusions of causation in favor of the probative studies. More recent trials have taken steps to reduce the level of bias (29, 30) and future studies may advance this effort further.
Specific Questions We Address By Use of the Best Available Evidence
Does an increase in SSB intake increase body weight or body mass index (BMI) in humans?
Does reduction of SSB intake reduce body weight or BMI in humans?
We now evaluate and summarize the currently available evidence that could potentially be probative with respect to drawing conclusions about the effects of SSB reduction on weight or obesity.
METHODS
See supplemental material online for details of the updated literature review, study selection, and data extraction methods. As the present paper was in review, an additional study meeting our criteria became public as a conference abstract (31). This trial tested the effects of home water delivery and an educational program to reduce SSB consumption in overweight, adult, Mexican women as compared to the education-only control group. Based on the available information in the abstract, we were unable to formally include this study result in our meta-analysis but we discuss the possible effects on our conclusions using estimates from data reported in the abstract in the next section on results.
RESULTS
The Extent of the Data Available: Studies Included and Excluded
Table 1 contains a brief listing and description of the six new studies (29, 30, 32–36) added for meta-analysis. We provide more details of each study in the supplementary material online. Figure S1 in the supplementary material online contains a flow chart of the screening and selection of recently published studies.
Table 1.
Studies published since January 2009 meeting the original inclusion criteria (37).
| Reference | Question that can be addressed regarding the effects of SSBs on weight | How meta-analyzed | Primary Outcome(s) and Analysis as Stated in Trials Registry and Paper |
|---|---|---|---|
| Njike et al., 2011 (30) | Added two servings per day of sugar-free cocoa, sugared cocoa, or placebo cocoa in obese adults in a crossover trial, six weeks each phase. | Meta-analyzed all response data for all phases (author provided raw data on request) – combined both caloric groups (sugared cocoa and placebo cocoa) and subtracted sugar-free group. |
Trial Registry: NCT00538083.
Paper:
|
| Vaz et al., 2011 (36) | Added choco-malt beverage mix to water and gave one serving per day to children in a parallel trial. | Meta-analyzed untreated control group versus unfortified group.* |
Trial Registry: NCT00876018
Paper:
|
| Maersk et al., 2012 (34) | Added 1 liter per day of milk, regular cola, diet cola, or water in overweight/obese adults in a parallel trial for six months. | Meta-analyzed regular cola group versus diet group. |
Trial Registry: NCT00777647
Paper:
|
| Ebbeling et al., 2012 (33) | Multi-component program to reduce/replace SSBs with noncaloric beverages in adolescents. | Meta-analyzed weight change at end of one-year intervention period. |
Trial Registry: NCT00381160
Paper:
|
| de Ruyter et al., 2012 (29) | Provided school children identically labeled SSB or non-caloric equivalent to consume one can/day. | Considered an SSB reduction study as inclusion criteria was current SSB consumers. |
Trial Registry: NCT00893529
Paper:
|
| Tate et al., 2012 (35) | Substituted SSBs with artificially sweetened equivalent or water in obese adults who drink two or more servings per day at baseline. | Meta-analyzed water and artificially sweetened groups together versus SSB group. |
Trial Registry: NCT01017783
Paper:
|
SSB – Sugar-sweetened Beverage
We originally excluded any types of beverages that had growth promotion as a function, but the unfortified beverage met our original inclusion criteria and is included in this analysis.
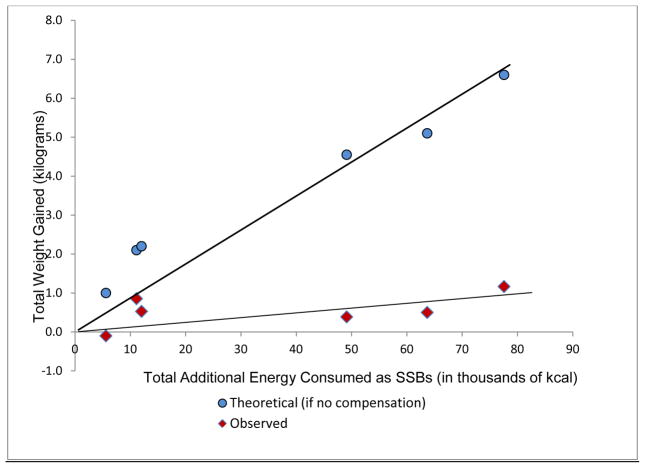
In the three new studies in which SSBs were added [90 to 500 kcal/day to the diets of adults (30, 34); 158 kcal/day in children (36)], statistically significant weight gain was observed in both adult trials, ranging from 0.39 to 1.14 kg (Table S1 in the supplementary material online). No significant difference in weight gain was observed in the study in children between the treatment and control participants (36). When we compared observed weight gain to theoretical weight gain from added SSBs in all RCTs published to date (Figure 2), compensation appeared to occur in longer-term studies.
Figure 2.
Observed (30, 34, 40–42, 63) versus theoretical (64) weight gain effect of mandatory sugar-sweetened beverage (SSB) consumption.
Notes: For observed values on the Y axis, weight change was determined by the change of those drinking more SSBs minus those drinking less. The X axis was determined by multiplying the added kcal per day times the duration of the study divided by 1000. Fit lines were generated by setting the origin to zero and by using the linear regression (least squares) options in Microsoft® Excel. The theoretical values (round markers) were generated by entering mean baseline values for each study sample into the NIDDK Body Weight Simulator (64) and adding the same number of calories per day for the same number of days as reported in the studies (30, 34, 40–42, 63). Activity settings in the simulator were at the lowest level of sedentary and no activity or dietary changes over the study duration were entered into the simulator. Observed data represent an average energy compensation rate of 85% (range = 57% – 110% compensation).
