Abstract
In the mammalian brain, the α7 nicotinic and NMDA receptor antagonist kynurenic acid is synthesized by irreversible enzymatic transamination of the tryptophan metabolite l-kynure-nine. d-kynurenine, too, serves as a bioprecursor of kynurenic acid in several organs including the brain, but the conversion is reportedly catalyzed through oxidative deamination by d-aminoacid oxidase. Using brain and liver tissue homogenates from rats and humans, and conventional incubation conditions for kynurenine aminotransferases, we show here that kynurenic acid production from d-kynurenine, like the more efficient kynurenic acid synthesis from l-kynurenine, is blocked by the aminotransferase inhibitor amino-oxyacetic acid. In vivo, focal application of 100 µM d-kynurenine by reverse microdialysis led to a steady rise in extracellular kynurenic acid in the rat striatum, causing a 4-fold elevation after 2 h. Attesting to functional significance, this increase was accompanied by a 36% reduction in extracellular dopamine. Both of these effects were duplicated by perfusion of 2 µM l-kynurenine. Co-infusion of amino-oxyacetic acid (2 mM) significantly attenuated the in vivo effects of d-kynurenine and essentially eliminated the effects of l-kynurenine. Thus, enzymatic transamination accounts in part for kynurenic acid synthesis from d-kynurenine in the brain. These results are discussed with regard to implications for brain physiology and pathology.
Keywords: α7 nicotinic receptor, aminotransferases, dopamine, microdialysis, NMDA receptor, schizophrenia
Kynurenic acid (KYNA), a neuroactive metabolite of the kynurenine pathway of tryptophan degradation with antiox-idant properties (Lugo-Huitrón et al. 2011), is synthesized by irreversible transamination of the pivotal kynurenine pathway metabolite l-kynurenine (l-KYN). In the mammalian brain, as in the periphery, this reaction is catalyzed by several well-characterized aminotransferases. In the context of the study of KYNA biology, these enzymes are now commonly referred to as kynurenine aminotransferases (KATs), though this terminology does not necessarily imply a strict preference for kynurenine as a substrate in a biological environment (Okuno et al. 1991; Cooper 2004; Guidetti et al. 2007a; Han et al. 2010). In mammals, KAT II is responsible for the majority of KYNA neosynthesis from l-KYN under physiological conditions (Guidetti et al. 2007a).
Originally described as a broad spectrum antagonist of excitatory amino acid receptors (Perkins and Stone 1982), KYNA is now known to inhibit the NMDA receptor (Ganong et al. 1983), and especially its glycine co-agonist site (Kessler et al. 1989), preferentially. In addition, KYNA also acts as an antagonist of the α7 nicotinic acetylcholine receptor (α7nAChR; Hilmas et al. 2001) and, as discovered most recently, stimulates the orphan G-protein coupled receptor GPR-35 (Wang et al. 2006). Hypothetically, endogenous KYNA might therefore target each of these receptors, separately or jointly, to mediate physiological effects in the mammalian brain. However, most studies to date favor the α7nAChR as KYNA’s preferential target in the brain in vivo. For example, the KYNA-induced reduction in extracellular dopamine in the rat striatum can be prevented by co-administration of a α7nAChR agonist and duplicated by a selective α7nAChR antagonist (Rassoulpour et al. 2005; Wu et al. 2007). Evidence for a functional role of endogenous KYNA in the brain also comes from studies showing bi-directional regulation of extracellular glutamate and related behavioral events by fluctuations in brain KYNA levels (Carpenedo et al. 2001; Wu et al. 2010; Pocivavsek et al. 2011). Notably, these phenomena may play a role in the pathophysiology of neurodegenerative diseases and schizophrenia, which present with abnormal brain KYNA metabolism (Beal et al. 1991; Ogawa et al. 1992; Baran et al. 1999; Sathyasaikumar et al. 2011).
Whereas KYNA does not contain an asymmetric carbon atom and therefore has no stereoisomers, its bioprecursor kynurenine exists in two enantiomeric forms. Interestingly, in vitro and in vivo studies in rats and rabbits have shown that d-KYN, like l-KYN, is converted to KYNA in peripheral tissues (Mason and Berg 1952; Loh and Berg 1971), and that the concentration of KYNA in plasma is elevated after systemic d-KYN administration in rats (Fukushima et al. 2009). Although transamination was originally proposed as the enzymatic mechanism involved (Mason and Berg 1952), more recent studies provided credible evidence that the conversion of d-KYN to KYNA is instead performed by d-aminoacid oxidase (d-AAO), an enzyme catalyzing the oxidative deamination of its substrate(s) (Loh and Berg 1971; Fukushima et al. 2009; Ishii et al. 2010).
Because of the recent surge in interest in the neurobiology of KYNA, we and others began to ask the question if d-KYN also serves as a bioprecursor of KYNA in the CNS (Amori et al. 2009; Pérez-De La Cruz et al. 2009; Ogaya et al. 2010) and, if KYNA production from d-KYN in the brain can be verified, to identify the biosynthetic mechanism(s) involved. In the present study, we decided to address this issue in tissue homogenates from rats and humans, and by in vivo microdialysis in the rat striatum, in most cases comparing the effects of d-KYN and l-KYN side-by-side. Both approaches focused specifically on the possible catalytic role of KAT rather than d-AAO, and the microdialysis experiments also included an investigation of the effects of newly produced KYNA on extracellular dopamine levels. Our results revealed that d-KYN can indeed be enzymatically transaminated to KYNA in both rat and human tissues, that KAT is partly responsible for the de novo formation of KYNA from d-KYN in the brain in vivo, and that KYNA produced from d-KYN is likely functionally identical to KYNA produced from l-KYN.
Materials and methods
Animals
Adult, male Sprague–Dawley rats (250–300 g; Charles-River Laboratories, Kingston, NY, USA) were used for this study. All animals were housed in a temperature-controlled, Association for Assessment and Accreditation of Laboratory Animal Care (AA-ALAC)-approved animal facility and were kept on a 12 h/12 h-light/dark cycle with free access to food and water.
Human tissue
Human brain and liver samples (kept frozen at −80°C until use) were provided by the Maryland Brain Collection, a tissue repository housed at the Maryland Psychiatric Research Center and maintained in collaboration with the Office of the Medical Examiner of the State of Maryland. Tissues (post-mortem interval ≤ 22 h) were obtained from individuals without a history of neurological or psychiatric disorders.
Chemicals
Kynurenic acid (KYNA), d-kynurenine (D-KYN; 98% pure, containing less than 0.05% l-kynurenine; Ogaya et al. 2010; X. Wang, unpublished data), amino-oxyacetic acid (AOAA) and pyridoxal-5’-phosphate were obtained from Sigma (St Louis, MO, USA). L-Kynurenine sulfate (l-KYN; purity: 99.4%), was purchased from Sai Advantium (Hyderabad, India). All other biochemicals and chemicals were acquired from various commercial suppliers and were of the highest available purity.
In vitro studies
Animals were killed (CO2), and their brain and liver were rapidly dissected out, frozen on dry ice and weighed. Human brain and liver were weighed in the frozen state.
