Abstract
Background
Fibromyalgia syndrome (FMS) is frequently associated with psychiatric conditions, particularly anxiety. Deficits in contingency learning during fear conditioning have been hypothesized to increase anxiety and, consequently, pain sensation in susceptible individuals. The goal of this study was to examine the relationship between contingency learning and pain experience in subjects with FMS and rheumatoid arthritis (RA).
Methods
Fourteen female FMS subjects, 14 age-matched female RA subjects and 14 age-matched female healthy controls (HCs) were included in a fear-conditioning experiment. The conditioned stimulus (CS) consisted of visual signs, the unconditioned stimulus (US) of thermal stimuli. CS− predicted low-temperature exposure (US), while CS+ was followed by low or high temperature.
Results
In the FMS group, only 50% of the subjects were aware of the US–CS contingency, whereas 86% of the RA subjects and all of the HCs were aware of the contingency. CS+ induced more anxiety than CS− in RA subjects and HCs. As expected, low-temperature exposure was experienced as less painful after CS− than after CS+ in these subjects. FMS subjects did not show such adaptive conditioning. The effects of the type of CS on heart rate changes were significant in the HCs and the aware FMS subjects, but not in the unaware FMS subjects.
Conclusions
Contingency learning deficits represent a potentially promising and specific, but largely unstudied, psychopathological factor in FMS. Deficits in contingency learning may increase anxiety and, consequently, pain sensation. These findings have the potential to contribute to the development of novel therapeutic approaches for FMS.
1. Introduction
Fibromyalgia syndrome (FMS) is characterized by chronic widespread pain, anxiety, fatigue, cognitive impairments and depression (Staud, 2006). The most effective treatments for FMS include behavioural treatments and psychopharmacological drugs (Thieme et al., 2006; Lesley, 2009). Therefore, and in contrast to rheumatoid arthritis (RA) (Schett and Firestein, 2010), central processes seem pathogenically more important than peripheral processes in FMS (Neeck, 2002), although not all findings in FMS clearly support this notion. Despite the growing evidence, there is a paucity of studies on the neuropsychiatric mechanisms underlying FMS pathogenesis.
The relationship between FMS and anxiety is particularly strong (Asmundson and Katz, 2009). In a community sample of women, subjects with FMS had a 20-fold increase in current rates of generalized anxiety disorder than did women without FMS (Karen et al., 2006). Anxiety disorders appear to precede the onset of chronic musculoskeletal pain (Asmundson and Taylor, 1996), and anxiolytic psychological and pharmacological interventions can reduce the pain associated with medical procedures (Park et al., 2008). Experimental studies have confirmed the enhancing effects of anxiety/fear on pain (Helmstetter and Bellgowan, 1993; Crown et al., 2000; Rhudy and Meagher, 2000; Meagher et al., 2001; Neugebauer et al., 2004; Meulders et al., 2012).
Conditioning, which plays a key role in the physiological and behavioural responses to pain, is an integral part of several models of chronic pain. These models, including the operant conditioning model, aversive emotional conditioning model and fear avoidance model, emphasize the role of conditioning in the origin and maintenance of chronic pain through its role on muscular tension or avoidance of physical activities (Flor and Turk, 1989; Vlaeyen and Linton, 2000; Leeuw et al., 2007). In classical conditioning, a previously neutral stimulus (later the conditioned stimulus = CS) paired with a biologically significant stimulus (unconditioned stimulus = US) elicits a conditioned response (CR) that resembles the response to the US, and this is the unconditioned response (UR). In the fear-conditioning paradigm, a CS (CS+) is presented together with an aversive US, while the other stimulus (CS−) is never paired with the US. Hence, CS+ acquires the same aversive qualities as the US, and the subject learns to fear the stimulus associated with the aversive event. The present study relied on an expectancy-based model of fear conditioning to examine the relationship between contingency learning and pain experience, and it was based on the following three observations. First, anxiety induced by pain expectation enhances pain (Al Absi and Rokke, 1991; Ploghaus et al., 2001). Second, conditioning is a process by which organisms learn contingency among stimuli (i.e., the US follows the CS+ but not the CS−), and they develop expectancies about the occurrence or non-occurrence of aversive events, which can lead to conditioned fear to the CS+ (Lovibond and Shanks, 2002). In differential conditioning experiments in which a CS+ is repeatedly paired with an aversive US (e.g., a shock) and a CS− is never reinforced with the US, the CS+ evokes aversive expectancy (fear), while the CS− evokes no aversive expectancy (no fear). Hence, during conditioning, a mildly aversive event is felt as more painful following a CS+ than a CS−. Third, given the finding that the development of expectancy during conditioning is a learning process (Chan and Lovibond, 1996), excessive fear may result from a failure to learn the correct CS–US contingency (Grillon, 2002). Generally, the inability to correctly learn predictive cues in the environment leaves the organism in a state of chronic anxiety because of the inability to identify safety periods. According to this perspective, contingency learning deficits may conceivably contribute to hyperalgesia in FMS subjects. Fear-conditioning experiments can be used to elicit both hypoalgesia (Flor and Grösser, 1999; Flor et al., 2002) or hyperalgesia (Al Absi and Rokke, 1991; Ploghaus et al., 2001). Whether the experiments elicit hypo- or hyperalgesia depends on the kind (stress related to the pain or not) and intensity of the stressor and the experiment duration (Al Absi and Rokke, 1991; Flor and Grösser, 1999; Rhudy and Meagher, 2000). Thus, to induce hyperalgesia in this study, we used pain-related and relatively mild stressors.