In the one new study of adults (35) and the two new studies of children (29, 32, 33) in which participants who drank some amount of SSBs at baseline were asked to eliminate or reduce their SSB consumption, standardized mean differences in percentage weight loss or BMI reduction ranged from 0.13 to 0.33 (Table S2 in the supplementary material online). The overall results for added SSBs (small but statistically significant weight gain; Figure 3) or for reduced SSBs in subjects of all weight ranges (small and not statistically significant weight loss; Figure 4) did not differ greatly from our earlier analysis (37).
Figure 3.
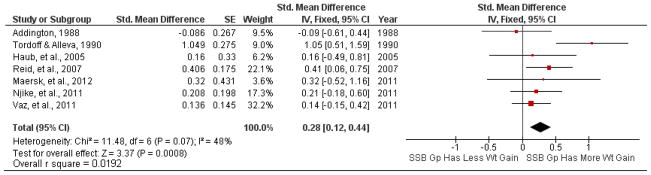
Forest plot comparing studies of added sugar-sweetened beverage (SSB) consumption.
Note: R square values were calculated from the overall standardized mean difference estimate (d) per the method found in (65).
Figure 4.
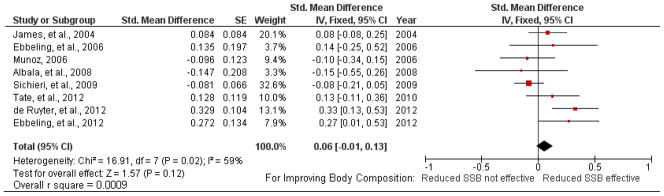
Forest plot comparing studies of reduced sugar-sweetened beverage (SSB) consumption; subjects in all weight categories included.
Note: R square values were calculated from the overall standardized mean difference estimate (d) per the method found in (65).
In new studies in which all participants were overweight or obese at baseline standardized mean differences ranged from 0.13 to 0.73 (Table S3 in the supplementary material online). In combination with earlier studies or subgroup analysis of the effects of reducing SSBs on overweight subjects (Figure 5), the overall standardized mean difference was 0.25 [95% confidence interval (CI): 0.13 to 0.38 standard deviations, p < 0.0001].
Figure 5.

Forest plot comparing studies of reduced sugar sweetened beverage (SSB) consumption; only subjects overweight/obese at baseline included.
Note: R square values were calculated from the overall standardized mean difference estimate (d) per the method found in (65).
In the newly published study by Hernández-Cordero, et al. (31), the authors reported no significant effect with a p-value of 0.50. Assuming this is a two-tailed p value, the reported sample size yields an effect size of either −0.086 or +0.086. The means were not reported so we cannot determine the direction. If the sample effect size were +0.086, then the summary statistic would not change at all from the summary estimate and confidence interval shown in Figure 4. Alternatively, if the sample effect size was −0.086, the summary estimate would be reduced towards zero (from 0.06 to 0.05) and remain statistically non-significant. Similarly, for the analysis shown in Figure 5 for subjects overweight at baseline, the addition of this study would shift the overall estimate from 0.25 to 0.21, or as low as 0.17 depending on direction of observed effect.
Assessment of Study-Level Risk of Bias
Figure S2 (supplementary material online) summarizes our cumulative assessment of potential areas of bias of the pertinent studies to date. The most important areas for risk of bias overall come from lack of participant blinding and selective reporting. Some study designs failed to adequately isolate treatment effects from the attention researchers paid to some groups. Additionally, only two studies’ protocols (29, 34) had an objective measure of participant compliance (returned containers, urinary sucralose measures), making cross-comparisons and estimates of true effects difficult. Failure to mention whether assessors were blinded was common (10 out of 15 studies), further clouding assessment of potential sources of bias.
Assessment of Publication Bias
Figures S3-S5 (supplementary material online) are funnel plots (38) for the assessment of potential publication bias from only the published studies and analyses for each of the three groups of designs or populations we analyzed (excluding some analyses we performed on data not published but received upon request). We also evaluated potential publication bias by using the rank correlation test (39). We found no present evidence of publication bias for studies on the effects of adding SSBs (30, 34, 36, 40–42); p = 0.805), for studies on the effects of reducing SSBs in all weight categories (29, 33, 35, 43–46); p = 0.976), or for studies on the effects of reducing SSBs in subjects who were overweight at baseline (33, 35, 43, 44, 46); p = 0.858).
Sensitivity Analysis
Age Differences
There was unequal representation of age groups among the types of trials. The added SSB studies were all on adults except one (36), and the reducing studies were predominantly in children with two exceptions (35, 47). Therefore, we evaluated the overall summary effects by excluding the studies referenced above. The overall standardized mean difference (SMD) for the added SSB studies (adults only) increased by 0.06 (to 0.34; 95% CI: 0.15 to 0.54). The overall SMD for the reduction of SSBs in children of all weight categories was reduced by 0.01 (to 0.07; 95% CI: −0.01 to 0.15). The overall SMD for the reduction studies in children only who were overweight or obese at baseline increased by 0.05 (to 0.30; 95% CI: 0.13 to 0.46). These results are not largely different from the combined analysis reported in Figures 4–6. Per the convention put forth by Cohen (48), these standardized effects would all be categorized as “small.”