After thawing, tissues were homogenized [1 : 10 (w/v) for brain, 1 : 300 (w/v) for liver] in 5 mM Tris-acetate buffer (pH 8.0) containing 50 µM pyridoxal-5’-phosphate and 10 mM 2-mercaptoethanol. Routinely, 80 µL of the homogenate were then incubated at 37°C for 2 h with d-KYN or l-KYN using 150 mM Tris-acetate buffer (pH 7.4) containing 1 mM pyruvate and 80 µM pyridoxal-5’-phosphate, in a total volume of 200 µL. Tissue dilutions and incubation times were varied in pilot experiments. Where indicated, AOAA (1 mM), a non-specific aminotransferase inhibitor, was added to the incubation mixture. The reaction was terminated by the addition of 20 µL of 50% (w/v) trichloroacetic acid and 1 mL of 0.1 M HCl. After centrifugation (16 000 g, 10 min), newly produced KYNA was determined in 20 µL of the supernatant. Blanks were obtained by measuring the KYNA content of the tissues used (i.e. tissues incubated in the absence of d-KYN or l-KYN) and the KYNA content of a buffer solution containing d-KYN or l-KYN.
In vivo microdialysis
Rats were anesthetized with chloral hydrate (360 mg/kg, i.p.) and placed in a stereotaxic frame (David Kopf, Tujunga, CA, USA). A guide cannula was positioned on top of the striatum (AP: 1.0 mm anterior to bregma, L: 2.5 mm from the midline, V: 3.0 mm below the dura) and secured to the skull with stainless steel screws and acrylic dental cement. On the next day, a microdialysis probe (CMA/ 10, membrane length: 3 mm, Carnegie Medicine, Stockholm, Sweden), extending 3 mm through the striatum, was inserted and connected to a microperfusion pump set to speed of 1 µL/min. The freely moving animals were then perfused with Ringer solution (pH 6.7) containing 144 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4 and 1.7 mM CaCl2. After the establishment of a stable baseline, test compounds were applied by reverse dialysis for 2 h. Subsequently, perfusion with Ringer solution continued for 4 h. Microdialysis samples were collected every 30 min. Extracellular KYNA and dopamine were determined in the same samples, and data were not corrected for recovery from the microdialysis probe (Wu et al. 2007).
KYNA determination
KYNA was determined by HPLC. Briefly, 20 µL of the supernatant recovered in the in vitro experiments (see above) or 15 µL of the microdialysate were directly applied to a 3-µm C18 reverse phase column (80 × 4.6 mm; ESA, Chelmsford, MA, USA), and KYNA was isocratically eluted using a mobile phase containing 250 mM zinc acetate, 50 mM sodium acetate and 3% acetonitrile (pH 6.2) at a flow rate of 1 mL/min. In the eluate, KYNA was detected fluorimetrically (excitation wavelength: 344 nm; emission wavelength: 398 nm; S200 fluorescence detector; Perkin-Elmer, Waltham, MA, USA). The retention time of KYNA under these conditions was ~7 min. In pilot experiments, the identity of de novo produced KYNA was also tested using a mobile phase containing 4% acetonitrile under otherwise identical conditions. This reduced the retention time of KYNA to ~5 min.
Dopamine determination
Dopamine was measured in microdialysis samples using HPLC analysis with electrochemical detection. To this end, 15 µL of the microdialysate were acidified with 10 µL 0.1 N HCl, and 15 µL of the resulting mixture were injected onto a PP-ODS column (30 × 4.6 mm; Eicom Corp., San Diego, CA, USA). Dopamine was eluted with a mobile phase containing 0.1 M sodium phosphate buffer (pH 6.0), 500 mg/L sodium dodecyl sulfate, 50 mg/L EDTA and 1% methanol at a flow rate of 0.5 mL/min and detected at +450 mV (HTEC 500; Eicom Corp.). The retention time of dopamine was ~2 min.
Data analysis
The effects of AOAA and temperature on KYNA formation in vitro were analyzed by Student’s t-test.
In microdialysis experiments, the average of four samples collected immediately prior to the administration of test compounds was defined as the baseline value (100%). Levels of the analytes (KYNA and dopamine) in these samples did not differ from each other by more than 10%. The effects of d-KYN and l-KYN, in the presence or absence of AOAA, were analyzed by two-way anova followed by Bonferroni’s post hoc test. The area under the curve (AUC) was calculated from time 2–8 h for each rat, and Student’s t-test was used to compare AUCs.
In all cases, a p-value < 0.05 was considered significant.
Results
KYNA production from d-KYN in the rat forebrain in vitro
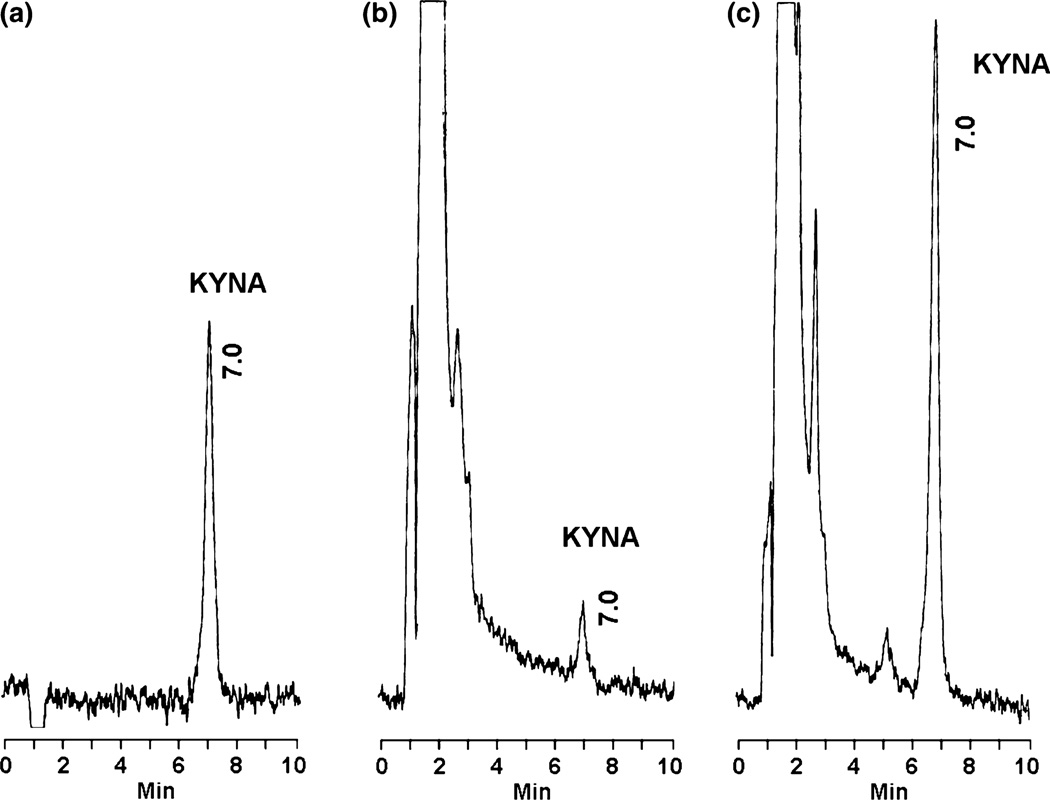
In pilot experiments, we tested the production of KYNA from d-KYN in rat forebrain tissue homogenate, using optimal assay conditions for the study of enzymatic KYNA synthesis from l-KYN in the rat brain in vitro (Okuno et al. 1991). Using 100 µM d-KYN as a substrate, de novo formation of KYNA was observed in the eluate from the HPLC column after a 2-h incubation period. The identity of the newly formed substance with both standard KYNA and endogenous brain KYNA was verified using both the mobile phase used routinely, containing 3% acetonitrile (Fig. 1), and a mobile phase containing 4% acetonitrile (data not shown).