We hypothesized that subjects with FMS would show deficits in contingency learning, which would result in excessive fear to the CS− and, therefore, the exacerbation of pain following the CS−. Hence, we expected healthy controls (HCs) and subjects with RA to experience an exposure to low temperature as less painful after the safety signal CS− than after the danger signal CS+. However, in FMS subjects, we expected reduced CS–US contingency learning (Odling Smee, 1975) that would result in similar levels of fear and pain after the safety signal (CS−) and the danger signal (CS+).
2. Methods and materials
2.1 Subjects
Given the predominance of women with FMS (Wolfe et al., 1995) and to reduce the heterogeneity of the study samples, we only included women in this study. In order to exclude the possibility that reactions deviating from those of the HC group that were found in this study were non-specifically related to chronic pain conditions rather than to FMS, we used two control groups, one of which comprised age-matched healthy women and the other of which comprised age-matched women with RA because neuropsychiatric mechanisms are not expected to play major roles in the pathophysiology of RA. The study sample of 42 female subjects who were aged 18 to 65 years included the three following age-matched diagnostic groups: 14 subjects with FMS, 14 subjects with RA and 14 HCs. The demographic and clinical characteristics across the diagnostic groups are provided in Table 1. Subjects provided written informed consent after receiving a full explanation of the study purpose, procedures and risks. The study was approved by the local ethical committee (Kantonale Ethikkommission Zürich).
Table 1.
Demographic and clinical characteristics of subjects with fibromyalgia (n = 14), healthy controls (n = 14) and rheumatoid arthritis (n = 14).
| Variable | Fibromyalgia
|
Healthy control
|
Arthritis
|
p* | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age | 48.8 | 9.1 | 48.9 | 4.7 | 54.5 | 6.5 | n.s. |
| State-Trait Anxiety Inventory | |||||||
| State | 47.2 | 14.3 | 33.3 | 5.6 | 33.6 | 5.7 | <0.001 |
| Trait | 46.3 | 11.9 | 32.8 | 6.6 | 32.9 | 5.7 | <0.001 |
| Beck Depression Inventory | 17.0 | 10.2 | 4.1 | 2.8 | 7.0 | 4.8 | <0.001 |
| Pain Disability Index | 41.1 | 12.9 | 2.7 | 3.4 | 17.1 | 12.1 | <0.001 |
|
| |||||||
| n | % | n | % | n | % | ||
|
| |||||||
| Education | |||||||
| No completed | 3 | 21.4 | 0 | 0.0 | 0 | 0.0 | n.s. |
| Apprenticeship | 9 | 64.3 | 9 | 64.3 | 11 | 78.6 | |
| College/university | 2 | 14.3 | 5 | 35.7 | 3 | 21.4 | |
| Occupational invaliditya | |||||||
| No | 6 | 42.9 | 14 | 100.0 | 11 | 78.6 | <0.01 |
| Yes (complete or partial) | 8 | 57.1 | 0 | 0.0 | 3 | 21.4 | |
| Any psychiatric diagnosisb | 8 | 57.1 | 0 | 0.0 | 2 | 14.3 | <0.01 |
| Major depressive disorder | 3 | 21.4 | 0 | 0.0 | 1 | 7.1 | |
| Any anxiety disorder | 7 | 50.0 | 0 | 0.0 | 2 | 14.3 | |
| Panic disorder | 3 | 21.4 | 0 | 0.0 | 1 | 7.1 | |
| Agoraphobia | 3 | 21.4 | 0 | 0.0 | 1 | 7.1 | |
| PTSD | 3 | 21.4 | 0 | 0.0 | 0 | 0.0 | |
| Medication | |||||||
| Antidepressants | 8 | 57.1 | 0 | 0.0 | 1 | 7.1 | |
| Anxiolytics | 1 | 7.1 | 0 | 0.0 | 1 | 7.1 | |
| Opioids | 1 | 7.1 | 0 | 0.0 | 1 | 7.1 | |
Chi-square test, Fisher’s exact test or analysis of variance when appropriate.
Compensation by the insurance.
Multiple diagnosis possible.
PTSD, posttraumatic stress disorder; SD, standard deviation.
2.2 Diagnostic and psychometric assessments
Rheumatologic diagnoses were established according to the American College of Rheumatology 1990 classification criteria (Wolfe et al., 1990) and the criteria for the classification of RA (Arnett et al., 1988). Dolorimetry was performed at 24 tender points (for classification ≥12/24 with ≤2 kg/ 1.27 cm2) and eight control points (for classification ≤3/8) (Dettmer and Chrostek, 1991). The method we adopted has been described elsewhere (Wolfe, 1997).
