Abstract
Studies in male rodents have shown that stress-induced increases in circulating corticosterone are increased by both CB1 receptor (CB1R) antagonist treatment and genetic deletion. The purposes of the current study were to determine whether female mice respond in the same manner as males, and whether indirect CB1R agonists accelerate the return of corticosterone to baseline. In agreement with earlier studies, CB1R null and rimonabant-treated male mice had significantly increased circulating corticosterone 30 minutes following the end of a restraint episode compared to wild type and vehicle-treated, respectively. Females treated with rimonabant had significantly higher circulating corticosterone compared to vehicle. However, corticosterone concentrations were not different between CB1R null and wild type females at 30 minutes recovery, although CB1R null mice had higher corticosterone concentrations at 90 minutes of recovery. Female CB1R null mice exhibited greater serum binding capacity for corticosterone than wild type. The monoacylglycerol lipase inhibitor, JZL184, attenuated corticosterone concentrations at restraint offset in male, and at 30 minutes recovery in female mice compared to vehicle. Male mice treated with JZL184 exhibited greater concentrations of circulating corticosterone at 120 minutes recovery, even in the absence of restraint. JZL184 had no effect on corticosterone concentrations in CB1R null mice. The fatty acid amide hydrolase inhibitor, URB597, did not affect corticosterone responses to restraint in male or female, wild type or CB1R null mice. These data suggest that 2-arachidonoylglycerol is the primary endocannabinoid involved in CB1R regulation of the recovery of the HPA axis from restraint stress. These data support a role for endocannabinoid-CB1R signaling in the regulation of the corticosterone response to restraint stress and suggest that female mice with life-long loss of the CB1R undergo compensatory changes that minimize the impact of loss of endocannabinoid signaling on circulating corticosterone.
Keywords: Endocannabinoid, rimonabant, JZL184, URB597, monoacylglycerol lipase, fatty acid amide hydrolase, corticosteroid-binding globulin
1. Introduction
The endocannabinoid signaling (ECS) system in the brain is composed of the type one cannabinoid receptor (CB1R) and the endocannabinoids (eCBs) N-arachidonylethanolamine (AEA) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Sugiura et al., 1995). AEA is metabolized in brain by fatty acid amide hydrolase (FAAH; (Cravatt et al., 1996)), whereas approximately 80% of 2-AG hydrolysis in brain is catalyzed by monoacylglycerol lipase (MAGL; (Blankman et al., 2007)). URB597 inhibits FAAH and produces significant increases in brain AEA (Kathuria et al., 2003), while JZL184 inhibits MAGL and increases brain 2-AG (Long et al., 2009). These drugs function as indirect agonists of CB1R signaling, and so could be expected to have different pharmacological effects compared to direct agonists. In addition, the indirect agonists can be used to interrogate the relative roles of AEA and 2-AG in processes regulated by tonic eCB signaling (Saario and Laitinen, 2007).
Activation of the hypothalamic-pituitary-adrenal (HPA) axis is a hallmark of the stress response in mammals. The HPA response must recover to baseline following offset of the threat so that homeostasis can be restored (McEwen, 2004). Negative feedback regulation of the HPA axis is mediated by neuronal and humoral circuits activated by glucocorticoids at the level of the pituitary, hypothalamus and higher brain regions (Keller-Wood and Dallman, 1984). In particular, the hippocampus and prefrontal cortex have high glucocorticoid receptor (GR) density and play major roles in HPA axis recovery (Herman et al., 2005).
ECS exerts bidirectional effects on the HPA axis. Multiple studies have shown that direct activation of CB1R, particularly with high concentrations of direct agonists, increases circulating glucocorticoid concentrations (Bloom and Kiernan, 1980; Johnson et al., 1978; Romero et al., 2002). A recent report from Haller and colleagues demonstrates that inhibition of MAGL with JZL184 also increases HPA axis activity in mice (Aliczki et al., 2013). On the other hand, activation of ECS dampens or reduces HPA axis activation by stress exposure (Ganon-Elazar and Akirav, 2009; Hill et al., 2009; Patel et al., 2004), likely through actions in the hypothalamus (Di et al., 2003) and amygdala (Hill et al., 2009). ECS is also involved in the recovery of the HPA axis to baseline, likely as an effector within the long feedback loops initiated by GR activation in the hippocampus (Wang et al., 2012) and prefrontal cortex (Hill et al., 2011). In particular, in vivo studies from our laboratory demonstrate that CB1R blockade in the PFC of male rodents prolongs the recovery of the HPA axis to baseline following stress (Hill et al., 2011). One purpose of the studies in this report was to examine HPA axis recovery under conditions in which ECS is enhanced by inhibition of FAAH and MAGL.
There are well described sex differences in the regulation of the HPA axis (Handa et al., 1994). Basal corticosterone concentrations are higher in female than male rats (Seale et al., 2004) and females have larger adrenal glands (Bastida et al., 2007; Malendowicz et al., 1986). Restraint stress activates the HPA axis to a greater degree in female compared to male mice, which is partially due to greater negative feedback in males (Buynitsky and Mostofsky, 2009). In support of this notion, males exhibit increased mRNA transcripts for GR in the hippocampus and glutamic acid decarboxylase, an enzyme responsible for GABA synthesis, in the BNST (Goel and Bale, 2010). Thus, males are hypothesized to have increased protein “machinery” to allow for more efficient activation of the hippocampal-BNST-PVN inhibitory feedback loop. Additionally, females in many species and strains have higher concentrations of corticosteroid-binding globulin (CBG), a protein that determines the percentage of free glucocorticoids in the circulation and available to enter the brain (Faict et al., 1986).