Fig. 1.
Chromatographic profiles of KYNA production from d-KYN. (a) KYNA standard (100 fmoles); (b, c) KYNA in rat forebrain tissue homogenate incubated in the absence (b) or presence (c) of 100 µM d-KYN for 2 h at 37°C (see text for experimental details).
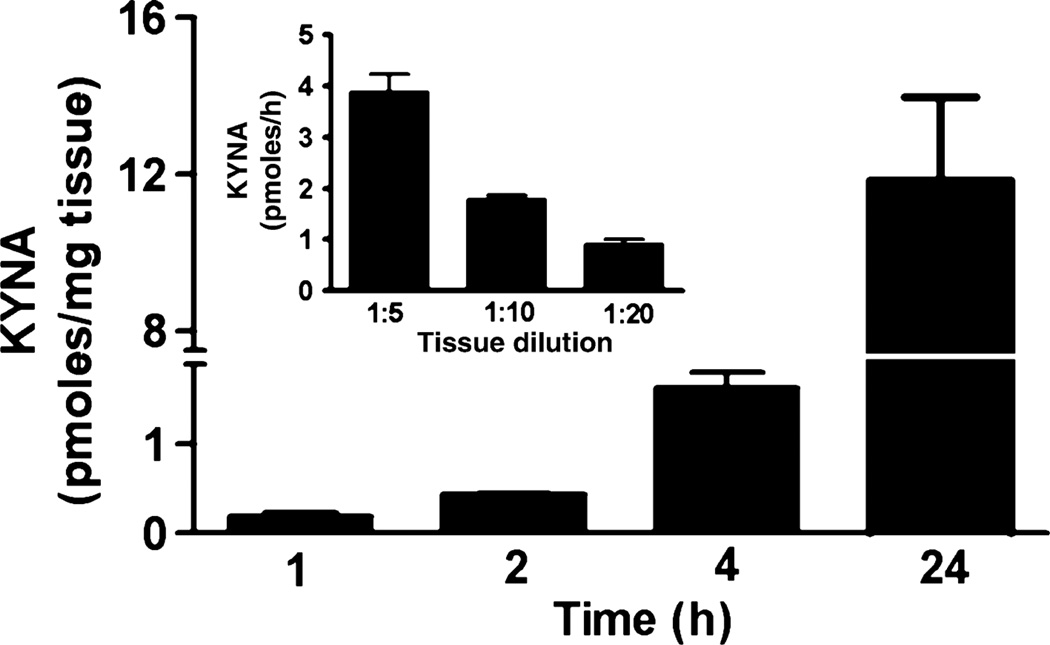
To characterize the conversion of d-KYN to KYNA in the rat brain, we first determined the time-, tissue concentration-and temperature dependency of the process, varying one parameter at a time and maintaining the routine incubation conditions otherwise. These experiments revealed that the neosynthesis of KYNA from d-KYN was linear up to 24 h (Fig. 2), decreased proportionally with increasing tissue dilution (Fig. 2, inset), and was reduced by 85.4 ± 0.8% compared with 37°C when the temperature of the incubation medium was lowered to 20°C (p < 0.05 vs. 37°C, n = 3; data not shown).
Fig. 2.
Effects of incubation time on KYNA production from d-KYN in rat forebrain homogenate. Tissue was incubated with 100 µM d-KYN. Data are the mean ± SEM of three experiments. Inset: effects of tissue dilution on KYNA production from d-KYN. Rat forebrain was homogenized (w/v) as indicated. Data are the mean ± SEM of three experiments.
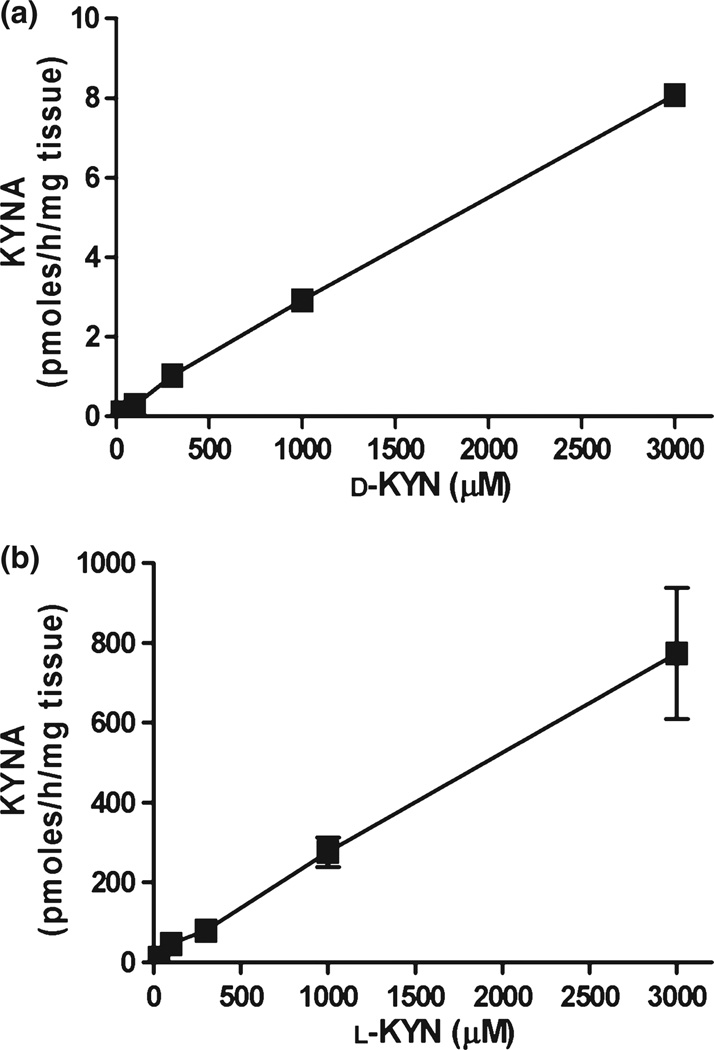
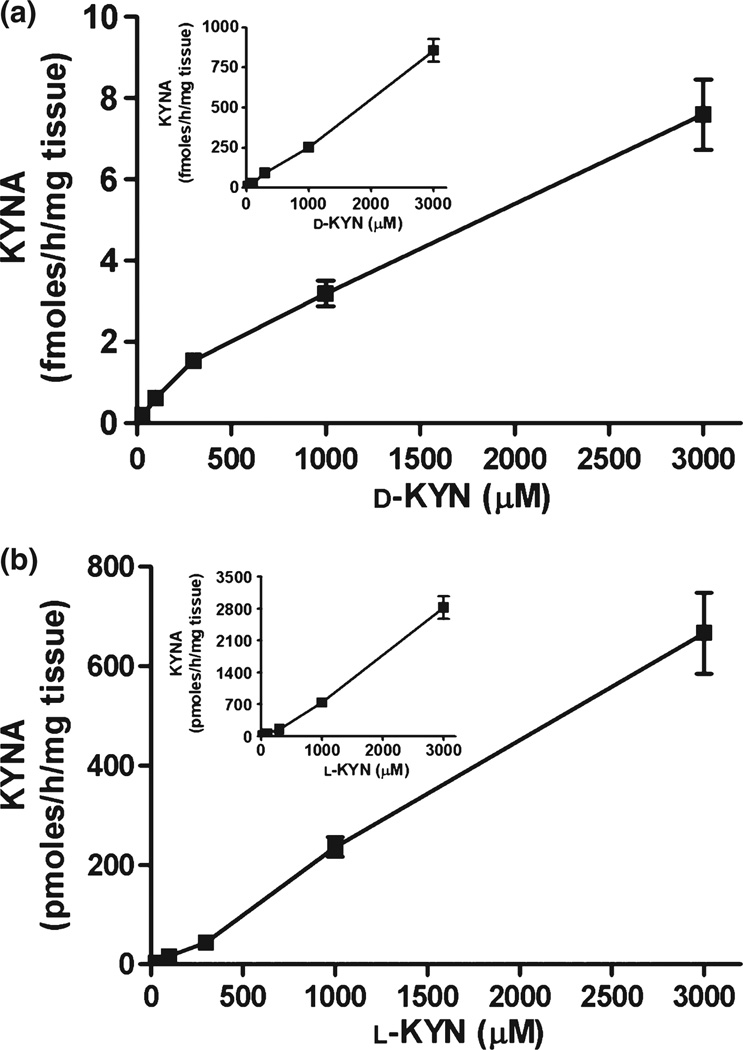
After defining standard assay conditions for brain tissue homogenate, that is, 2-h incubation of tissue homogenate diluted 1 : 10 (w/v) at 37°C in conventional buffer used for KAT assays (Okuno et al. 1991), we compared KYNA formation from d-KYN and l-KYN over a wide concentration range (30 µM to 3 mM). Concentration-dependent KYNA production was seen for both enantiomers, with l-KYN being far more potent than d-KYN (Fig. 3a and b and Table 1). Specifically, l-KYN was 100–200 times and ~70 times more effective than d-KYN in producing KYNA in forebrain and liver, respectively.