Psychiatric diagnoses were established with the Mini International Neuropsychiatric Interview (Sheehan et al., 1998), which is a short structured diagnostic interview for 17 Axis-I diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders, version IV and the International Classification of Diseases-10 criteria. The FMS and RA subjects were recruited through the outpatient clinical services of the Department of Rheumatology of Zurich University Hospital, and the HC were recruited by advertisements in local newspapers. The clinical evaluation included electrocardiography (ECG) and laboratory tests. Exclusion criteria included major medical illnesses other than FMS and RA, pregnancy, psychosis, suicidal ideation or suicide attempts within the previous 8 weeks, substance abuse within the past year, and a lifetime history of substance dependence. Prior to the experiment, clinical characteristics were assessed with the State-Trait Anxiety Inventory (Laux and Vossel, 1982), the Beck Depression Inventory (Beck et al., 1994) and the Pain Disability Index (Tait et al., 1990; Dillmann et al., 1994). The Pain Disability Index measures pain-related interference with role functioning in seven areas (occupational, home/family, recreational, social, sexual, activities of daily living and life support), which are all rated on 11-point Likert-type scales (0, no disability; 10, complete disability). The average ongoing chronic pain, which was defined as the average pain that subjects had suffered during the 2 weeks prior to the measurement, was assessed with a visual analogue scale (VAS) that went from 0 (no pain) to 100 (worst possible pain).
2.3 Pain stimuli
Thermal stimuli were applied to the thenar of the non-dominant hand with a 27-mm-diameter thermal contact thermode (CHEPS, Medoc Ltd, Ramat Yishai, Israel). The CHEPS thermode has a heating rate of 70 °C/s and a cooling rate of 40 °C/s. The same heating and cooling rate was applied during the whole experiment. Pain threshold estimation was based on five thermal stimuli that slowly increased in temperature (1 °C/s) until it was stopped by a button press or when the maximum temperature of 52 °C was reached. In the second step, a temperature that was rated as moderate pain (approximately 50 mm on the VAS) was individually assessed for each subject and used as the low-temperature stimulus (USlow). The temperature that was established for USlow was increased by 2.5 °C to obtain the high-temperature stimulus (UShigh). This substantial increase in temperature has been found to clearly discriminate between UShigh and all other painful stimulations (Ploghaus et al., 2001). UShigh was only presented during the experiment itself. There was a 30-min interval between the pain threshold estimation and the conditioning experiment.
2.4 Protocol
No explicit information about the CS–US relationship was given to the participants prior to conditioning. Prior to the experiment, subjects were instructed that they would see shapes on the screen and feel heat bursts on their hand. Thermal stimulation and the recording of physiologic activity (heart rate, HR) was controlled by a commercial device (Presentation software, Neurobehavioral Systems, Inc., Albany, CA, USA). Visual stimuli (simple black squares and triangles on a white background) were presented on a monitor that was located 2 m in front of the subjects. Subjects were presented thermal stimuli of different temperatures for a duration of 6 s. During the experiment, the perceived pain intensity and fear levels were assessed with a VAS that consisted of a 100-mm line that was anchored from 0 (no pain) to 100 (worst possible pain) and 0 (no fear) to 100 (maximal fear) (Scott and Huskisson, 1976). It was explained to the subjects that the pain ratings explicitly concerned their perceived sensory intensity and not the unpleasantness of the pain. The levels of fear that were explained to be related to the CS (and not to the pain) were used to measure whether the conditioning was successful or not. The scale was presented for a period of 5 s after the offset of the thermal stimulus.
The experimental paradigm, which is displayed in Fig. 1, used delay-conditioning contiguities. The whole paradigm consisted of 20 trials with 10 for each condition (CS−, CS+). One visual signal (CS−) was always followed by the USlow. This signal came to evoke low fear about the impending pain. The other visual signal was followed in a pseudorandomized way in half of the trials by USlow (CS+ low) and in the other half of the trials by the higher temperature pain stimulus, UShigh (CS+ high). This signal (CS+ high) came to elicit higher fear about the impending pain. The delay between signal onset and the onset of thermal stimulation (CS–US interval) was randomized with a range between 8 and 15 s in order to make the CS–US associative learning more efficient. The intertrial interval was 30 s.
Figure 1.
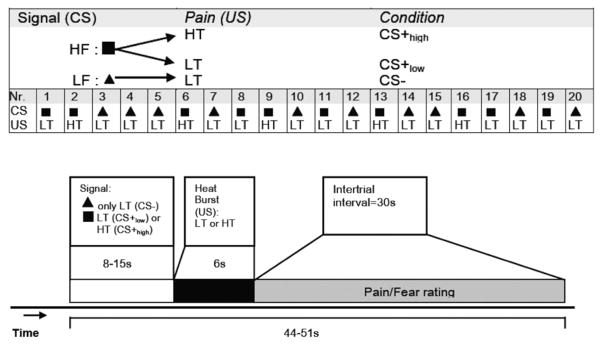
Experimental paradigm and time course of the conditioning experiment. Experimental paradigm (top): Visual cues predicted exposure to painful heat stimulation. Painful stimulation was delivered at a low temperature (LT) or at a higher, more painful temperature (HT). One visual cue (here: triangle) was consistently followed by a LT. Another cue (here: square) was followed by LT or HT. LF: low fear/ pain signal (CS−); HF: high fear/pain signal (CS+ low or CS+ high). The conditioned stimuli were presented during a variable amount of time (8 to 15 s) in order to increase the unpredictability of the unconditioned stimuli. The duration of the exposure to heat stimulation was 6 s. Self-reported fear and pain ratings were assessed in the intertrial intervals of 30 s. CS, conditioned stimulus; US, unconditioned stimulus.