Several lines of evidence suggest there are sex differences in ECS. Female rats exhibit lower CB1R density in mesencephalon, hypothalamus and basal ganglia (Gonzalez et al., 2005; Riebe et al., 2010; Rodriguez de Fonseca et al., 1994). The affinity of ligands for limbic forebrain CB1R is significantly lower in females than males (Rodriguez de Fonseca et al., 1994). In addition, females are more affected by prolonged cannabinoid exposure during adolescence (Mateos et al., 2011; Viveros et al., 2012) and female rodents self-administer more of a cannabimimetic drug than males (McGregor and Arnold, 2007). Despite the higher prevalence for cannabis use among men, women progress to cannabis dependence more rapidly (Fattore et al., 2009). In a study of the adverse effects of the CB1R antagonist rimonabant in humans, all of those reporting depression as a serious adverse effect were women (Van Gaal et al., 2008). These findings suggest that females have lower ECS than males and, as a result, are more likely to be affected by reduced ECS than males.
ECS modifies the circadian rhythm of the HPA axis in a sexually dimorphic manner, such that CB1R blockade increases corticosterone near the circadian nadir and peak in male rats, but only near the nadir in female rats (Atkinson et al., 2010). Although CB1R blockade and deletion have similar effects to increase corticosterone in male and female rodents exposed to a variety of stressors (Steiner and Wotjak, 2008), there are no studies that directly compare the role of ECS in stress recovery between the sexes.
The purpose of the current studies is to evaluate the role of ECS in the restoration of baseline HPA axis activity following an episode of acute stress. We hypothesize that sex differences in the recovery of the HPA axis are partially due to differences in ECS signaling and that potentiation of 2-AG-mediated signaling through pharmacological inhibition of MAGL accelerates the return of HPA axis activity to baseline at early times after stress, but also exerts a delayed, CB1R-dependent increase in corticosterone concentrations.
2. Methods
2.1 Animals
Male and female outbred ICR (CD-1) mice aged 2–4 months were used in these studies. In some studies, mice were obtained from Harlan Laboratories (Madison, WI, USA) and allowed to acclimate to on-site housing for at least 36 hours prior to experimentation. In other studies, wild-type (WT) and CB1 receptor knock out mice (CB1R−/−) were bred in-house from founders obtained from Roche Bioscience (Palo Alto, CA, USA) that were crossed from 129/SvJ to the ICR background for more than 9 generations; genotypes were determined as previously described (Ibrahim et al., 2003). All mice were provided ad libitum access to standard mouse chow and water and were housed on a 12:12 hour light:dark cycle with lights on at 0600 hours. Experiments were carried out in the light phase. All experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Drug treatment
All drugs were administered 30 minutes before the initiation of restraint via intraperitoneal (i.p.) injection in a volume of 0.1 ml/25 g body weight. Rimonabant was obtained from the NIDA Research Resources Drug Supply Program (Research Triangle Institute, North Carolina, USA); URB597 and JZL184 were obtained from Cayman Chemical Company (Ann Arbor, Michigan, USA). Rimonabant (5 mg/kg) and URB597 (0.1 mg/kg) were administered using a 1:1:18 ethanol:cremaphor:saline vehicle while JZL184 (1 and 16 mg/kg) was administered in 1:1:18 dimethyl sulfoxide:cremaphor:saline following previously published methods (Cradock et al., 1973). Cremaphor is a castor oil based emulsifier (Spectrum Chemicals, Gardena, CA 90248, USA).
2.2 Restraint protocol and blood collection
Mice were restrained by anchoring the proximal portion of the tail to a bench top for 30 minutes as described previously (Hill et al., 2011). After restraint, the mice were returned to their home cages to recover. For the collection of tail blood samples, the mice were placed under an inverted beaker, with the tail protruding from under the spout as described previously (Hill et al., 2011). A 0.1–0.4 cm cut was made at the tip of the tail using a sharp scissors. Tail blood was collected in heparinized micro-hematocrit capillary tubes (Fisherbrand). No more than 3 minutes elapsed during the blood collection procedure and the blood sample formed a clot. Samples were obtained immediately before restraint and immediately after the end of the restraint. Additional blood samples were obtained during home cage recovery. No more than three blood samples were taken from a single animal and only one blood sample was taken during the recovery period. In some studies, trunk blood was collected following decapitation and allowed to clot, yielding serum. Blood samples were centrifuged at 10,000 × g for 30 seconds and plasma or serum was removed and frozen at −20°C until assayed.
2.3 Measurement of total corticosterone
Total corticosterone concentrations were determined by radioimmunoassay (MP Biomedicals, Solon, OH). Compared corticosterone concentrations were measured in the same assay. The intra-assay coefficient of variation for the assay (provided by the manufacturer) is 4.4 – 10.3%.
2.4 Serum corticosterone binding capacity
Serum CBG was estimated by measuring the capacity of corticosterone to bind serum proteins obtained from trunk blood, as described previously (D'Agostino and Henning, 1981; Raff et al., 2003). Briefly, serum was obtained from trunk blood and was stripped of endogenous steroids by adding 30 µL dextran-coated charcoal to 0.1 mL serum. The solution was centrifuged for 5 minutes at 2000 × g and the resulting supernatant was removed. Stripped serum (15 µL) was diluted 1:100 in 40 mM sodium phosphate buffer (pH 7.9). This mixture was incubated in tubes containing 15 µL [3H]corticosterone (20 µCi/mL; 80 Ci/mmol; MP Biomedicals, Solon, OH, USA) and 37.5 µL non-labeled corticosterone (1 µg/mL) that was evaporated to dryness. Nonspecific binding of [3H]corticosterone was determined in the presence of 10 µL unlabeled corticosterone (100 µg/mL; 289 nM). Samples were incubated for 90 minutes at 4°C. Following incubation, free and bound steroids were separated by adding 30 µL of dextran-coated charcoal followed by centrifugation for 5 minutes at 2000 g. Bound [3H]corticosterone was counted in 0.2 mL of each supernatant in 6 mL scintillation fluid. Binding capacity was calculated as the difference between bound [3H]corticosterone in the presence and absence of unlabeled corticosterone.