Fig. 3.
Concentration-dependency of KYNA production from d-KYN (a) and l-KYN (b) in rat forebrain tissue homogenate under standard incubation conditions, as detailed in the text. Data are from three rats and are the mean ± SEM.
Table 1.
In vitro production of KYNA from d-KYN and l-KYN in rat forebrain and liver: effect of AOAA. Tissue homogenates (n = 3 per group) were incubated for 2 h at 37°C with 100 µM d-KYN or l-KYN in the absence or presence of AOAA (1 mM). Data are the mean ± SEM
| KYNA (pmoles/h/mg tissue) | d-KYN | d-KYN + AOAA | % inhibition | l-KYN | l-KYN + AOAA | % inhibition |
|---|---|---|---|---|---|---|
| Forebrain | 0.26 ± 0.01 | 0.03 ± 0.01 | 88.1 | 67.52 ± 4.07 | 0.26 ± 0.03 | 99.6 |
| Liver | 22.76 ± 3.79 | 0.46 ± 0.19 | 97.7 | 1635.27 ± 217.81 | 18.55 ± 0.32 | 98.8 |
Effect of AOAA on the de novo synthesis of KYNA in rat forebrain and liver in vitro
We next tested the ability of AOAA, a compound that blocks the activity of aminotransferases non-specifically by attacking the Schiff base linkage between the obligatory co-factor pyridoxal-5’-phosphate and the enzyme (Beeler and Churchich 1976), to inhibit KYNA production from d-KYN and l-KYN in rat tissue homogenates (Table 1). Addition of 1 mM AOAA to the incubation mixture containing 100 µM of d-KYN or l-KYN prevented the neosynthesis of KYNA in rat liver homogenate (each p < 0.05 vs. control). Similarly, 1 mM AOAA abolished the formation of KYNA from l-KYN and d-KYN in the forebrain (each p < 0.05 vs. control). Thus, under in vitro conditions that are optimized for the measurement of KAT activity in rat tissues, essentially all KYNA synthesis from both d-KYN and l-KYN is catalyzed by KATs.
Effect of d-KYN and l-KYN on extracellular KYNA and dopamine in the rat striatum in vivo
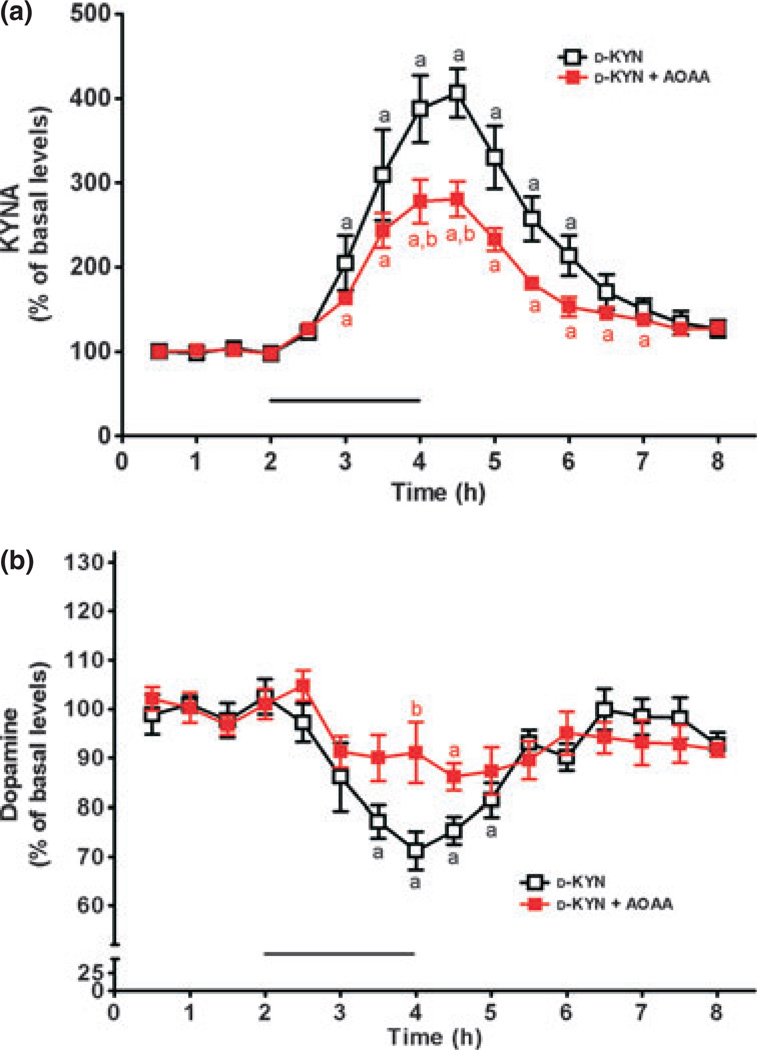
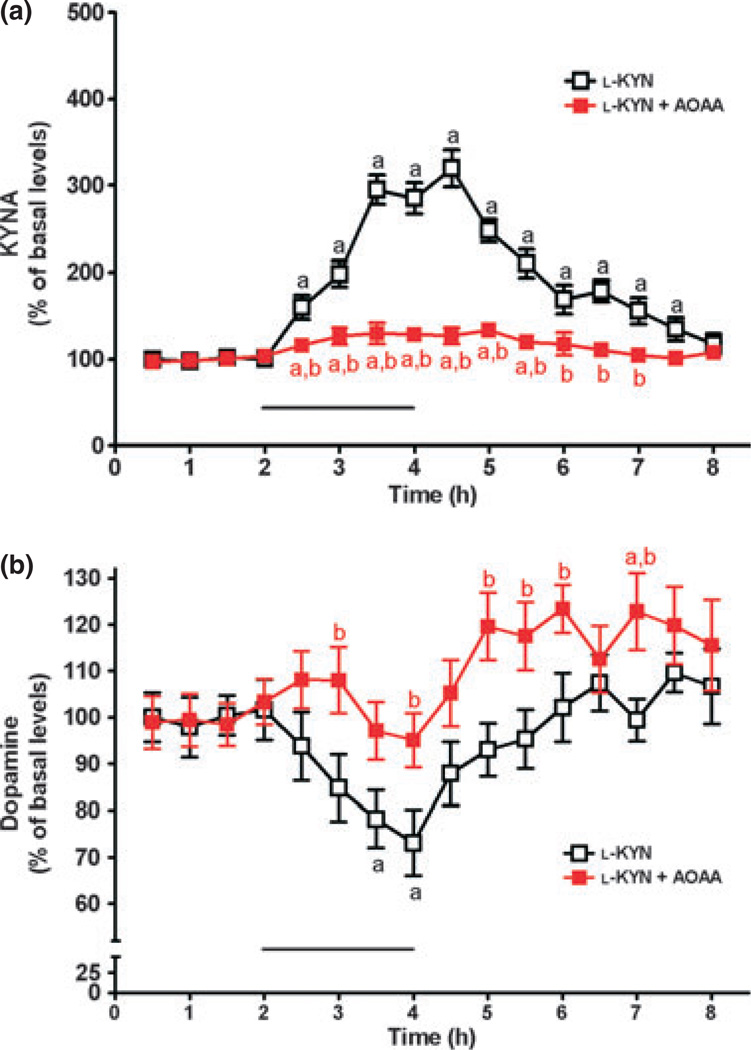
Microdialysis was performed to investigate the conversion of d-KYN or l-KYN to KYNA in vivo, and to simultaneously assess possible effects on extracellular dopamine levels (cf. Rassoulpour et al. 2005). To this end, the precursors were applied for 2 h by reverse dialysis, and the content of both KYNA and dopamine was monitored in microdialysates for a total of 6 h after baseline measurements. Basal levels, collated from all animals used in the microdialysis experiments (n = 23), were 2.6 ± 0.1 and 2.4 ± 0.1 nM for KYNA and dopamine, respectively. Perfusion with 100 µM d-KYN significantly raised extracellular KYNA levels (p < 0.05 vs. basal levels; n = 8), reaching a maximum of 406 ± 29% of baseline values between 2 and 3 h after the beginning of the d-KYN application. KYNA levels then gradually returned to baseline values (Fig. 4a). In the same samples, dopamine levels were significantly reduced (nadir: 64 ± 5% of basal values after 1.5 h; p < 0.05), before returning to basal values 3.5 h after the beginning of the d-KYN infusion (Fig. 4b).
Fig. 4.
Effect of reverse dialysis of d-KYN (100 µM; bar) on extracellular KYNA (a) and dopamine (b) levels in the rat striatum. d-KYN was infused alone (open symbols, n = 8) or together with AOAA (2 mM; red symbols, n = 7). KYNA and dopamine were measured in the same microdialysate samples. See text for absolute baseline values. Data are the mean ± SEM. ap < 0.05 vs. baseline; bp < 0.05 vs. d-KYN alone (two-way anova followed by Bonferroni’s post hoc test).