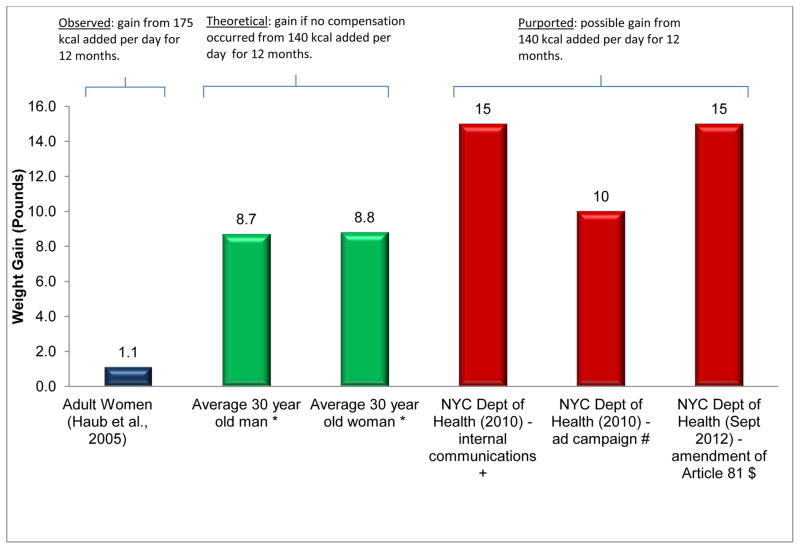
Figure 6.
Comparison of weight gain attributed to consumption of sugar-sweetened beverages for one year from various sources.
Note: For the Haub study, the weight change shown above is adjusted by subtracting the control group weight change.
* Body Mass Index of 27.8 kg/m2 (NHANES 2010 50th percentile for both men and women in the United States (66) entered into NIDDK body weight simulator (64).
Study Heterogeneity in Reduction Studies
Because the heterogeneity statistic was significant (Figure 4) in the reduction studies in both weight groups, we evaluated which study exerted the most influence for its effects on the overall SMD (46). Exclusion of this study resulted in a nonsignificant heterogeneity statistic (χ2(6) = 10.15, P = 0.12, I2 = 41%) and an increased overall SMD of 0.13 (95% CI: 0.04 to 0.22). These analyses shifted the overall statistics by relatively small amounts when considering the observed shifts in body weight among the analysis groups.
Interpreting the Magnitude of Effects
At this juncture, it may be helpful to express the estimated effect sizes for SSB reduction on BMI in some additional metrics which may ease interpretation. One such metric is the probability that a randomly selected person from a hypothetical population in which SSB reduction was implemented will be better off (with respect to BMI) than a randomly selected person from a hypothetical population that is the same in all ways except that SSB reduction has not been implemented. Without intervention, the probability is 0.50 that a person from one population weighs more than a person from the other population. After the interventions included in our analysis, these probabilities would change slightly. The probability that a randomly selected person from the reduced SSB population will have lower BMI than a person randomly selected from the control population would be 0.52. The probability that a randomly selected overweight person from the reduced SSB population will have a lower BMI than an overweight person randomly selected from the control population would be 0.57.
Another way to place the effect sizes in perspective is to consider the r2 metric shown in Figures 3–5. Increasing consumption of SSBs explains 1.92% of the variance in body weight or BMI change. Reducing consumption of SSBs in persons of all weight categories explains 0.09% of the variance in body weight or BMI change. Among persons who are overweight or obese at baseline, reducing the consumption of SSBs explains 1.54% of the variance in body weight or BMI change. It is possible to apply other methods such as risk analysis for evaluating potential effects on population levels of obesity (49), but that is beyond the scope of the present analysis.
ADDITIONAL CONSIDERATIONS
Having demonstrated that, although the conjecture that decreasing SSB consumption will decrease obesity and obesity-related diseases is reasonable, the pertinent data testing the hypothesis are equivocal (i.e., the pooled results are nearly but not quite statistically significant), we now address several related questions.
If the data are as weak as we have shown, why do some members of the public and the scientific community seem to perceive that the proposition has been proven?
We suggest three major reasons for this confusion.
Emotion-Raising Language
Emotion-raising language has often been used in discussions of SSBs and obesity. Some authors have used words like “plague” (50), “toxic” (51, 52), “hazardous” (4, 53), and “deadly” (4, 54) when describing SSBs or the sugars they contain and have tried to promote perceived connections between SSB marketers and the worst behavior of tobacco marketers (55). Although such words may help to advance an agenda (56), they do not educate or inform the public. Moreover, they likely raise emotions and impair logical reasoning (57). As Kersh and Morone (56) wrote, “Scientific findings never carry the same political weight as does a villain threatening American youth. If critics successfully cast portions of the industry in this way, far-reaching political interventions are possible, even likely. When an industry becomes demonized, plausible counterarguments (privacy, civil liberties, property rights, and the observation that “everyone does it”) begin to totter.”
Distortion of Scientific Information
A second factor that has likely contributed to misperceptions in this area is the distortion of scientific information by some authors and commentators. Table 2 lists some of the types of distortion that have occurred with quantitative or anecdotal documentation. Figure 6 depicts disparities in projected versus actual outcomes of the effects of added SSBs over 1 year. Clearly, such practices mislead and have likely contributed to misperceptions in the scientific and lay communities about the strength of the evidence regarding the proposition debated here.
Table 2.