2.5 Heart rate
HR was continuously recorded simultaneously with a Lifeshirt-System® (VivoMetrics® Inc., Ventura, CA, USA). The device, which applies a proprietary algorithm to detect the peak of the R-wave from the digitized ECG, has been shown to be highly accurate in the detection of R-waves and in providing an accurate timing of R–R intervals (Heilman and Porges, 2007). The LifeShirt samples the ECG at 200 Hz. R-waves were detected in the digitized ECG, and interbeat intervals (cardiac time) were converted to beats per min (BPM, real time) every 500 ms. BPM during the first 8 s of the CS were analysed s-by-s and were expressed relative to the baseline that was taken during a 1-s window before CS onset. BPM changes were then converted back into HR changes, which were computed by subtracting HR during the s before CS (i.e., the last s of the intertrial interval) from the HR during the highest acceleration within the CS (i.e., between the fourth and seventh second).
2.6 Contingency learning
Following the experiment, contingency awareness was assessed by asking the subjects which of the two visual cues (CS+ and CS−) had previously been associated with the painful high-temperature stimulation. If the subjects reported that they were completely or fairly certain that the CS+ was paired with the high-temperature stimulus and CS− was not paired with the high-temperature stimulus, they were considered aware.
2.7 Data analysis
In order to analyse the self-reported experiences of pain intensity, fear and the HR data, linear mixed-model analyses were computed with SPSS 19 for Windows (IBM Corporation, Armonk, NY, USA). Group differences in the pain and fear ratings, as well as the HR responses, were tested with full factorial models with group and signal (type of CS) or group and temperature (type of US) as fixed factors. Interaction effects were used to evaluate the differences in the reactions and responses between the groups. The model-predicted estimated marginal means of the measures in each group allowed for comparisons of the levels of signal and temperature. In all models, a first-order autoregressive covariance structure was appropriate for the repeated measures. A restricted maximum likelihood model estimation was used. The significance level was set to p <0.05.
In order to evaluate the effects of contingency learning (i.e., predictability of the impending stimulus) on pain perception, only CS− and CS+ low trials (i.e., CS followed by low-temperature US) were used. The reason for excluding CS+ high trials was that pain and fear were assessed retrospectively, and they therefore would have potentially biased the comparison of CS− and CS+ ratings and one of the main questions of this study, which was whether the same pain stimulus (USlow) would be rated differently depending on the condition (CS−, CS+). CS+ high trials were included in all other mixed-model analyses. This was because the effects of temperature on the pain ratings and HR responses could be tested only when the high-temperature stimuli were included and because HR responses directly following the CS were supposed to be unbiased by the subsequently applied heat bursts. Because there was no learning history before the first trial, it was consistently excluded from the data analyses.
3. Results
3.1 Pain stimuli and awareness of CS–US relationship
The mean temperature for the USlow stimulus was 46.26 °C (SD, 1.20) for the FMS subjects, 46.98 °C (SD, 0.29) for the RA subjects, and 46.99 °C (SD, 0.55) for the HC group (F, 3.98; df, 2, 39; p = 0.03). At the end of the conditioning experiment, only 7 out of the 14 (50%) FMS subjects reported that they were completely or fairly certain that the CS+ high signal was paired with the higher temperature stimulus (UShigh) and were designated aware (see Fig. 2 for the learning curves). In 12 out of the 14 (86%) RA subjects and in the whole (100%) HC group, the subjects were aware of the meanings of the conditioned signals. Accordingly, awareness significantly differed between the diagnostic groups (Fisher’s exact test, p = 0.005). Based on our hypothesis, we expected an increased rate of unaware FMS subjects because of their fear-learning deficits, and we did not exclude them from further analyses. There was no association between awareness and psychiatric comorbidities in the FMS group (Fisher’s exact test, p = 0.63). In the FMS group, 10 of the 14 patients were being treated with psychotropic substances. However, there was no association between awareness and medication (Fisher’s exact test, p = 0.56).
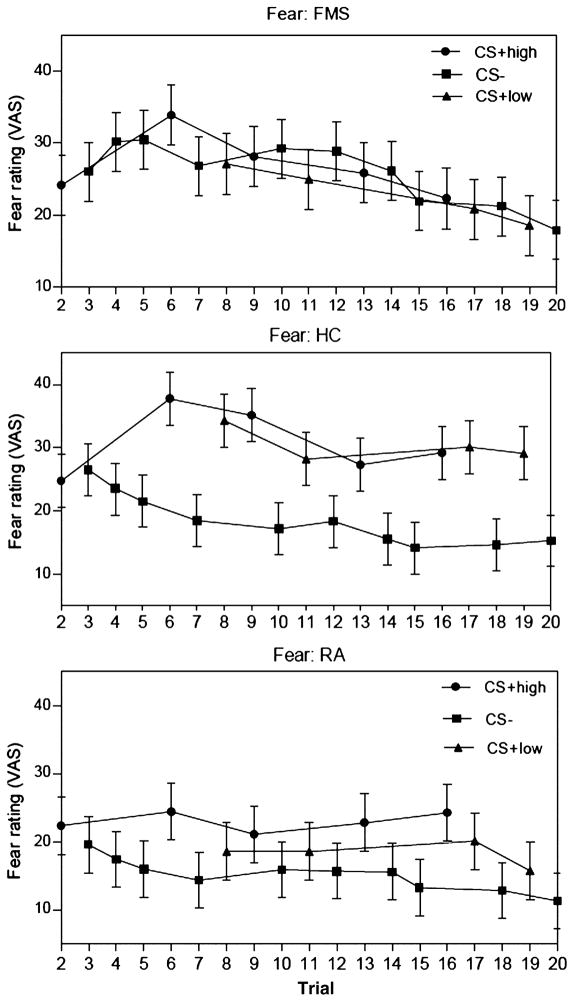
Figure 2.