In separate rats, AOAA (2 mM) was co-applied with 100 µM d-KYN. As illustrated in Fig. 4a, co-infusion of d-KYN and AOAA caused a maximal increase of 280% compared with baseline KYNA levels (p < 0.05; n = 7). Measured in the same striatal microdialysates, dopamine levels were reduced from baseline values, reaching statistical significance after 2.5 h (p < 0.05). Expressed differently, that is, in comparison to the effects of 100 µM d-KYN alone, co-perfusion of d-KYN + AOAA reduced the AUC of KYNA by 39% (p < 0.05) and the AUC of dopamine by 34% (p = 0.226) (Fig. 4a and b). Thus, in contrast to the results obtained in vitro (Table 1), AOAA clearly attenuated, but failed to abolish, the neosynthesis of KYNA from d-KYN in vivo.
Identical experiments were conducted using intrastriatal dialysis of l-KYN (2 µM), alone or in conjunction with AOAA (2 mM) (n = 4 each). The lower concentration of l-KYN was used to approximate the effects of the two enantiomers on KYNA in quantitative terms (cf. Wu et al. 2007). As illustrated in Fig. 5a and b, respectively, administration of l-KYN raised extracellular KYNA levels to a maximum of 320 ± 21% of baseline values by 2.5 h and reduced extracellular dopamine levels correspondingly to a nadir of 73 ± 7% of basal values (p < 0.05 each). The effects of l-KYN on both analytes then gradually decreased over time, and both KYNA and dopamine levels eventually returned to baseline values after the l-KYN infusion was terminated (Fig. 5a and b).
Fig. 5.
Effect of reverse dialysis of l-KYN (2 µM; bar) on extracellular KYNA (a) and dopamine (b) levels in the rat striatum. l-KYN was infused alone (open symbols) or together with AOAA (2 mM; red symbols). KYNA and dopamine were measured in the same microdialysate samples. See text for absolute baseline values. Data are the mean ± SEM (n = 4 per group). ap < 0.05 vs. baseline; bp < 0.05 vs. l-KYN alone (two-way anova followed by Bonferroni’s post hoc test).
As shown in Fig. 5a, co-perfusion of AOAA (2 mM) did not entirely abolish the rise in KYNA levels caused by 2 µM l-KYN, as modest increases compared with baseline values were seen for 3.5 h (p < 0.05). However, measured in the same dialysate samples, AOAA completely blocked the l-KYN-induced reduction in extracellular dopamine (p > 0.05 vs. baseline; Fig. 5b). Comparison of the AUCs corresponding to the effects of l-KYN alone and l-KYN + AOAA revealed that co-perfusion of AOAA inhibited KYNA production from l-KYN by 82% (p < 0.05) and prevented the l-KYN-induced reduction in extracellular dopamine (p < 0.05) (Fig. 5a and b).
De novo synthesis of KYNA in human cortex and liver in vitro: effect of AOAA
The final set of experiments was designed to examine and characterize the conversion of d-KYN to KYNA in human brain and liver. To this end, tissue homogenates were incubated under the same assay conditions used for the study of rat tissues, using different concentrations of d-KYN (30 µM to 3 mM) (Fig. 6a). In both tissues, d-KYN was converted to KYNA in a dose-dependent manner up to the highest concentration used. KYNA production was approximately 50 times more efficient in liver than in cortex (Fig. 6a inset). For comparison, the effects of l-KYN were determined in parallel experiments using the same concentration range. As in rat tissues (see Fig. 3 and Table 1), l-KYN was more effective than d-KYN as a precursor of KYNA, exceeding the potency of its enantiomer by 50- and 3-fold in cortex and liver, respectively (Fig. 6b and inset, and Table 2).
Fig. 6.