Some types of distortion of information that have occurred regarding SSBs and obesity
| Type of Distortion | Where it Occurs | Documentation of Occurrence | Comments |
|---|---|---|---|
| Papers citing original RCTs that investigated the effect of SSB reduction on weight exaggerated the extent of the evidence supporting a beneficial effect | In the scientific peer-reviewed literature | Cope and Allison (14) documented in a quantitative analysis of the literature that this exaggerated reporting was the norm rather than an exception. | This is not a criticism of the original RCTs, but rather the manner in which subsequent authors cite them. |
| Association studies are described by using language that indicates a cause and effect relationship has been found | In the scientific peer-reviewed literature [e.g.,(70)], in government-sponsored newsletters [e.g., (71)], and in mass media articles (72–74) | “A new study…suggests a key way to reduce childhood obesity could be to limit your child’s salt intake. The study looked at 4,000 children in Australia and found kids who ate more salt also had more cravings for sugary-sweetened drinks like soda and juice.” (73) | “Cravings” were not mentioned in the published study (75), which did not overstate the findings, but were exaggerated in media coverage. This misleading practice is common in the obesity field overall (76). |
| Public statements that contradict available evidence | Communications from public health agencies | In 2010, the New York Times ran an article in which, through e-mails obtained under the Freedom of Information Act, they showed that the NYC Department of Health was knowingly making exaggerated statements about the amount of weight gain expected from drinking SSBs. Even after this expose (September 2012), the NYC Department of Health made even more exaggerated and evidence-contradicted statements about the amount of weight gain expected from drinking SSBs. | See Figure 11 for details and specific references. |
| Changing what is considered the primary endpoint or analysis in an RCT | In the scientific peer-reviewed literature | An example (34) of this occurred in an RCT published in the American Journal of Clinical Nutrition (AJCN). In the paper, the authors state “Our main aim was to test the hypothesis that sucrose-sweetened cola increases ectopic fat including VAT4, total body fat accumulation, and metabolic risk factors…,” whereas the registration in ClinicalTrials.gov states “Primary Outcome Measures: Body Weight; MR spectroscopy; MRI; DEXA scan.” Similarly, in ClinicalTrials.gov, the title of the trial is “Effect of Carbonated Soft Drinks on the Body Weight,” whereas in the article the title is “Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study.” The fact that there was no significant effect on weight was not mentioned in the abstract of the paper. | This does not conform to the CONSORT guidelines for publishing RCTs to which authors publishing in AJCN are expected to adhere. |
| Conclusion statements from paper do not match the results | In peer-reviewed papers, press releases, and mass media interviews | An example from the peer-reviewed literature occurred in a paper in AJCN (35) in which the results section of the abstract stated “Mean (±SEM) weight losses at 6 mo were −2.5 ± 0.45% in the DB group, −2.03 ± 0.40% in the Water group, and −1.76 ± 0.35% in the AC group; there were no significant differences between groups.” Yet, the conclusion section of the abstract stated “Replacement of caloric beverages with non-caloric beverages as a weight-loss strategy resulted in average weight losses of 2% to 2.5%.” Given the nonsignificant result, it does not seem justifiable to state there is any weight loss as a result of the treatment. Even if point estimates were being provided in a merely descriptive manner, the unbiased estimates of treatment effects in an RCT are the control-subtracted means, not the raw means in the treatment group. Examples from press releases and media interviews can be found in (14, 77) and in these sources (78, 79). | Though a trained scientist carefully reading the original papers will understand the results, journalists, regulators, clinicians, and scientists who only rapidly read an abstract are likely to be misled. |
| Publication bias | In the scientific peer-reviewed literature | Cope and Allison (14) showed that in observational epidemiologic studies of the association of SSB consumption and obesity, a standard test of publication bias was significant, suggesting that investigators are more likely to publish positive statistically significant findings than to publish null findings. | This is why we wrote earlier in this paper that the observed magnitude of association is likely biased upwards. Interestingly, Cope and Allison found that this publication bias seemed to occur among non-industry-funded authors and not among industry-funded authors. |
The Mere Exposure Effect
The final factor that we believe has led to the erroneous perception that the evidence showing that the proposition of this debate has been unequivocally proven is the “mere exposure effect.” The mere exposure effect is the label psychologists use for the phenomenon that the more a person is exposed to an idea, the more they come to like and accept it. As the Nobel Prize-winning economist Daniel Kahneman described, “A reliable way to make people believe in falsehood is frequent repetition, because familiarity is not easily distinguished from truth. Authoritarian institutions and marketers have always known this fact. But it was psychologists who discovered that you do not have to repeat the entire statement of a fact or idea to make it appear true” (58).
The number of articles on SSBs and obesity and the number of statements that SSBs are especially problematic in obesity are extraordinary, especially in comparison to the modest amount of probative data (3). Thus, opinions about SSBs may have been offered so often that these opinions have become accepted as fact by many in the scientific community, media, and lay public.
Are we alone in the view that a beneficial effect of SSB reduction on obesity has not been demonstrated?
In a word, no. As the quotations in Table 3 reveal, our views are concordant with those of other individual scientists and authoritative expert panels.
Table 3.
Quotations illustrating that others do not believe the benefits of interventions aimed at SSB reduction on weight have been established.
| Person(s) or Body Offering Statement | Statement | Reference |
|---|---|---|
| United States Department of Agriculture Dietary Guidelines Advisory Committee |
“Thus, there are mixed results on this topic. RCTs report that added sugars are not different from other calories in increasing energy intake or body weight. Prospective studies report some relationship with SSB and weight gain, but it is not possible to determine if these relationships are merely linked to additional calories, as opposed to added sugars per se. The systematic reviews in this area are also inconsistent, probably based on different measures used to determine added sugars intake or intake of SSB.”