Learning curves. Self-reported fear ratings after each trial classified by diagnostic group and kind of CS stimulus. The panels illustrate adaptive differential conditioning by higher fear responses to CS+ than to CS− in healthy controls and rheumatoid arthritis subjects, but not in fibromyalgia subjects. Error bars indicate standard errors of means (SEM).
3.2 Effect of CS on anxiety and pain experience
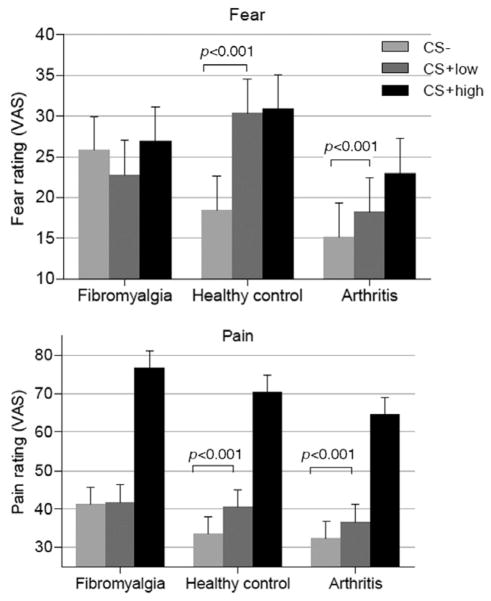
The ratings of subjective pain and fear perception as assessed by the VAS during the conditioning experiment are presented in Fig. 3 as well as in Tables 2 and 3 (means and standard deviations of self-reported fear and pain after CS− /low-temperature stimulation, CS+ /low-temperature stimulation and CS+ /high-temperature stimulation). Mixed-model analyses that were used to examine the effects of the group and the type of conditioned stimulus (CS− vs. CS+ low) on fear and pain ratings revealed significant interaction effects between group and the type of CS on both ratings (fear: F, 42.1; df, 2, 343.0; p < 0.001; pain: F, 6.27; df, 2, 356.1; p = 0.002), indicating that the diagnostic groups showed significantly different patterns of fear and pain responses to CS presentation, which corresponded to our hypothesis. Pairwise comparisons of the estimated marginal means revealed that fear and pain experiences differed significantly between CS− and CS+ low in HC (fear: F, 127.9; df, 1, 346.7; p < 0.001; pain: F, 37.8; df, 1, 356.1; p < 0.001) and RA (fear: F, 11.4; df, 1, 341.2; p < 0.001; pain: F, 15.7; df, 1, 356.1; p < 0.001), but not in the FMS group (fear: F, 2.26; df, 1, 341.2; p = 0.13; pain: F, 1.33; df, 1, 356.1; p = 0.25). Thus, the low-temperature stimulation was experienced as less painful when it was predicted by the CS− (safety cue) in the HC and the RA group but not in the FMS group. Even after excluding the unaware FMS subjects (n = 7), the responses to CS− and CS+ low were not statistically different (fear: F, 0.12; df, 1, 276.1; p = 0.73; pain: F, 2.42; df, 1, 277.4; p = 0.12; Table 3).
Figure 3.
Behavioural results. The means of the self-reported fear and pain ratings after CS− /low-temperature stimulation, CS+ /low-temperature stimulation and CS+ /high-temperature stimulation classified by diagnostic group (14 fibromyalgia subjects, 14 healthy controls, 14 rheumatoid arthritis subjects). Error bars indicate standard errors of means (SEM).
Table 2.
Means and standard deviations of pain and fear rating, and heart rate changes of subjects with fibromyalgia (n = 14), healthy controls (n = 14) and rheumatoid arthritis (n = 14).
| Variable | Fibromyalgia
|
Healthy control
|
Arthritis
|
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Fear | ||||||
| CS− | 25.8 | 25.1 | 18.5 | 14.1 | 15.2 | 11.2 |
| CS+ low | 22.8 | 20.6 | 30.4 | 20.7 | 18.2 | 15.0 |
| CS+ high | 26.9 | 24.8 | 30.9 | 21.1 | 23.0 | 14.7 |
| Pain | ||||||
| CS− | 41.2 | 20.5 | 33.6 | 20.7 | 32.4 | 18.4 |
| CS+ low | 41.9 | 19.6 | 40.5 | 25.0 | 36.7 | 21.3 |
| CS+ high | 76.7 | 16.8 | 70.3 | 22.5 | 64.5 | 19.8 |
| HR change (bpm) | ||||||
| CS− | 3.75 | 4.74 | 4.23 | 4.68 | 4.10 | 5.35 |
| CS+ | 4.44 | 4.75 | 5.28 | 4.85 | 4.62 | 4.91 |
CS, conditioned stimulus; HR, heart rate; SD, standard deviation.
Table 3.