Concentration-dependency of KYNA production from d-KYN (a) and l-KYN (b) in human cortex and liver (insets). Tissues were incubated for 2 h at 37°C, as described in the text. Data are the mean ± SEM (n = 3–5).
Table 2.
In vitro production of KYNA from d-KYN and l-KYN in human cortex and liver: effect of AOAA. Tissue homogenates were incubated for 2 h at 37°C with 100 µM d-KYN or l-KYN in the absence or presence of AOAA (1 mM). Data are the mean ± SEM (n = 3–5)
| KYNA (pmoles/h/mg tissue) | d-KYN | d-KYN + AOAA | % inhibition | l-KYN | l-KYN + AOAA | % inhibition |
|---|---|---|---|---|---|---|
| Cortex | 0.61 ± 0.09 | 0.14 ± 0.03 | 69.9 | 16.20 ± 2.78 | 0.36 ± 0.13 | 97.3 |
| Liver | 27.58 ± 0.84 | 7.91 ± 2.92 | 71.1 | 60.15 ± 5.17 | 1.20 ± 0.68 | 98.1 |
As shown in Table 2, addition of AOAA (1 mM) to the incubation mixture containing 100 µM of d-KYN reduced KYNA formation by 69.9 ± 10.1% and 71.1 ± 11.2% in human cortex and liver, respectively (each p < 0.05 vs. control). In both tissues, AOAA also abolished KYNA production from l-KYN (by 97.3 ± 1.5% and 98.1 ± 1.0% in cortex and liver, respectively; each p < 0.05 vs. control).
Discussion
The studies described here were designed to characterize the conversion of d-KYN, the enantiomer of the pivotal kynurenine pathway metabolite l-KYN, to KYNA in the brain, at times using liver tissue for comparative purposes. Our results revealed not only that the rat brain has the capacity to generate KYNA when exposed to d-KYN, confirming a recent report by Ogaya et al. (2010), but demonstrated that the conversion can also take place in human brain tissue. Moreover, we showed for the first time, by in vivo microdialysis in the rat striatum, that KYNA which was newly formed from d-KYN was functionally significant, causing a reduction in extracellular dopamine in the same manner as l-KYN-derived KYNA (Wu et al. 2007). Finally, by using the aminotransferase inhibitor AOAA as an experimental tool, we demonstrated that enzymatic transamination accounts in part for KYNA synthesis from d-KYN in the mammalian brain. Because of the increasingly appreciated role of KYNA as a neuromodulator involved in physiology and pathology (see Introduction), these results have implications for both basic and clinical neurobiology.
Although d-amino acids were frequently studied alongside their l-isomers in the early years of biochemical research (see Berg 1953; for review) and are present to various degrees in higher organisms, only a few are currently believed to carry out important biological functions in mammals. Of these, d-serine has undoubtedly gained most prominence, especially due to its significant role in the brain (Wolosker et al. 2008), but there is also credible evidence for biological roles of d-aspartate and d-alanine in mammalian brain and peripheral organs (Errico et al. 2009; Hamase et al. 2009; Miyoshi et al. 2011). No such role has so far been attributed to d-tryptophan. However, in the context of the present study it is noteworthy that exogenously provided d-tryptophan can be readily transformed into KYNA – including brain KYNA – in mammals (Loh and Berg 1971; Triebwasser et al. 1976; Ishii et al. 2010, 2011). Although this process may alternatively involve the enzymatic inversion of d-tryptophan to l-tryptophan by d-AAO and subsequent sequestration to l-KYN and further to KYNA (Loh and Berg 1971), it is more likely that d-KYN serves as an intermediate (Ishii et al. 2010). Indeed, the production of d-KYN from exogenously supplied d-tryptophan in rabbits and rats was shown several decades ago (Kotake and Ito 1937; Mason and Berg 1952; Higuchi and Hayaishi 1967) and has been verified (Langner and Berg 1955) or implied (Hankes et al. 1972) in humans as well. Although definitive studies are still outstanding, this conversion must begin with the oxidative opening of the indole ring of d-tryptophan by either tryptophan-2,3-dioxy-genase (formerly tryptophan pyrrolase) or indoleamine-2,3-dioxygenase, two enzymes that recognize either l- or d-tryptophan as a substrate, albeit with different kinetic characteristics (Watanabe et al. 1980; Capece et al. 2010). So far, however, and possibly because of analytical limitations, the presence of endogenous d-KYN in mammalian tissues has not been reported.
The irreversible transamination of l-KYN to KYNA in brain and liver, catalyzed by KATs, has been thoroughly investigated by us and others, and the functional role of l-KYN-derived KYNA in the brain is increasingly understood and appreciated (see Introduction). In our attempt to evaluate the stereospecificity of this enzymatic transamination in vitro, we optimized the incubation conditions, specifically by the addition of the obligatory co-factor pyridoxal-5′-phosphate and the co-substrate pyruvate (Snell and Jenkins 1959; Fonnum et al. 1964). Also supported by the effective inhibition afforded by AOAA, which forms a Schiff base with pyridoxal-5′-phosphate (Beeler and Churchich 1976), these experiments provided clear evidence for an aminotransferase-catalyzed formation of KYNA from d-KYN in both brain and liver. Inversion of d-KYN to l-KYN, a possible intermediate step in this process, has not been reported so far and is also ruled out by our recent studies in which the efficient conversion of d-KYN to KYNA was shown using purified KAT preparations (manuscript in preparation).
Our microdialysis experiments revealed, importantly, that transamination of d-KYN was not limited to a situation favoring optimal conditions in vitro. Similar to the report of Ogaya et al. (2010), who recently demonstrated the de novo formation of KYNA from d-KYN in the rat prefrontal cortex, we found that d-KYN was approximately 30 times less potent than l-KYN as a bioprecursor of KYNA in the rat striatum. The effect of d-KYN was partially inhibited (by 39%) by the inclusion of AOAA in the perfusion solution, whereas the production of KYNA from l-KYN was almost totally abolished in the presence of the transaminase inhibitor (Figs 4a and 5a). Together with our preliminary observation that the conversion of d-KYN to KYNA is substantially attenuated in mice lacking KAT II (Amori et al. 2009), these results leave no doubt that enzymatic transamination plays a significant part in the cerebral biosynthesis of KYNA from both enantiomers of kynurenine under physiological conditions. In the case of d-KYN, however, an additional enzymatic mechanism, namely oxidative deamination by d-AAO, is involved in the process, accounting in part for the de novo formation of KYNA in the brain in vivo (Ishii et al. 2010; Ogaya et al. 2010). Experiments currently in progress are designed to evaluate the relative contributions of the two biosynthetic routes in the healthy and diseased brain using pharmacological and genetic tools.
The reduction in extracellular dopamine seen in the striatum following perfusion with d-KYN attests to the functional significance of d-KYN as a bioprecursor of KYNA in the brain in vivo and deserves special consideration. KYNA formation induced by l-KYN (Wu et al. 2007) or by a diet high in tryptophan (Okuno et al. 2011) leads to very similar reductions in extracellular dopamine. This effect of l-KYN, which was replicated in the course of the present study (Fig. 5b), has been traced to a sequence of events involving the release of KYNA from astrocytes (which harbor KAT II; Guidetti et al. 2007b), reduced glutamate release following the inhibition of pre-synaptic a7nAChRs by KYNA and, finally, diminished activation of glutamate receptors on dopaminergic nerve terminals (Wu and Schwarcz 2009). As d-KYN likely enters astrocytes using the same transporter as l-KYN (Speciale et al. 1989), and as astrocytes also contain d-AAO (Moreno et al. 1999), it is possible that the same cascade, including the initial synthesis of KYNA in astrocytes, also operates when striatal tissue is exposed to d-KYN. Alternative explanations for the d-KYN-induced reduction in striatal dopamine described here cannot be ruled out without further experimentation, however. Yet regardless of the precise mechanisms involved, the present results raise the possibility that d-KYN may also duplicate several other neurochemical and behavioral phenomena that are triggered by l-KYN (Nilsson et al. 2006; Chess et al. 2009; Pocivavsek et al. 2011; Trecartin and Bucci 2011).