[We should] “Conduct well-controlled and powered research studies testing interventions that are likely to improve energy balance in children at increased risk of childhood obesity, including dietary approaches that reduce …sugar-sweetened beverages” [because] “very few solid data are available on interventions in children.” |
(12) |
| European Food Safety Authority | “The Panel concludes that a cause and effect relationship has not been established between total sugar intake and body weight gain, and that a cause and effect relationship has not been established between the consumption of foods and beverages in which sugars have been replaced by intense sweeteners and contribution to the maintenance or achievement of a normal body weight.” | (80) |
| Lisa Te Morenga, Simonette Mallard, Jim Mann | “Trials in children, which involved recommendations to reduce intake of sugar sweetened foods and beverages, had low participant compliance to dietary advice; these trials showed no overall change in body weight.” | (81) |
| German Nutrition Society | “From 2 of the 4 available meta-analyses the conclusion is drawn that increased consumption of sugar-sweetened beverages in children and adolescents is associated with a higher risk of obesity. In contrast, another meta-analysis judges the effect as almost zero. The cohort studies published since then verify this risk-increasing effect only in part. The most recent meta-analysis concludes that the risk-increasing effect is limited to individuals with initially already increased BMI or existing overweight, respectively.” | (82) |
| Thomas Baranowski | “Another concern is the behavior or behaviors targeted for change. Many obesity prevention interventions have targeted increasing fruit and vegetable intake and decreasing sweetened beverage intake. Systematic reviews, however, showed no consistent evidence that increased fruit and vegetable intake protected against obesity or that sweetened beverage intake contributed to it.” | (83) |
| Joint statement from American Heart Association and the American Diabetes Association | “At this time, there are insufficient data to determine conclusively whether the use of NNS [non-nutritive sweeteners] to displace caloric sweeteners in beverages and foods reduces added sugars or carbohydrate intakes, or benefits appetite, energy balance, body weight, or cardiometabolic risk factors.” | (84) |
What would it take to shift the balance of evidence?
In a possibly apocryphal interchange, a devotee of Karl Popper’s philosophy of science once challenged the great mathematical geneticist J. B. S. Haldane to specify what it would take to change his views about the validity of evolutionary theory. Haldane reportedly retorted “Fossil rabbits in the Precambrian!” Though a poetic retort, Haldane was effectively specifying objective empirical evidence that would be sufficient for him to change his view, something any scientist addressing empirical questions should be prepared to do.
In the debate at The Obesity Society Meeting (September 20, 2012), the senior author [DBA] stated:
“The day that multiple RCTs are published that
are well designed, executed, and analyzed;
show statistically significant outcomes in preplanned analyses of the total randomized sample on measures of total body weight, BMI, or total body fat and clearly support the value of reducing SSBs; and
are sufficient in inferential weight to outweigh the existing RCT data;
I will be delighted to modify my opinion.”
The day after the debate (September 21, 2012), two new RCTs were published (29, 33). These two publications together met some (but not all) of the criteria specified above as we discussed earlier. Most notably, their collective evidential weight moved the integrated meta-analytic estimate for the effects of SSB reduction very close to the border of the conventional 0.05 level of statistical significance. For this reason, we believe that these two new studies can be described as “tilting the needle” in the direction of demonstrating the obesity-reducing benefit of SSB reduction, but that the data remain equivocal. Nevertheless, we remain open-minded that future RCTs (and according to ClinicalTrials.gov some will be forthcoming) may fulfill the criteria above and offer unequivocal support for the proposition.
We also suggest that the following approaches can increase the transparency of, and confidence in, RCTs in this area: 1) registering all RCTs in advance in ClinicalTrials.gov; 2) making the raw data from all RCTs publicly available for common and open analyses, regardless of the source of funding; 3) providing documentation via ClinicalTrials.gov as to which analyses are (were) preplanned; and 4) publishing all results regardless of outcome. These are laudable practices in all situations, but especially important in an area that has become so contentious.
How does the strength of evidence for conclusions relate to support for actions?
As we mentioned earlier, we are not addressing whether any particular policy or program should or should not be implemented. Rather, our sole purpose has been to present a synthesis of the currently available literature that provides an estimate of the degree of evidence for the debate proposition. Moreover, it is important to note that our paper assessed the evidence for effect of reducing SSB consumption, which should not be conflated with the effects of particular policies (e.g., taxes, bans, advertising campaigns, etc.) intended to reduce SSB consumption. The effects of any such policies represent a different question and not one for which we have evaluated the evidence.
The question of whether the available evidence is sufficiently strong to justify a particular action is a subjective one subject to societal perceptions, values, goals, and the plausibility of unintended consequences (59) (60). This is illustrated by quotations from two authoritative sources on this point as food for thought:
“Since taking office, the President has emphasized the need to use evidence and rigorous evaluation in budget, management, and policy decisions to make government work effectively. …Where evidence is strong, we should act on it. Where evidence is suggestive, we should consider it. Where evidence is weak, we should build the knowledge to support better decisions in the future.” (61)
“On fair evidence we might take action on what appears to be an occupational hazard, e.g. we might change from probably carcinogenic oil to a non-carcinogenic oil in a limited environment and without too much injustice if we are wrong. But we should need very strong evidence before we made people burn a fuel in their homes that they do not like or stop smoking the cigarettes and eating the fats and sugar that they do like.” (1)
CONCLUSIONS
Our updated meta-analysis shows that the currently available randomized evidence for the effects of reducing SSB intake on obesity is equivocal. Even if statistical significance is ignored, the point estimates of effects on BMI reduction are small, accounting for only 1.5% of the variance observed in those who were overweight at baseline. Therefore, we conclude that the debate proposition cannot be supported at this time. Of course, absence of evidence is not evidence of absence. The lower limit of the confidence interval around the estimated effect of SSB reduction is very close to the border of statistical significance. It is certainly possible that additional, larger, or otherwise stronger studies will in the future provide clear and convincing evidence that lowering SSB consumption will reduce obesity and obesity-related disease prevalence. We are certainly not arguing against the common-sense recommendation that for individuals who wish to lose weight and who presently drink large amounts of SSBs, reducing intake of these and other sources of energy seems wise.
We greatly respect our debate opponent, Dr. Hu, for addressing these issues in a manner that is both thoroughly scientific and equally collegial. We are hopeful that this debate may be seen not only as a careful consideration of the evidence regarding SSBs and obesity, but also as an exemplar of and call to a more informed, unexaggerated, open-minded, rational, and civil dialogue on the many public health issues around obesity that, like SSB-related issues, have become so contentious.