2.5 Data analyses
Statistical analyses were performed using Sigma Plot software (San Jose, CA, USA). ANOVA were utilized to inform differences in groups; however, we hypothesized differences a priori between male and female animals and among the effects of MAGL and FAAH inhibitors, so we carried out those planned comparisons regardless of the ANOVA results. Post hoc tests were done using the Holm-Šídák method. Data are presented as the mean ± SEM. Comparisons in which p values were less than 0.05 were considered statistically significant.
3. Results
3.1 Studies in CB1R knock out mice
We examined the corticosterone responses to restraint in male and female, wild type and CB1R−/− mice. In agreement with studies of others (Handa et al., 1994), female wild type mice exhibit an augmented increase in circulating corticosterone than male wild types in response to 30 minutes of restraint (Fig. 1A). Post hoc tests revealed that females had higher corticosterone concentrations compared to males at stress offset (p<0.001) and 30 minutes later (p<0.01). Female mice exhibited an accelerated reduction in corticosterone during the first 60 minutes compared to males since corticosterone concentrations were not different between male and female mice at 60 minutes after restraint offset.
Figure 1.
Stress-induced serum corticosterone concentrations, where zero minutes represents the offset of restraint, in (A) ICR WT male and female; two-way ANOVA results: time (F4,127 = 92, p<0.0001), sex (F1,127 = 25, p<0.0001) and interaction (F4,130 = 15, p<0.0001). (B) Male WT and CB1R−/−; two way ANOVA results: time (F4,130 = 99, p<0.0001), genotype (F1,130 = 6.7, p<0.05) and interaction (F4,130 = 2.2, n.s.). (C) Female WT and CB1R−/−; two way ANOVA results: time (F4,125 = 105, p<0.0001), genotype (F1,125 = 2.2, n.s.) and interaction (F4,125 = 1.0, n.s.). (D) CB1R−/− male and female; two-way ANOVA results: time (F4,128 = 92, p<0.0001), sex (F1,128 = 22, p<0.0001) and interaction (F4,128 = 15, p<0.0001). **p<0.01, ***p<0.001, #p<0.05, ###p<0.01, +p<0.05, +++p<0.001; significantly different from the other group on the graph at the same time point by Holm-Šídák t tests. Sample size ranged from 22 – 27 mice at the −30 and 0 minute time points and 4 – 12 mice at the other time points.
We compared the time course of corticosterone responses to restraint between male wild-type and male CB1R−/− mice (Fig. 1B). Post hoc comparisons revealed that corticosterone concentrations were significantly higher in male CB1R−/− mice compared to wild-type 30 minutes after termination of the restraint stress (p<0.001).
The corticosterone responses of female wild type and CB1R−/− mice to restraint were compared (Fig. 1C). Planned post hoc tests showed that the CB1R−/− mice had significantly higher plasma corticosterone concentrations 90 minutes after the end of restraint compared to wild-type mice (p<0.05).
The responses of male and female CB1R−/− mice are compared directly in Fig. 1D. Post hoc tests revealed female CB1R−/− mice had significantly higher corticosterone than male at restraint offset (0 minute; p<0.001) but not at 30 minutes of recovery. Females also had higher corticosterone at 90 minutes (p<0.05). These data support the conclusion that CB1R signaling does not contribute to the lower corticosterone response in males at the end of the restraint, but suggest that CB1R signaling contributes to initial recovery of the HPA axis to baseline in male but not female mice.
3.2 Pharmacological inhibition of CB1R signaling
Male and female mice were injected 30 minutes prior to the onset of restraint with the CB1R antagonist, rimonabant (5 mg/kg), or an equivalent volume of vehicle (Fig. 2A). Planned post hoc tests demonstrate rimonabant-treated male mice had higher corticosterone concentrations compared to vehicle-treated males prior to the restraint and 30 minutes after restraint offset (both p<0.001).
Figure 2.
Stress-induced serum corticosterone concentrations, where zero minutes represents the offset of restraint in (A) ICR WT males treated with vehicle or rimonabant (5 mg/kg), two-way ANOVA results: time (F4,125 = 26, p<0.0001), drug treatment (F1,125 = 21, p<0.0001) and interaction (F4,125 = 2.1, p=0.08); (B) females treated with vehicle or rimonabant, two way ANOVA results: time (F4,125 = 29, p<0.0001), treatment (F1,125 = 25, p<0.0001) and interaction (F4,125 = 1.0, n.s.). (C) vehicle-treated male and female mice; two-way ANOVA results: time (F4,122 = 33, p<0.0001), sex (F1,122 = 3.9, p=0.05) and interaction (F4,122 = 2.6, p<0.05). (D) rimonabant-treated males and females; two way ANOVA results: time (F4,128 = 22, p<0.0001), sex (F1,128 = 13, p<0.001) and interaction (F4,128 = 2.41, p=0.05). *p<0.05, ***p<0.001, #p<0.05, ##p<0.01, ###p<0.001, ++p<0.01 +++p<0.001; significantly different from the other group on the graph at the same time point by Holm-Šídák t tests. Sample size ranged from 22 – 23 at the −30 and 0 minute time points and 6 – 9 at the other time points.