Means and standard deviations of pain and fear rating, and heart rate of subjects with fibromyalgia: Comparison of aware (n = 7) with unaware subjects (n = 7).
| Variable | Aware
|
Unaware
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Fear | ||||
| CS− | 17.4 | 17.0 | 34.3 | 28.9 |
| CS+ low | 17.9 | 9.7 | 27.7 | 26.8 |
| Pain | ||||
| CS− | 36.0 | 15.7 | 46.4 | 23.4 |
| CS+ low | 38.6 | 15.6 | 45.1 | 22.7 |
| Heart rate change | ||||
| CS− | 4.77 | 5.62 | 2.72 | 3.40 |
| CS+ | 6.37 | 5.00 | 2.51 | 3.61 |
CS, conditioned stimulus; SD, standard deviation.
The average ongoing chronic pain rating in the FMS group was 7.1 (SD, 1.3), and it was 4.6 (SD, 2.4) in the RA group. There was no significant difference regarding the ongoing chronic pain rating between the aware and unaware FMS subjects (t, − 0.19; df, 12; p = 0.85). In the FMS or in the RA groups, ongoing pain did not correlate significantly with pain or fear ratings during each trial.
3.3 Effect of US on pain experience
There was a significant main effect of the type of US (low vs. high temperature) on self-reported pain (F, 1, 086.1; df, 1, 598.5; p < 0.001) and a non-significant interaction effect between subject group and the type of US in this mixed-model analysis (F, 1.75; df, 2, 598.5; p = 0.17), suggesting that, in all diagnostic groups, high-temperature stimulation induced higher pain experience than low-temperature stimulation.
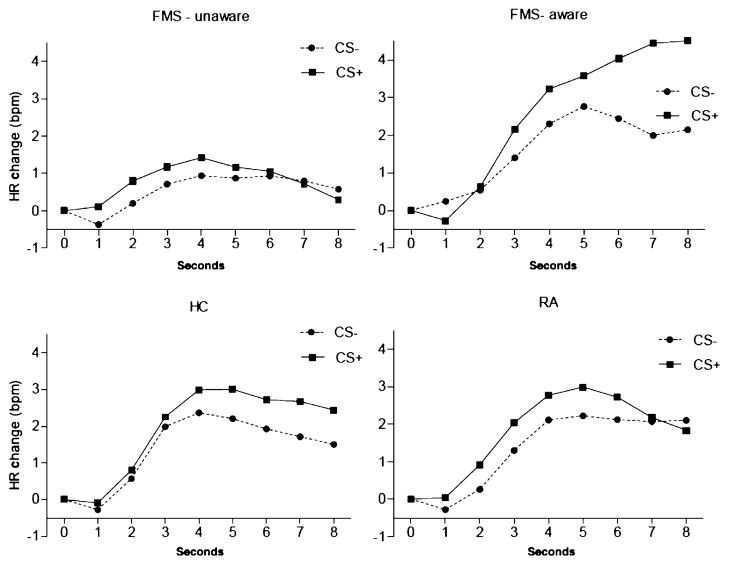
3.4 Heart rate during CS
The HR changes during each CS are shown in Fig. 4. The maximum changes were calculated as the highest acceleration between 4 and 7 s post-CS. The means and SDs of the HR changes that were related to conditioned stimuli (CS− and CS+) are reported in Table 2. The group-by-CS type interaction was not significant (F, 0.33; df, 2, 580.3; p = 0.72), while the main effect of CS type was significant (F, 6.91; df, 1, 580.3; p = 0.009), indicating that the acceleration of HR after CS+ was more or less similar in all diagnostic groups. After excluding the unaware FMS subjects from the analysis, there was a significantly increased HR change in the aware FMS subjects after CS+ compared to CS− (F, 4.89; df, 1, 468.4; p = 0.03). In contrast, unaware FMS subjects showed no such difference (F, 0.09; df, 1, 471.4; p = 0.77; Table 3).
Figure 4.
Heart rate results. Heart rate changes during the conditioned stimuli CS+ and CS−. The panels illustrate the heart rate change waveforms for the CS+ and CS− trials. Because the present study used a range of CS–US intervals (8–15 s), only the initial 8 s were used to estimate heart rate responses to the CS. The time point 0 s represents the s before CS onset. Heart rate responses of aware (7) and unaware (7) FMS subjects were analysed separately.
4. Discussion
Consistent with the findings of the study by Ploghaus et al. (2001), HCs and subjects with RA experienced less fear following CS− than following CS+, and they experienced exposures to low temperature as less painful when presented after CS− than after CS+. These results demonstrated that the experience of pain may be modulated by fear. Consistent with our hypothesis, subjects with FMS showed deficits in contingency learning at the behavioural and physiological level (HR). Most likely, because they did not know which CS predicted the high temperature, the CS− induced the same level of fear as the CS+. Unlike the control and RA groups, the experience of the low-temperature stimulation in the FMS group was not modulated by the CS. Specifically, they showed increased levels of pain during the CS− low, probably because the CS− did not act as a safety cue.