Supplementary Material
Acknowledgments
Supported in part by NIH grant P30DK056336. The opinions expressed are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated. This paper is based on a debate held at The Obesity Society 2012 Annual Meeting. The authors thank Sigrid Gibson; Drs. Michelle Bohan Brown, Richard Forshee, Richard Mattes and Douglas Weed for their suggestions on drafts of this manuscript. The authors also thank Dr. Marc Reitman for the use of Figure 1. The authors are also grateful to those who kindly responded to our request for additional data about their studies: Dr. Valentine Njike, Dr. David Katz, Dr. Mario Vaz, Dr. Tinku Thompson, Ms. Janne de Ruyter, Dr. Sonia Hernández-Cordero and Dr. Martijn Katan.
Footnotes
Competing Interests: In the last 36 months, Dr. Allison has received consulting fees from Kraft Foods. The University of Alabama at Birmingham has received gifts and grants from multiple organizations including but not limited to The Coca-Cola Company, PepsiCo, Red Bull, and Kraft Foods. Drs. Kaiser, Keating, and Shikany have no competing interests to report.
Ethical Approval: Not required.
Author Contributions: KAK: performed an updated systematic review, reviewed papers for inclusion criteria, extracted data from papers, wrote summaries of new studies included in appendix, checked meta-analysis calculations, assessed risk of bias for newly included studies, generated tables, generated figures, and wrote a significant portion of the text; KDK: extracted data from papers, analyzed supplemental data received from authors, generated new meta-analysis statistics, and verified prior data reported; JMS: reviewed papers for inclusion criteria, assessed risk of bias for newly included studies, wrote summaries of new studies included in appendix, and reviewed and edited text; DBA: conceived of the project scope, developed debate arguments, directed meta-analysis methods, reviewed papers for inclusion criteria, and edited and wrote a significant portion of the text.
References
- 1.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Preventive Services Task Force. US Preventive Services Task Force Procedure Manual. 2008. [Google Scholar]
- 3.Casazza K, Allison DB. Stagnation in the Clinical, Community, and Public Health Domain of Obesity: The Need for Probative Research. Clinical Obesity. 2012;2:83–85. doi: 10.1111/j.1758-8111.2012.00052.x. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA. Fructose: pure, white, and deadly? Fructose, by any other name, is a health hazard. J Diabetes Sci Technol. 2010;4:1003–1007. doi: 10.1177/193229681000400432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [Accessed: 12-14-2012];Bottled Water Consumption Per Person in the United States, 1976–2007. Posted: 12-7-2007. Available at: http://www.earth-policy.org/index.php?/data_center/C21/
- 6.Ogden CL, Carroll MD. [Accessed: 12-14-2012];Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2007–2008. Posted: 2010. Available at: http://www.cdc.gov/NCHS/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf.
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr. 2009;89:438–439. doi: 10.3945/ajcn.2008.26980. [DOI] [PubMed] [Google Scholar]
- 9.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drewnowski A, Bellisle F. Liquid calories, sugar, and body weight. Am J Clin Nutr. 2007;85:651–661. doi: 10.1093/ajcn/85.3.651. [DOI] [PubMed] [Google Scholar]
- 12.Dietary Guidelines Advisory Committee. [Accessed: 12-19-2012];Report of the DGAC on the Dietary Guidelines for Americans, 2010: Part D - Section 5: Carbohydrates. Posted: 2010. Available at: http://www.cnpp.usda.gov/Publications/DietaryGuidelines/2010/DGAC/Report/D-5-Carbohydrates.pdf.
- 13.Hu FB. In: Obesity Epidemiology. Hu FB, editor. New York: Oxford University Press; 2008. [Google Scholar]
- 14.Cope MB, Allison DB. White hat bias: examples of its presence in obesity research and a call for renewed commitment to faithfulness in research reporting. Int J Obes (Lond) 2010;34:84–88. doi: 10.1038/ijo.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24:794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 16.Tieken SM, Leidy HJ, Stull AJ, Mattes RD, Schuster RA, Campbell WW. Effects of solid versus liquid meal-replacement products of similar energy content on hunger, satiety, and appetite-regulating hormones in older adults. Horm Metab Res. 2007;39:389–394. doi: 10.1055/s-2007-976545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassady BA, Considine RV, Mattes RD. Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr. 2012;95:587–593. doi: 10.3945/ajcn.111.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattes RD. Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiol Behav. 1996;59:179–187. doi: 10.1016/0031-9384(95)02007-1. [DOI] [PubMed] [Google Scholar]
- 19.McKiernan F, Hollis JH, Mattes RD. Short-term dietary compensation in free-living adults. Physiol Behav. 2008;93:975–983. doi: 10.1016/j.physbeh.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almiron-Roig E, Chen Y, Drewnowski A. Liquid calories and the failure of satiety: how good is the evidence? Obes Rev. 2003;4:201–212. doi: 10.1046/j.1467-789x.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 21.Almiron-Roig E, Flores SY, Drewnowski A. No difference in satiety or in subsequent energy intakes between a beverage and a solid food. Physiol Behav. 2004;82:671–677. doi: 10.1016/j.physbeh.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Houchins JA, Burgess JR, Campbell WW, et al. Beverage vs. solid fruits and vegetables: effects on energy intake and body weight. Obesity (Silver Spring) 2012;20:1844–1850. doi: 10.1038/oby.2011.192. [DOI] [PubMed] [Google Scholar]
- 23.Kulze AG. [Accessed: 12-19-2012];Eat Right for Life: Chapter 5 - Drink the Right Beverages. Posted: 2013. Available at: http://www.welcoa.org/store/resources/documents/er-sample-chapter.pdf.