The effects of rimonabant treatment on restraint-induced changes in circulating corticosterone were examined in female mice (Fig. 2B). Planned post hoc tests indicate rimonabant-treated female mice had higher corticosterone compared to vehicle-treated females prior to restraint (p<0.01), at restraint offset (p<0.05), and 30 minutes after restraint offset (p<0.01). There was also significantly higher corticosterone in rimonabant-treated compared to vehicle-treated females 90 minutes after restraint ended (p<0.05); similar to the results seen in the CB1R−/− mice, rimonabant-treated females exhibited a delayed increase in corticosterone that was not observed in the vehicle-treated female mice.
Overall, acute pharmacological blockade of the CB1R resulted in patterns of increased corticosterone that were similar in male and female mice. This finding is supported when the data for both sexes are plotted by treatment (vehicle: Fig. 2C and rimonabant: Fig. 2D). Post hoc tests demonstrated that vehicle-treated females had higher corticosterone compared to males at restraint onset (p<0.05) and offset (p<0.001) but not at the other times examined. Post hoc t-tests revealed female rimonabant-treated mice had significantly greater corticosterone compared to males treated with rimonabant at restraint onset (p<0.01) and offset (p<0.001). Female mice had a trend towards higher corticosterone at 90 minutes (p=0.057), suggesting a greater role for the CB1R in the late stages of recovery in female compared to male mice.
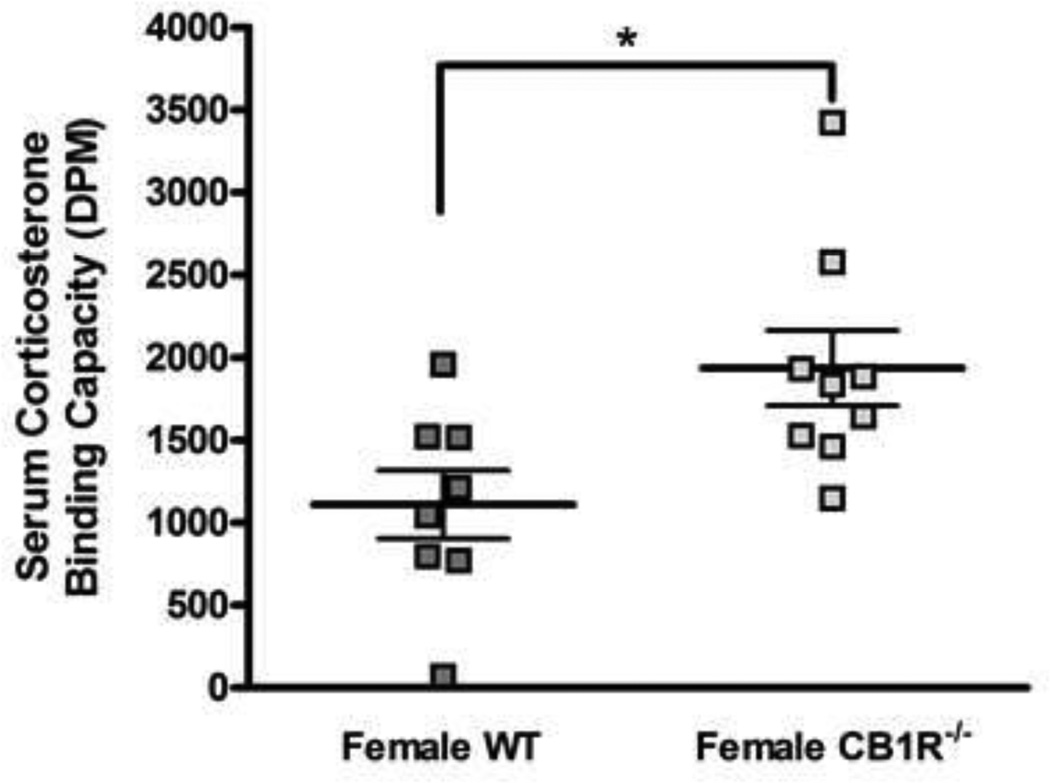
3.3 CB1R knock out Females have Increased Serum Binding Capacity for Corticosterone
We hypothesized that the lack of effect of genetic deletion of the CB1R in female mice on stress-induced changes in circulating corticosterone is due to changes in the percent of corticosterone bound to plasma proteins in the female CB1R−/− mice. That is, a change in binding with no change in plasma total corticosterone measured by RIA would indicate a change in free (bioavailable) corticosterone. To test this hypothesis, we measured corticosterone binding capacity in serum samples obtained from female wild type and CB1R−/− mice (Fig. 3). CB1R−/− females had significantly higher corticosterone binding than WT females by unpaired t test (t = 2.7, p<0.05).
Figure 3.
Serum binding capacity for corticosterone in ICR WT and CB1R−/− female mice. Serum was obtained from trunk blood and stripped of endogenous steroids with charcoal followed by incubation with unlabeled and [3H]corticosterone. Binding capacity was calculated as the difference between bound [3H]corticosterone in the presence and absence of unlabeled corticosterone. Sample size is 8 – 9 in each group. *p<0.05; significantly different by unpaired t tests.
3.4 Inhibition of MAGL, but not FAAH, Alters Restraint-induced HPA axis Activation
The specific MAGL inhibitor, JZL184 (16 mg/kg), or vehicle were administered to male mice to explore the role of 2-AG in CB1R regulation of the HPA axis. Mice were restrained 30 minutes after injection and corticosterone concentrations were determined before and at various time intervals following stress offset (Fig. 4). Post hoc t-tests demonstrated JZL184-treated male mice (Fig. 4A) had significantly lower corticosterone concentrations compared to vehicle-treated males at 0 minutes (p<0.05), but higher concentrations at 90 (p<0.05) and 120 minutes after stress offset (p<0.01). There were trends toward significance at pre-restraint (p=0.10) and 30 minutes recovery (p=0.06). In female mice (Fig. 4B), t-tests demonstrated a significant difference between corticosterone concentrations in vehicle and JZL184-treated mice at 30 minutes (p<0.05) and a trend at 120 minutes (p=0.076).