Conditioning theories provide a conceptual framework for understanding reactions to threats, assuming that learning is based on the information value of predictive cues and that fear/anxiety is based on expectancy about the occurrence of aversive events (Mathews and MacLeod, 1985). Perceived unpredictability, which is the failure to learn about the contingency, is fundamental to sustained anxiety (Grillon et al., 2004) and to an increase in contextual anxiety (Grillon, 2002). Deficits in the learning about contingencies in the environment make the environment unpredictable, which is anxiogenic and may contribute to increased chronic pain perception. Additionally, unpredictability may contribute to hyperalgesia in FMS subjects, given the strong relationship between FMS and anxiety (Asmundson and Katz, 2009) and the increased reactivity to threat-related pictures in these individuals (Bartley et al., 2009). The origin of perceived unpredictability is still obscure. In conditioning experiments, subjects with trait anxiety have shown slower rates at the cognitive (awareness) and physiological (skin conductance) level for differentiating between CS+ and CS− than do control subjects (Chan and Lovibond, 1996). This learning deficit is associated with a bias towards a higher expectancy of aversive events. Subsequent studies have shown that deficits in explicit cue fear conditioning result in an increased perception of unpredictability, enhanced physiological signs of anxiety and behavioural avoidance (Grillon, 2002). Such a learning deficit may conceivably contribute to attentional bias, anxiety sensitivity, avoidance, reduced activity levels and higher pain levels in FMS subjects (Asmundson and Katz, 2009). Evidence from neuroimaging studies seems to confirm a shared pathogenic mechanism in anxiety and pain regarding the perception of unpredictability. In healthy volunteers, the anticipation of unpredictably administered electric shocks induces sustained anxiety along with an increase in blood flow in the hippocampus (Hasler et al., 2007). In the Ploghaus study (Ploghaus et al., 2001) on anxiety-induced hyperalgesia, hippocampal activation was associated with an exacerbation of pain by anxiety in healthy volunteers. In FMS subjects, a recent imaging study that used magnetic resonance diffusion-tensor imaging and voxel-based morphometry found microstructural changes in the bilateral hippocampi (Lutz et al., 2008). In addition, they found structural abnormalities in the amygdala, which plays a central role in explicit cue fear conditioning (Buchel and Dolan, 2000). These findings support our hypothesis that fear-related mechanisms might play an important role in FMS pathogenesis.
Another explanation for our findings is that the repeated administration of thermal stimuli in the FMS subjects resulted in central sensitization. Therefore, over time, their perceived intensities of the nociceptive stimulus increased (Clifford, 2011). However, this explanation is not likely true because there was no increase in the perceived pain intensity in FMS subjects.
The strengths of our study include the relatively close matching among the diagnostic groups regarding gender, age and group size and the inclusion of a positive control group with RA, which represents a chronic pain condition other than FMS and allows for evaluating the specificity of the results for FMS. The conditioning experiment described by Ploghaus et al. (2001) was well suited for studying the pathophysiology of FMS because the use of pain as an US is likely relevant in FMS subjects and the experiment allowed for the examination of pain exacerbation by anxiety.
This study has several methodological limitations. While Ploghaus et al. (2001) obtained fear and pain ratings in separate groups of subjects, we obtained these ratings in the same-subject groups because of the difficulty in recruiting two samples of FMS subjects. Hence, the self-report assessments of both processes in the same subject may have led to an overestimation of pain–fear correlations. Because the pain- and fear-rating data in HCs in this study were similar to those in the Ploghaus study, bias due to our rating method seems unlikely. Additionally, fear ratings may not only reflect fear related to the CS, but may also be influenced by general anxiety or uncertainty in the experiment. Further, this was not a traditional differential conditioning study because we did not use a true safety signal (no pairing with the US). Because we were interested in the influence of fear on pain experience in different conditions, we used a low-temperature stimulus for the CS− condition. Another concern is the clinical heterogeneity of the FMS sample as more than half the FMS subjects had comorbid anxiety or depressive disorder, which corresponded to the comorbidity pattern found in representative samples. Given that learning deficits have been reported for individuals with anxiety disorders (Lissek et al., 2009) and that subjects with major depressive disorder show abnormal reactivity during the anticipation of heat pain (Strigo et al., 2008), specific pathogenic mechanisms underlying FMS may not have solely contributed to the fear-learning deficits found in this group. Consistent with the high prevalence of psychiatric disorders among FMS subjects, nine subjects in the FMS group were being treated with antidepressant drugs and one was being treated with anxiolytic drugs, which may additionally have influenced fear conditioning. We did not, however, find an association between the presence of psychiatric comorbidities and the awareness of the CS–US relationship in our FMS sample (Fisher’s exact test, p = 0.63). Additionally, there was no association between awareness and medication (Fisher’s exact test, p = 0.56). We found no correlations between the levels of ongoing chronic pain and the fear ratings during the experiment in the FMS group and the RA group, suggesting that chronic pain may not have interfered with fear conditioning. Another concern was that we did not specifically test neuropsychological deficits, which could be related to the awareness deficits in the FMS group. The RA subjects may not represent an ideal control croup because they have a higher prevalence of mental disorders than the general population (Lok et al., 2010). However, in this study, RA subjects had a relatively low rate of psychopathology.
The failure of contingency learning associated with increased pain experience in FMS subjects suggested that fear-learning deficits play an important role in the FMS pathogenesis. When a danger signal (CS+) predicts a painful experience, the absence of the danger signal (e.g., context) predicts periods of safety. Subjects who are unable to identify danger signals (or are unable to learn safety cues) remain in a chronic state of fear because they cannot identify safety periods. In this model, fear conditioning is a process in which generalized fear of the context becomes stimulus specific and predictable. Fear remains generalized when aversive events occur unpredictably or when subjects fail to identify stimulus contingency (Grillon and Davis, 1997). In FMS subjects, a sustained and excessive anticipation of unpredictable painful events (bodily symptoms or external events) may contribute to the experience of chronic and widespread pain, low levels of physical activity and dependence on the relatively predictable effects of pain-contingent analgesics.