- 24. [Accessed: 12-18-2012];Nutrition Action Healthletter: Pour Better or Pour Worse. Posted: 6-1-2006. Available at: http://www.cspinet.org/nah/06_06/beverage.pdf.
- 25.Pan A, Hu FB. Effects of carbohydrates on satiety: differences between liquid and solid food. Curr Opin Clin Nutr Metab Care. 2011;14:385–390. doi: 10.1097/MCO.0b013e328346df36. [DOI] [PubMed] [Google Scholar]
- 26.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 27.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97:667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 30.Njike VY, Faridi Z, Shuval K, et al. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int J Cardiol. 2011;149:83–88. doi: 10.1016/j.ijcard.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Cordero S, Barquera S, Sentered Rodriguez-Ramirez, et al. Water intake and metabolic syndrome risk: a randomized clinical trial. Experimental Biology Conference; Boston. Abstract presented 4-22-2013. [Google Scholar]
- 32.de Ruyter JC, Olthof MR, Kuijper LD, Katan MB. Effect of sugar-sweetened beverages on body weight in children: design and baseline characteristics of the Double-blind, Randomized INtervention study in Kids. Contemp Clin Trials. 2012;33:247–257. doi: 10.1016/j.cct.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Ebbeling CB, Feldman HA, Chomitz VR, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–1416. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maersk M, Belza A, Stodkilde-Jorgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283–289. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 35.Tate DF, Turner-McGrievy G, Lyons E, et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr. 2012;95:555–563. doi: 10.3945/ajcn.111.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaz M, Pauline M, Unni US, et al. Micronutrient supplementation improves physical performance measures in Asian Indian school-age children. J Nutr. 2011;141:2017–2023. doi: 10.3945/jn.110.135012. [DOI] [PubMed] [Google Scholar]
- 37.Mattes RD, Shikany JM, Kaiser KA, Allison DB. Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes Rev. 2011;12:346–365. doi: 10.1111/j.1467-789X.2010.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copenhagen: The Nordic Cochrane Centre. Review Manager (RevMan) [Computer Program] Version 5.1.6. The Cochrane Collaboration; 2008. 2011. [Google Scholar]
- 39.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 40.Haub MD, Simons TR, Cook CM, Remig VM, Al-Tamimi EK, Holcomb CA. Calcium-fortified beverage supplementation on body composition in postmenopausal women. Nutr J. 2005;4:21. doi: 10.1186/1475-2891-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr. 2007;97:193–203. doi: 10.1017/S0007114507252705. [DOI] [PubMed] [Google Scholar]
- 42.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51:963–969. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 43.Albala C, Ebbeling CB, Cifuentes M, Lera L, Bustos N, Ludwig DS. Effects of replacing the habitual consumption of sugar-sweetened beverages with milk in Chilean children. Am J Clin Nutr. 2008;88:605–611. doi: 10.1093/ajcn/88.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117:673–680. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- 45.James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomised controlled trial. BMJ. 2004;328:1237. doi: 10.1136/bmj.38077.458438.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sichieri R, Paula TA, de Souza RA, Veiga GV. School randomised trial on prevention of excessive weight gain by discouraging students from drinking sodas. Public Health Nutr. 2009;12:197–202. doi: 10.1017/S1368980008002644. [DOI] [PubMed] [Google Scholar]
- 47.Munoz D. The efficacy of two brief interventions to reduce soda consumption in a college population (Doctoral dissertation) Albany: New York State University of New York; 2006. [Google Scholar]
- 48.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1988. [Google Scholar]
- 49.Forshee RA, Storey ML, Ginevan ME. A risk analysis model of the relationship between beverage consumption from school vending machines and risk of adolescent overweight. Risk Anal. 2005;25:1121–1135. doi: 10.1111/j.1539-6924.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- 50.Popkin BM. Sugary beverages represent a threat to global health. Trends Endocrinol Metab. 2012;23:591–593. doi: 10.1016/j.tem.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Lustig RH, Gupta S. [Accessed: 12-7-2012];Is sugar toxic? Available at: http://www.cbsnews.com/8301-18560_162-57407294/is-sugar-toxic/
- 52.Lustig RH, Schmidt LA, Brindis CD. Public health: The toxic truth about sugar. Nature. 2012;482:27–29. doi: 10.1038/482027a. [DOI] [PubMed] [Google Scholar]
- 53.Bray GA. Soft drink consumption and obesity: it is all about fructose. Curr Opin Lipidol. 2010;21:51–57. doi: 10.1097/MOL.0b013e3283346ca2. [DOI] [PubMed] [Google Scholar]
- 54.Yudkin JS. Pure, White and Deadly. London: Penguin Books; 1986. [Google Scholar]
- 55.Brownell KD, Warner KE. The perils of ignoring history: Big Tobacco played dirty and millions died. How similar is Big Food? Milbank Q. 2009;87:259–294. doi: 10.1111/j.1468-0009.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kersh R, Morone J. The politics of obesity: seven steps to government action. Health Aff (Millwood ) 2002;21:142–153. doi: 10.1377/hlthaff.21.6.142. [DOI] [PubMed] [Google Scholar]
- 57.Schmeichel BJ, Vohs KD, Baumeister RF. Intellectual performance and ego depletion: role of the self in logical reasoning and other information processing. J Pers Soc Psychol. 2003;85:33–46. doi: 10.1037/0022-3514.85.1.33. [DOI] [PubMed] [Google Scholar]
- 58.Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus & Giroux; 2011. [Google Scholar]
- 59.Sharpe KM, Staelin R, Huber J. Using Extremeness Aversion to Fight Obesity: Policy Implications of Context Dependent Demand. J Consumer Res. 2008;35:406–422. [Google Scholar]
- 60.Wansink B, Hanks D, Just DR, et al. [Accessed: 12-7-2012];From Coke to Coors: A Field Study of a Sugar-Sweetened Beverage Tax and its Unintended Consequences. Posted: 5-26-2012. Available at: http://dx.doi.org/10.2139/ssrn.2079840.