Figure 4.
Effect of MAGL inhibitor on stress-induced serum corticosterone concentrations. Zero minutes represents the offset of restraint; mice were treated with vehicle or 16 mg/kg JZL184 30 minutes prior to the beginning of the restraint period. (A) Male wild type mice; two way ANOVA results: time (F5,171 = 21, p<0.0001), treatment (F1,171 = 0.25, n.s.) and interaction (F5,171 = 4.4, p<0.001). (B) Female wild type mice; two way ANOVA results: time (F5,169 = 23, p<0.0001); treatment (F1,169 = 0.03, n.s.) and interaction (F5,169 = 1.7, p=0.14). (C) Male CB1R−/− mice; two-way ANOVA results: time (F5,110 = 28, p<0.0001); treatment (F1,110 = 0.21, n.s.) and interaction (F5,110 = 0.58, n.s.) (D) Female CB1R−/− mice; two way ANOVA time (F5,129 = 25, p<0.0001), treatment (F1,129 = 0.9, n.s.) and interaction (F5,129 = 0.2, n.s.). #p<0.05, ##p<0.01; significantly different by Holm-Šídák t tests. Sample size is 17 – 35 per group at the −30 and 0 minute time points and 3– 12 per group at each of the other time points.
The temporally biphasic effect of JZL184 on circulating corticosterone observed in male mice could be the result of a combination of CB1R and non-CB1R mediated effects. To determine whether JZL184 has non-CB1R effects, we examined the corticosterone responses to restraint in CB1R−/− male (Fig. 4C) and female (Fig. 4D) mice pretreated with JZL184 (16 mg/kg) or vehicle. There were no significant differences between the treatment groups at any time, suggesting that all of the observed effects of JZL184 on circulating corticosterone are CB1R-mediated.
Previous studies have shown that JZL184 treatment can increase corticosterone concentrations at 40 min following drug administration (Aliczki et al., 2013). In accord with these data, treatment with 16 mg/kg JZL184 significantly elevates plasma corticosterone concentrations 3 hours after injection compared to vehicle injected mice (Table 1).
Table 1.
The MAGL inhibitor, JZL184, produces a long-lasting increase in circulating corticosterone concentrations in male mice
| Corticosterone (ng/ml) | |||
|---|---|---|---|
| Treatment | 1.5 hr post injection | 2.5 hr post injection | 3 hr post injection |
| Control (no injection) | 25.7 ± 9 | 28.3 ± 12 | 25.5 ± 4 |
| Vehicle injection | 71.5 ± 19 | 82.5 ± 13 | 108 ± 22 |
| JZL184 (16 mg/kg) | 140.4 ± 59 | 243.5 ± 64 | 300.5 ± 67* |
Male mice were injected i.p. with either vehicle (0.1 ml/25 g body weight) or JZL184 and trunk blood was collected at the times indicated after injection. Control mice were left undisturbed in their cages. Each value is the mean of 6 replicates except for the control, 3 hour time point which is the mean of 3. Data are shown as mean ± SEM. Two way ANOVA indicates that treatment had a significant main effect (F2,43 = 18.6, p<0.0001) while neither time (F2,43 = 1.88, n.s.) nor the interaction (F4,43 = 1.1, n.s.) were significant. Planned comparisons between vehicle and JZL184 treated groups using t-tests reveal a significant effect of JZL184 treatment at the 3 hour time point
p<0.05.
The specific FAAH inhibitor URB597 (0.1 mg/kg) or vehicle were administered to mice 30 minutes prior to restraint. Corticosterone concentrations were determined in blood obtained before the restraint period and at various subsequent times (Fig. 5). URB597 treatment had no significant effect on corticosterone concentrations at any time before or after the stress in either male or female mice.
Figure 5.
Effect of inhibition of FAAH on stress-induced serum corticosterone concentrations. Zero minutes represents the offset of restraint. Mice were treated with vehicle or 0.1 mg/kg URB597 30 minutes prior to restraint. (A) Male, wild type mice; two way ANOVA results: time (F4,66 = 18, p<0.0001), treatment (F1,66 = 1.2, n.s.) and interaction (F4,66 = 0.42, n.s.). (B) Female, wild type mice; two way ANOVA results: time (F4,130 = 8.5, p<0.0001), treatment (F1,130 = 0.03, n.s.) and interaction (F4,130 = 0.46, n.s.). (C) Male, CB1R−/− mice; two way ANOVA results: time (F3,46 = 5.7, p<0.005), treatment (F1,46 = 0.8, n.s.) and interaction (F3,46 = 0.57, n.s.). (D) Female, CB1R−/− mice; two way ANOVA results: time (F3,61 = 24.3, p<0.0001), treatment (F1,61 = 0.16, n.s.) and interaction (F3,61 = 0.44, n.s.). Sample size is 5 – 24 per group.
There is evidence that AEA can activate cellular targets other than the CB1R. Transient receptor potential vanilloid receptors (TRPV1) have been found in similar brain regions as the CB1R (Cristino et al., 2006; Grueter et al., 2010) and can be activated by high concentrations of AEA and result in neuronal signaling opposite to the CB1R (Ross, 2003). To test the hypothesis that inhibition of FAAH produces non-CB1R mediated effects on corticosterone that oppose effects mediated by CB1R activation, we measured restraint-induced corticosterone in CB1R−/− male and female mice pretreated with URB597 (0.1 mg/kg). URB597 treatment had no significant effect on corticosterone concentrations at any time before or after the stress in either male or female mice.