The presence of endogenous d-KYN in mammalian tissues has not been documented so far (see above), but is, in fact, not unlikely. d-tryptophan, which has been identified in human urine (Zhao and Liu 2001), is neosynthesized by microorganisms (Lam et al. 2009) and may thus gain access to higher animals for further sequestration to d-KYN by tryptophan-2,3-dioxygenase and/or indoleamine-2,3-dioxy-genase in the gut or elsewhere in the body, as described earlier. In light of the abundance of infiltrating microorganisms in the digestive tract (Kaplan et al. 2011), this process probably occurs under physiological conditions but may not be of substantive biological relevance. In contrast, d-KYN levels can be envisioned to be elevated as a consequence of microbial infections, especially since the challenges to the host immune system are consistently associated with a substantial up-regulation of indoleamine-2,3-dioxygenase (Johnson et al. 2009; Prendergast et al. 2011). d-KYN may therefore be at least in part responsible for the significant increases in brain KYNA levels seen under inflammatory conditions (Heyes and Lackner 1990; Holtze et al. 2008).
These considerations may be relevant for the pathophysiology of depressive disorders (O’Connor et al. 2009; Laugeray et al. 2010) and may be of special interest for schizophrenia research. Thus, maternal infections are increasingly recognized as a major risk factor in the disease (Brown et al. 2005; Mortensen et al. 2007; Pedersen et al. 2011), and there is evidence for genetic anomalies in kynurenine pathway enzymes, including tryptophan-2,3-dioxygenase (Miller et al. 2004, 2006; Aoyama et al. 2006; Wonodi et al. 2011). Together, these risk factors may promote the production of d-KYN, eventually increasing the level of KYNA in brain and cerebrospinal fluid (Erhardt et al. 2001; Schwarcz et al. 2001) and thus contributing to pathological downstream events (see Introduction). As also pointed out in recent studies by Fukushima and his collaborators (Ogaya et al. 2010; Ishii et al. 2011), these events may be further exacerbated in persons with a genetic defect leading to enhanced d-AAO activity (Verrall et al. 2007; Burnet et al. 2008; Madeira et al. 2008), because these individuals would have an increased ability to convert d-KYN to KYNA.
Acknowledgements
This work was supported in part by USPHS grant NS057715. We are grateful to Dr Robert McMahon for his assistance with statistical analyses.
Abbreviations used
- 3-HK
3-hydroxykynurenine
- AOAA
amino-oxyacetic acid
- AUC
area under the curve
- d-AAO
d-aminoacid oxidase
- d-KYN
d-kynurenine
- KAT
kynurenine aminotransferase
- KYNA
kynurenic acid
- l-KYN
l-kynurenine
- α7nAChR
α7 nicotinic acetylcholine receptor
References
- Amori L, Pérez-De La Cruz V, Wu H-Q, Notarangelo FM, Schwarcz R. Conversion of D-kynurenine to kynurenic acid in rodent brain and liver. Soc. Neurosci. Abstr. 2009;34:34–11. [Google Scholar]
- Aoyama N, Takahashi N, Saito S, et al. Association study between kynurenine 3-monooxygenase gene and schizophrenia in the Japanese population. Genes, Brain Behav. 2006;5:364–368. doi: 10.1111/j.1601-183X.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer’s disease. J. Neural Transm. 1999;106:165–181. doi: 10.1007/s007020050149. [DOI] [PubMed] [Google Scholar]
- Beal MF, Swartz KJ, Hyman BT, Storey E, Finn SF, Koroshetz W. Aminooxyacetic acid results in excitotoxin lesions by a novel indirect mechanism. J. Neurochem. 1991;57:1068–1073. doi: 10.1111/j.1471-4159.1991.tb08258.x. [DOI] [PubMed] [Google Scholar]
- Beeler T, Churschich JE. Reactivity of the phosphopyridoxal groups of cystathionase. J. Biol. Chem. 1976;251:5267–5271. [PubMed] [Google Scholar]
- Berg CP. Physiology of the D-amino acids. Physiol. Rev. 1953;33:145–189. doi: 10.1152/physrev.1953.33.2.145. [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am. J. Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Bristow GC, Godlewska BR, Sikka P, Walker M, Harrison PJ. D-amino acid oxidase activity and expression are increased in schizophrenia. Mol. Psychiatry. 2008;13:658–660. doi: 10.1038/mp.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capece L, Arrar M, Roitberg AE, Yeh SR, Marti MA, Estrin DA. Substrate stereo-specificity in tryptophan dioxygenase and indoleamine 2,3-dioxygenase. Proteins. 2010;78:2961–2972. doi: 10.1002/prot.22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, Moroni F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur. J. Neurosci. 2001;13:2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav. Brain Res. 2009;201:325–331. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Cooper AJ. The role of glutamine transaminase K (GTK) in sulfur and alpha-keto acid metabolism in the brain, and in the possible bioactivation of neurotoxicants. Neurochem. Int. 2004;44:557–577. doi: 10.1016/j.neuint.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- Errico F, Napolitano F, Nistico R, Centonze D, Usiello A. D-aspartate: an atypical amino acid with neuromodulatory activity in mammals. Rev. Neurosci. 2009;20:429–440. doi: 10.1515/revneuro.2009.20.5-6.429. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Haavaldsen R, Tangen O. Transamination of aromatic amino acids in rat brain. J. Neurochem. 1964;11:109–118. doi: 10.1111/j.1471-4159.1964.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Sone Y, Mitsuhashi S, Tomiya M, Toyo’oka T. Alteration of kynurenic acid concentration in rat plasma following optically pure kynurenine administration: a comparative study between enantiomers. Chirality. 2009;21:468–472. doi: 10.1002/chir.20620. [DOI] [PubMed] [Google Scholar]
- Ganong AH, Lanthorn TH, Cotman CW. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983;273:170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J. Neurochem. 2007a;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine ami-notransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007b;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- Hamase K, Morikawa A, Etoh S, Tojo Y, Miyoshi Y, Zaitsu K. Analysis of small amounts of D-amino acids and the study of their physiological functions in mammals. Anal. Sci. 2009;25:961–968. doi: 10.2116/analsci.25.961. [DOI] [PubMed] [Google Scholar]
- Han Q, Cai T, Tagle DA, Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010;67:353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankes LV, Brown RR, Leklem J, Schmaeler M, Jesseph J. Metabolism of C14 labeled enantiomers of tryptophan, kynurenine and hydroxykynurenine in humans with scleroderma. J. Invest. Dermatol. 1972;58:85–95. doi: 10.1111/1523-1747.ep12551699. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Lackner A. Increased cerebrospinal fluid quinolinic acid, kynurenic acid, and L-kynurenine in acute septicemia. J. Neurochem. 1990;55:338–341. doi: 10.1111/j.1471-4159.1990.tb08857.x. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Hayaishi O. Enzymic formation of D-kynurenine from D-tryptophan. Arch. Biochem. Biophys. 1967;120:397–403. doi: 10.1016/0003-9861(67)90256-1. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtze M, Asp L, Schwieler L, Engberg G, Karlsson H. Induction of the kynurenine pathway by neurotropic influenza A virus infection. J. Neurosci. Res. 2008;86:3674–3683. doi: 10.1002/jnr.21799. [DOI] [PubMed] [Google Scholar]