- 61.Zients JD. [Accessed: 12-19-2012];Memorandum to the Heads of Executive Departments and Agencies. Posted: 2012. Available at: http://www.whitehouse.gov/sites/default/files/omb/memoranda/2012/m-12-14.pdf.
- 62.Ogden CL, Carroll MD. [Accessed: 12-14-2012];Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2007–2008. Posted: 2010. Available at: http://www.cdc.gov/NCHS/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf.
- 63.Addington EA. Doctoral dissertation. Manhattan, Kansas: Kansas State University; 1998. Aspartame-or sugar-sweetened beverages. Effects on food appetites and mood in young adults. [Google Scholar]
- 64.Hall KD. [Accessed: 12-14-2012];Body Weight Simulator. Posted: 4-4-2012. Available at: http://bwsimulator.niddk.nih.gov/
- 65.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 66.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 67.Hartocollis A. [Accessed: 12-7-2012];E-Mails Reveal Dispute Over City’s Ad Against Sodas. Posted: 10-28-2010. Available at: http://www.nytimes.com/2010/10/29/nyregion/29fat.html?pagewanted=all.
- 68.New York City Department of Health and Mental Hygiene. Man Drinking Fat. NYC Health Anti-Soda Ad. Are You Pouring on the Pounds? Posted: 12-14-2009. Available at: http://www.youtube.com/watch?v=-F4t8zL6F0c.
- 69.Kansangra S. [Accessed: 12-7-2012];Maximum Size For Sugary Drinks: Proposed Amendment of Article 81 - Response to Comments. Posted: 9-13-2012. Available at: http://www.nyc.gov/html/doh/downloads/pdf/boh/article81-response-to-comments-ppt.pdf.
- 70.Davis JN, Whaley SE, Goran MI. Effects of breastfeeding and low sugar-sweetened beverage intake on obesity prevalence in Hispanic toddlers. Am J Clin Nutr. 2012;95:3–8. doi: 10.3945/ajcn.111.019372. [DOI] [PubMed] [Google Scholar]
- 71.Unattributed. [Accessed: 12-18-2012];Could Kids’ Salt Intake Affect Their Weight? Posted: 2012. Available at: http://www.nlm.nih.gov/medlineplus/news/fullstory_132089.html.
- 72.Azuz C. Lots of salt means lots of soda. Posted: 12-10-2012. Available at: http://www.cnn.com/video/?hpt=he_mid#/video/health/2012/12/10/hm-salt-and-soda.cnn.
- 73.Mackey L. [Accessed: 12-18-2012];Lowering salt intake may be key to lowering childhood obesity rates. Posted: 12-10-2012. Available at: http://wtvr.com/2012/12/10/lowering-salt-intake-may-be-key-to-lowering-childhood-obesity-rates/
- 74.McKenzie A. [Accessed: 12-18-2012];Study says salt may be a catalyst for childhood obesity. Posted: 12-13-2012. Available at: http://triad.news14.com/content/top_stories/676361/study-says-salt-maybe-a-catalyst-for-childhood-obesity.
- 75.Grimes CA, Riddell LJ, Campbell KJ, Nowson CA. Dietary Salt Intake, Sugar-Sweetened Beverage Consumption, and Obesity Risk. Pediatrics. 2012 doi: 10.1542/peds.2012-1628. e-pub. [DOI] [PubMed] [Google Scholar]
- 76.Cofield SS, Corona RV, Allison DB. Use of causal language in observational studies of obesity and nutrition. Obes Facts. 2010;3:353–356. doi: 10.1159/000322940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cope MB, Allison DB. White hat bias: a threat to the integrity of scientific reporting. Acta Paediatr. 2010;99:1615–1617. doi: 10.1111/j.1651-2227.2010.02006.x. [DOI] [PubMed] [Google Scholar]
- 78.James G. [Accessed: 12-10-2012];Are Diet Drinks The Key To Weight Loss? Posted: 2-15-2012. Available at: http://www.huffingtonpost.co.uk/2012/02/15/diet-drinks-water-key-to-weight-loss_n_1279488.html.
- 79.Lane P. [Accessed: 12-10-2012];Press Release: Weighing the difference: switching to water, diet beverages can tip the scales. Posted: 2012. Available at: http://uncnews.unc.edu/content/view/5088/71/
- 80.European Food Safety Authority. [Accessed: 12-18-2012];Scientific Opinion on the substantiation of health claims related to intense sweeteners and contribution to the maintenance or achievement of a normal body weight. Posted: 2012. Available at: http://www.efsa.europa.eu/en/efsajournal/doc/2229.pdf.
- 81.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 82.Hauner H, Bechthold A, Boeing H, et al. Evidence-based guideline of the German Nutrition Society: carbohydrate intake and prevention of nutrition-related diseases. Ann Nutr Metab. 2012;60 (Suppl 1):1–58. doi: 10.1159/000335326. [DOI] [PubMed] [Google Scholar]
- 83.Baranowski T. School-based obesity-prevention interventions in low- and middle-income countries: do they really work? Am J Clin Nutr. 2012;96:227–228. doi: 10.3945/ajcn.112.043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive Sweeteners: Current Use and Health Perspectives : A Scientific Statement from the American Heart Association and the American Diabetes Association. Circulation. 2012;126:509–519. doi: 10.1161/CIR.0b013e31825c42ee. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.