4. Discussion
Previous studies carried out in male rodents demonstrate that inhibition of ECS results in increased circulating corticosterone concentrations following stress exposure (Evanson et al., 2010; Newsom et al., 2012; Patel et al., 2004). Data in the current study are consistent with these findings and demonstrate that pharmacological inhibition of CB1R signaling results in increased corticosterone concentrations in both male and female mice 30 minutes after stress offset. Results in the male CB1R−/− mice were similar to those obtained with pharmacological CB1R blockade; however, corticosterone concentrations were not different between female CB1R−/− and WT mice in the early recovery period. We hypothesized that compensatory processes in females, including increased CBG concentrations that would alter the plasma free (bioavailable) corticosterone, could mitigate the occurrence of prolonged HPA axis activity when ECS is absent. The second purpose of these studies was to examine whether enhanced ECS reduces corticosterone responses to stress. We found that inhibition of MAGL reduced activation early in the recovery period, while FAAH inhibition had no effect. Interestingly, however, MAGL inhibition produced a sustained increase in corticosterone concentrations 120 minutes after stress offset (3 hours after drug administration) that was paralleled by a significant increase in corticosterone concentrations in non-restrained mice at the same time. These data indicate that increased 2-AG-mediated ECS can dampen the HPA axis response to stress, but also produces a time-dependent increase in circulating corticosterone that eventually results in a hyperactive HPA axis response.
Pharmacological antagonism of the CB1R indicates a role for ECS to regulate the return of circulating corticosterone concentrations to baseline after stress in both male and female mice. However, female mice exhibited a difference in the effects of pharmacological inhibition of the CB1R and global, life-long CB1R deletion. While the CB1R antagonist prolonged the HPA axis response to restraint, there was no difference in the corticosterone response to restraint between wild type and CB1R−/− females. One possible explanation is that female mice have compensated for the genetic deletion of CB1R. Several lines of evidence support the hypothesis that genetic deletion of components of ECS can induce compensation, which is not surprising in light of the diverse systems regulated by ECS. For example, CB1R−/− mice have decreased function of hippocampal and cingulate GABAA and GABAB receptors (Uriguen et al., 2011). Since a predominant effect of ECS in these brain regions is to reduce GABA release, these biochemical changes would be expected to mitigate the loss of CB1R retrograde regulation of GABA release by dampening GABA signaling. We found that CB1R−/− females had higher serum protein binding capacity for corticosterone than wild type females although total corticosterone concentrations were the same. These data suggest that CB1R−/− females have lower free corticosterone concentrations than wild type; a difference that could represent a compensatory mechanism since only free corticosterone is active. Although female CB1R−/− mice do not exhibit a difference in total circulating corticosterone, their increased CBG capacity could allow for reduced biologically active corticosterone concentrations compared to wild type females. As a result, the increased free glucocorticoid concentrations that would result from loss of the CB1R “brake” are mitigated in female CB1R−/− mice through an increase in CBG.
Our studies evaluated the roles of 2-AG versus AEA in restraint-induced HPA axis activation through comparison of the pharmacological effects of selective inhibitors of MAGL and FAAH, respectively. Pharmacological inhibition of MAGL produced modest but significant decreases in circulating corticosterone early in recovery, although the times at which significant differences were observed were not the same in males and females. Later in the recovery from restraint, JZL184-treated male mice had increased corticosterone concentrations compared to vehicle-treated controls. There was a trend to the same pattern in females, but the changes were not statistically significant. Both the effect of JZL184 to dampen corticosterone concentrations early in recovery and enhance them later did not occur in CB1R−/− mice, indicating that both changes are CB1R mediated effects. Male mice treated with JZL184 but not subjected to restraint also exhibited an increase in circulating corticosterone, reaching significance 3 hours after injection. The increase in corticosterone concentrations produced by JZL184 injection in the absence of restraint were very large; in fact were approximately the same as corticosterone concentrations in restrained mice treated with vehicle at stress offset. Taken together, these data suggest that increased 2-AG-mediated CB1R activation exerts a biphasic effect on corticosterone concentrations. At early times following JZL184 injection, the changes are primarily inhibitory of HPA axis activation, while after several hours, 2-AG-mediated CB1R activation results in increased circulating corticosterone. The current studies cannot delineate the requirement for stress in the effects of JZL184 because the i.p. injection is itself a significant stress.