- Ishii K, Ogaya T, Song Z, Iizuka H, Fukushima T. Changes in the plasma concentrations of D-kynurenine and kynurenic acid in rats after intraperitoneal administration of tryptophan enantiomers. Chirality. 2010;22:901–906. doi: 10.1002/chir.20850. [DOI] [PubMed] [Google Scholar]
- Ishii K, Iizuka H, Ogaya T, Song Z, Fukushima T. Comparative study on kynurenic acid production in the rat striatum by tryptophan enantiomers: An in vivo microdialysis study. Chirality. 2011;23(Suppl. 1):E12–E15. doi: 10.1002/chir.20938. [DOI] [PubMed] [Google Scholar]
- Johnson BA, III, Baban B, Mellor AL. Targeting the immunoregulatory indoleamine 2,3 dioxygenase pathway in immunotherapy. Immunotherapy. 2009;1:645–661. doi: 10.2217/IMT.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr. Res. 2011;69:465–472. doi: 10.1203/PDR.0b013e318217638a. [DOI] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J. Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kotake Y, Jr, Ito N. Studien u¨ber den intermediären Stoffwechsel des Tryptophans XXV. Isolierung des d-Kynurenins. J. Biochem. 1937;25:71–77. [Google Scholar]
- Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 2009;325:1552–1555. doi: 10.1126/science.1178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner RR, Berg CP. Metabolism of D-tryptophan in the normal human subject. J. Biol. Chem. 1955;214:699–707. [PubMed] [Google Scholar]
- Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav. Brain Res. 2010;210:84–91. doi: 10.1016/j.bbr.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Loh HH, Berg CP. Production of D-kynurenine and other metabolites from D-tryptophan by the intact rabbit and by rabbit tissue. J. Nutr. 1971;101:465–475. doi: 10.1093/jn/101.4.465. [DOI] [PubMed] [Google Scholar]
- Lugo-Huitrón R, Blanco-Ayala T, Ugalde-Muniz P, et al. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Terat. 2011;33:538–547. doi: 10.1016/j.ntt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr. Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Mason M, Berg CP. The metabolism of d- and l-tryptophan and d- and l-kynurenine by liver and kidney preparations. J. Biol. Chem. 1952;195:515–524. [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol. Dis. 2004;15:618–629. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Hamase K, Okamura T, Konno R, Kasai N, Tojo Y, Zaitsu K. Simultaneous two-dimensional HPLC determination of free d-serine and d-alanine in the brain and periphery of mutant rats lacking d-amino-acid oxidase. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:3184–3189. doi: 10.1016/j.jchromb.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Moreno S, Nardacci R, Cimini A, Ceru MP. Immunocytochemical localization of D-amino acid oxidase in rat brain. J. Neurocytol. 1999;28:169–185. doi: 10.1023/a:1007064504007. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Yolken RH. Early infections of Toxo-plasma gondii and the later development of schizophrenia. Schizophr. Bull. 2007;33:741–744. doi: 10.1093/schbul/sbm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Erhardt S. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J. Neural Transm. 2006;113:557–571. doi: 10.1007/s00702-005-0343-z. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, Saso S. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology. 1992;42:1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- Ogaya T, Song Z, Ishii K, Fukushima T. Changes in extracellular kynurenic acid concentrations in rat prefrontal cortex after D-kynurenine infusion: an in vivo microdialysis study. Neurochem. Res. 2010;35:559–563. doi: 10.1007/s11064-009-0099-1. [DOI] [PubMed] [Google Scholar]
- Okuno E, Schmidt W, Parks DA, Nakamura M, Schwarcz R. Measurement of rat brain kynurenine aminotransferase at physiological kynurenine concentrations. J. Neurochem. 1991;57:533–540. doi: 10.1111/j.1471-4159.1991.tb03783.x. [DOI] [PubMed] [Google Scholar]
- Okuno A, Fukuwatari T, Shibata K. High tryptophan diet reduces extracellular dopamine release via kynurenic acid production in rat striatum. J. Neurochem. 2011;118:796–805. doi: 10.1111/j.1471-4159.2011.07369.x. [DOI] [PubMed] [Google Scholar]
- Pedersen MG, Stevens H, Pedersen CB, Norgaard-Pedersen B, Mortensen PB. Toxoplasma infection and later development of schizophrenia in mothers. Am. J. Psychiatry. 2011;168:814–821. doi: 10.1176/appi.ajp.2011.10091351. [DOI] [PubMed] [Google Scholar]
- Pérez-De La Cruz V, Amori L, Notarangelo FM, Schwarcz R. Production of kynurenic acid from D-kynurenine in human brain and liver. Soc. Neurosci. Abstr. 2009;34:34–12. [Google Scholar]
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Wu H-Q, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr. Med. Chem. 2011;18:2257–2262. doi: 10.2174/092986711795656072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulpour A, Wu H-Q, Ferreé S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J. Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull. 2011;37:1147–1156. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Snell EE, Jenkins WT. The mechanism of the transami-nation reaction. J. Cell. Comp. Physiol. 1959;54:161–177. doi: 10.1002/jcp.1030540413. [DOI] [PubMed] [Google Scholar]
- Speciale C, Hares K, Schwarcz R, Brookes N. High-affinity uptake of L-kynurenine by a Na+-independent transporter of neutral amino acids in astrocytes. J. Neurosci. 1989;9:2066–2072. doi: 10.1523/JNEUROSCI.09-06-02066.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecartin KV, Bucci DJ. Administration of kynurenine during adolescence, but not during adulthood, impairs social behavior in rats. Schizophr. Res. 2011;133:156–158. doi: 10.1016/j.schres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebwasser KC, Swan PB, Henderson LM, Budny JA. Metabolism of D- and L-tryptophan in dogs. J. Nutr. 1976;106:642–652. doi: 10.1093/jn/106.5.642. [DOI] [PubMed] [Google Scholar]
- Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW. d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur. J. Neurosci. 2007;26:1657–1669. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Fujiwara M, Yoshida R, Hayaishi O. Stereospecificity of hepatic L-tryptophan 2,3-dioxygenase. Biochem. J. 1980;189:393–405. doi: 10.1042/bj1890393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Dumin E, Balan L, Foltyn VN. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatry. 2011;68:665–674. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-Q, Schwarcz R. AMPA receptors regulate kynurenate-induced dopamine release in the rat striatum. Soc. Neurosci. Abstr. 2009;34:748–12. [Google Scholar]
- Wu H-Q, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J. Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- Wu H-Q, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J. Mol. Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Liu YM. Electrophoretic separation of tryptophan enantiomers in biological samples. Electrophoresis. 2001;22:2769–2774. doi: 10.1002/1522-2683(200108)22:13<2769::AID-ELPS2769>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]