Recent studies from Haller and colleagues have also shown a significant temporal component to the effects of JZL184 on mice (Aliczki et al., 2012; Aliczki et al., 2013). They found that JZL184 produced immobility and effects on elevated plus maze (epm) behavior at long but not short times after administration (Aliczki et al., 2012). In partial agreement with the studies reported herein, that group found that JZL184 treatment increased corticosterone at 40 min but not 3 hours after the injection (Aliczki et al., 2013). Since JZL184 is a covalent inhibitor of MAGL (Long et al., 2009), it is likely that 2-AG concentrations increase progressively with time after treatment. Earlier work from our laboratory demonstrated that CB1R agonists exert dose-related effects on restraint-induced HPA axis activation such that low doses decrease, while higher doses enhance restraint-induced changes in circulating corticosterone (Patel et al., 2004). Thus, it is our current hypothesis that 2-AG concentrations in the first hour or so after JZL184 administration, like low doses of direct agonists, activate a pool of CB1R that dampen HPA axis activation. However, after several hours, the amount of 2-AG increases to high enough concentrations to activate a pool of CB1R that are less sensitive to agonists and couples to increased HPA axis activation. Support for CB1R pools that both inhibit and activate the HPA axis come from studies showing that CB1R agonist administration into the basolateral amygdala decreases restraint-induced corticosterone, while microinjections into the medial amygdala cause an increase (Hill et al., 2009). A related possibility is that CB1R mediated signaling exerts opposing effects on the recovery circuit by acting on both GABA and glutamatergic synapses (Hill et al., 2007). Alternatively, the time-dependent effect of JZL184 could have been due to rapid desensitization (or tachyphylaxis) of CB1R signaling by high concentrations of 2-AG. Studies of ECS in hippocampal cultures demonstrate that 2-AG induces rapid desensitization of CB1R signaling (Straiker and Mackie, 2007) and mice genetically lacking MAGL exhibit down-regulation of the CB1R (Chanda et al., 2010). JZL184 has been shown to have effects on anxiety-like behavioral patterns in rodents. Rats treated with 8 mg/kg JZL184 demonstrate reduced anxiety-like behavior in the epm 30 min following injection but only under conditions of high stress (Sciolino et al., 2011). Interestingly, male mice treated with 16 mg/kg JZL184 also exhibit decreased anxiety-like behavior in the epm, but this effect is blocked by CB2R but not CB1R antagonist co-treatment (Busquets-Garcia et al., 2011). Male mice exhibit reduced anxiety in the marble burying test following 16 mg/kg JZL184 that is partially reversed by CB1R blockade (Kinsey et al., 2011). Recent data suggests that the anti-anxiety effects of JZL184 are secondary to its ability to increase HPA axis activation since inhibition of corticosterone synthesis with metyrapone abolished the effects of JZL184 to increase open time in the epm (Aliczki et al., 2013). Thus, the putative anti-anxiety effects of JZL184 could be a combination of effects on CB2Rs and a CB1R-dependent increase in glucocorticoid concentrations.
There were no significant effects of FAAH inhibition in either sex at any time point examined. These data are at odds with earlier findings from our laboratory (Patel et al., 2004). In the earlier study, pretreatment of male ICR mice with 0.1 mg/kg URB597 reduced corticosterone concentrations measured in trunk blood harvested immediately following the end of restraint stress. In the earlier study, mice were restrained in rigid, plastic conical tubes which produce a greater degree of immobility than the restraint used in this study. Another difference between the two methods is their potential impact on thermoregulation since plastic tubing traps heat, whereas exposure to open counter tops at room temperature (20–23 degrees Celsius) allows convective heat losses. In either case, we speculate that the two stress methods differentially recruit AEA-mediated ECS and thus exhibit different sensitivities to URB597-mediated FAAH inhibition.
5. Conclusion
Our studies provide further support for the hypothesis that blockade of ECS increases the release of glucocorticoids in response to an acute stress. Given the physiological and psychological consequences of chronically heightened HPA axis activity (McEwen, 2004), these data suggest that inhibited ECS would result in pathophysiological consequences. Indeed, rimonabant use in humans is associated with increased anxiety, depression and suicidality (Nissen et al., 2008; Van Gaal et al., 2008). However, our findings indicate that activation of ECS by blockade of eCB catabolism during a stress episode has only a modest effect on the HPA axis response. The data from JZL184-treated, non-restrained mice indicate that activation of CB1R signaling, likely through time-dependent accumulation of 2-AG, can activate the HPA axis in a manner similar to direct cannabinoid agonists such as THC (Pertwee, 1974). Our findings also reveal a potential sex difference in susceptibility to CB1R desensitization, such that males exhibit a rapid tachyphylaxis that is not observed in female mice during the same time frame. Females also have the capacity to resist or compensate for suppression of CB1R signaling by complex mechanisms, which we show here is partially due to increased CBG in CB1R−/− mice. While this could make females less vulnerable to stress disorders, the compensatory process could be detrimental in the long-term, and actually contribute to psychopathology. This is a tempting speculation since hypoactivity of the HPA axis is seen in atypical depression.
Highlights.
CB1 receptor blockade increases, while monoacylglycerol lipase inhibition decreases, circulating corticosterone in both male and female mice during the thirty minute period after a restraint stress episode
While CB1 receptor null male mice exhibit increased circulating corticosterone concentrations following restraint stress compared to wild type, female mice null for the CB1 receptor exhibit the same pattern of corticosterone response as wild type
Inhibition of fatty acid amide hydrolase does not affect the concentration of circulating corticosterone post-restraint stress in male or female mice
These data offer further support for the hypothesis that endocannabinoid signaling is essential for the return of the HPA axis to baseline following stress
Acknowledgements
This work was supported by NIDA grants R21 DA022439 and R01 DA026996. CJR was partially supported by T32 GM080202 and T32 GM089586. Partial funding was provided through the Research and Education Program, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AEA
N-arachidonylethanolamine
- CB1R
cannabinoid type one receptor
- CBG
corticosteroid-binding globulin
- CRH
corticotrophin-releasing hormone
- eCB
endocannabinoid
- ECS
endocannabinoid signaling
- epm
elevated plus maze
- FAAH
fatty acid amid hydrolase
- HPA
hypothalamic-pituitary-adrenocortical
- MAGL
monoacylglycerol lipase
- TRPV1
transient receptor potential vanilloid type one receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliczki M, Balogh Z, Tulogdi A, Haller J. The temporal dynamics of the effects of monoacylglycerol lipase blockade on locomotion, anxiety, and body temperature. Behav Pharmacol. 2012;23:348–357. doi: 10.1097/FBP.0b013e3283564dfa. [DOI] [PubMed] [Google Scholar]
- Aliczki M, Zelena D, Mikics E, Varga ZK, Pinter O, Bakos NV, et al. Monoacylglycerol lipase inhibition-induced changes in plasma corticosterone levels, anxiety and locomotor activity in male CD1 mice. Horm Behav. 2013;63:752–758. doi: 10.1016/j.yhbeh.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Leggett JD, Wood SA, Castrique ES, Kershaw YM, Lightman SL. Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocrinology. 2010;151:3720–3727. doi: 10.1210/en.2010-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida CM, Cremades A, Castells MT, Lopez-Contreras AJ, Lopez-Garcia C, Sanchez-Mas J, et al. Sexual dimorphism of ornithine decarboxylase in the mouse adrenal: influence of polyamine deprivation on catecholamine and corticoid levels. Am J Physiol Endocrinol Metab. 2007;292:E1010–E1017. doi: 10.1152/ajpendo.00316.2006. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AS, Kiernan CJ. Interaction of ambient temperature with the effects of Δ9-tetrahydrocannabinol on brain catecholamine synthesis and plasma corticosterone levels. Psychopharmacol. 1980;67:215–219. doi: 10.1007/BF00431259. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009;33:1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Chanda PK, Gao Y, Mark L, Btesh J, Strassle BW, Lu P, et al. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- Cradock JC, Davignon JP, Litterst CL, Guarino AM. An intravenous formulation of 9-tetrahydrocannabinol using a non-ionic surfactant. J Pharm Pharmacol. 1973;25:345. doi: 10.1111/j.2042-7158.1973.tb10024.x. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- D'Agostino J, Henning SJ. Hormonal control of postnatal development of corticosteroid-binding globulin. Am J Physiol. 1981;240:E402–E406. doi: 10.1152/ajpendo.1981.240.4.E402. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faict D, De Moor P, Bouillon R, Heyns W, Heiniger HJ, Corrow D, et al. Transcortin and vitamin D-binding protein levels in mouse serum. J Endocrinol. 1986;109:141–147. doi: 10.1677/joe.0.1090141. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009;34(Suppl 1):S227–S236. doi: 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci. 2009;29:11078–11088. doi: 10.1523/JNEUROSCI.1223-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Bale TL. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151:1784–1794. doi: 10.1210/en.2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Mena MA, Lastres-Becker I, Serrano A, de Yebenes JG, Ramos JA, et al. Cannabinoid CB(1) receptors in the basal ganglia and motor response to activation or blockade of these receptors in parkin-null mice. Brain Res. 2005;1046:195–206. doi: 10.1016/j.brainres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, et al. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Dewey WL, Ritter KS, Beckner JS. Cannabinoid effects on plasma corticosterone and uptake of 3H-corticosterone by mouse brain. Eur J Pharmacol. 1978;47:303–310. doi: 10.1016/0014-2999(78)90238-8. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, O'Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2011;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malendowicz LK, Robba C, Nussdorfer GG. Sex differences in adrenocortical structure, function. XXII Light- and electron-microscopic morphometric studies on the effects of gonadectomy and gonadal hormone replacement on the rat adrenal cortex. Cell Tissue Res. 1986;244:141–145. doi: 10.1007/BF00218391. [DOI] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, et al. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors. J Psychopharmacol. 2011;25:1676–1690. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Arnold JC. Cannabis reward: biased towards the fairer sex? Br J Pharmacol. 2007;152:562–564. doi: 10.1038/sj.bjp.0707469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom RJ, Osterlund C, Masini CV, Day HE, Spencer RL, Campeau S. Cannabinoid receptor type 1 antagonism significantly modulates basal and loud noise induced neural and hypothalamic-pituitary-adrenal axis responses in male Sprague-Dawley rats. Neuroscience. 2012;204:64–73. doi: 10.1016/j.neuroscience.2011.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Rodes-Cabau J, Cannon CP, Deanfield JE, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Tolerance to the effect of delta1-tetrahydrocannabinol on corticosterone levels in mouse plasma produced by repeated administration of cannabis extract or delta1-tetrahydrocannabinol. Br J Pharmacol. 1974;51:391–397. doi: 10.1111/j.1476-5381.1974.tb10674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff H, Hong JJ, Oaks MK, Widmaier EP. Adrenocortical responses to ACTH in neonatal rats: effect of hypoxia from birth on corticosterone, StAR, and PBR. Am J Physiol Regul Integr Comp Physiol. 2003;284:R78–R85. doi: 10.1152/ajpregu.00501.2002. [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Romero EM, Fernandez B, Sagredo O, Gomez N, Uriguen L, Guaza C, et al. Antinociceptive, behavioural and neuroendocrine effects of CP 55,940 in young rats. Brain Res Dev Brain Res. 2002;136:85–92. doi: 10.1016/s0165-3806(02)00306-1. [DOI] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saario SM, Laitinen JT. Therapeutic potential of endocannabinoid-hydrolysing enzyme inhibitors. Basic Clin Pharmacol Toxicol. 2007;101:287–293. doi: 10.1111/j.1742-7843.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 2011;64:226–234. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, et al. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol. 2004;16:516–524. doi: 10.1111/j.1365-2826.2004.01195.x. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol. 2007;578:773–785. doi: 10.1113/jphysiol.2006.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Uriguen L, Garcia-Gutierrez MS, Manzanares J. Decreased GABAA and GABAB receptor functional activity in cannabinoid CB1 receptor knockout mice. J Psychopharmacol. 2011;25:105–110. doi: 10.1177/0269881109358204. [DOI] [PubMed] [Google Scholar]
- Van Gaal L, Pi-Sunyer X, Despres JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31(Suppl 2):S229–S240. doi: 10.2337/dc08-s258. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, Lopez-Gallardo M, de Fonseca FR. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J Psychopharmacol. 2012;26:164–176. doi: 10.1177/0269881111408956. [DOI] [PubMed] [Google Scholar]
- Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol. 2012;26:56–70. doi: 10.1177/0269881111409606. [DOI] [PMC free article] [PubMed] [Google Scholar]