FMS subjects did not report significantly more fear and pain in the CS+ high condition than the HCs did (p = 0.26, p = 0.27, respectively); this would have been a direct demonstration of fear-learning deficits causing excessive fear and hyperalgesia. However, our study was not designed to show such relationships. Painful stimuli (US) were adjusted for each subject to her individual pain threshold, resulting in systematically less intense stimulation in FMS subjects than in HCs and RA subjects, which is a confounding factor when comparing pain and fear across groups. Therefore, we focused on the differential fear and pain response, which showed significant group differences. Qualitatively, the results on pain that are displayed in Fig. 3 suggested that fear-learning deficits result in relatively more pain in the CS− condition in FMS subjects than in HCs and RA subjects. Furthermore, when we analysed aware and unaware FMS subjects separately, unaware FMS subjects reported higher fear and pain ratings than did aware FMS, HCs and RA subjects (Tables 2 and 3). Aware FMS subjects, however, showed higher HR acceleration compared to the other groups, suggesting an enhanced and sustained fear of pain.
This study encouraged the use of neuroimaging techniques to elucidate the neural substrate of fear-learning deficits and anxiety-induced hyperalgesia in FMS. Based on the studies by Hasler et al. (2007) on unpredictability and Ploghaus et al. (2001) on anxiety-related hyperalgesia and the structural imaging work in FMS (Lutz et al., 2008), one may hypothesize that a hippocampal network is importantly involved in fear-related mechanisms in the pathogenesis of FMS. Moreover, the results of this study may translate into the development of specific therapeutic approaches to FMS. Given the evidence in animals that D-cycloserine enhances the ability to discriminate between conditioned danger cues and conditioned safety cues (Land and Riccio, 1999), D-cycloserine may enhance conditioning discrimination and reduce unpredictability and anxiety-related pain in FMS subjects.
In summary, fear-learning deficits represent a potentially promising and specific, but largely unstudied, psychopathological marker in FMS. Consistent with our predictions, the ability of FMS subjects to differentiate between a cue predicting a risk of painful thermal stimulation and a safety cue led to relatively increased fear and pain in the context of the safety cue. The findings of this study may contribute to elucidating the pathophysiology of FMS and may lead to the development of novel therapeutic approaches.
What’s already known about this topic?
Classical conditioning plays a key role in the physiological response, subjective feeling and behavioural response to pain, and, consequently, conditioning is an integral part of several models of chronic pains. Such models emphasize the role of conditioning in the origin and maintenance of chronic pain via its role on muscular tension or avoidance.
What does this study add?
The present study relies on an expectancy-based model of aversive conditioning to elucidate the role of anxiety in the pathogenesis of fibromyalgia syndrome (FMS) compared to rheumatoid arthritis. Specifically, the study examined the role of conditioned fear evoked by expectation of pain on the experience of pain in FMS.
Fear-learning deficits in a conditioning experiment as a potentially promising and specific psychopathological marker in fibromyalgia syndrome.
Deficits in fear learning may increase anxiety and, as a consequence, pain sensation. These findings have the potential to contribute to the development of novel therapeutic approaches for fibromyalgia.
Acknowledgments
Funding sources
This work was supported by the Hermann Klaus Foundation, Zurich, Switzerland; Werner Alfred Selo Foundation, Zurich, Switzerland; and the Kurt and Senta Herrmann Foundation, Zurich, Switzerland.
Footnotes
Conflicts of interest
None declared.
Author contributions
- Conception and design: Josef Jenewein, Hanspeter Moergeli, Haiko Sprott, Dominik Ettlin, Konrad Bloch, Mike Brügger, Kyrill Schwegler, Christian Grillon, Gregor Hasler
- Data acquisition: Josef Jenewein, Haiko Sprott, David Honegger, Luzia Brunner, Dominik Ettlin, Konrad Bloch, Mike Brügger, Kyrill Schwegler
- Analysis and interpretation of data: Josef Jenewein, Hanspeter Moergeli, Sonja Schumacher, Haiko Sprott, Christian Grillon, Gregor Hasler, Konrad Bloch, David Honegger, Luzia Brunner, Mike Brügger
- Drafting the article or revising it critically for important intellectual content: Josef Jenewein, Hanspeter Moergeli, Haiko Sprott, Christian Grillon, Sonja Schumacher, Gregor Hasler
- Final approval of the version to be published: Josef Jenewein, Hanspeter Moergeli, Haiko Sprott, David Honegger, Luzia Brunner, Dominik Ettlin, Christian Grillon, Konrad Bloch, Mike Brügger, Kyrill Schwegler, Sonja Schumacher, Gregor Hasler.
All authors discussed the results and commented on the manuscript.
References
- Al Absi M, Rokke PD. Can anxiety help us tolerate pain? Pain. 1991;46:43–51. doi: 10.1016/0304-3959(91)90032-S. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: State-of-the-art. Depress Anxiety. 2009;26:888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Taylor S. Role of anxiety sensitivity in pain-related fear and avoidance. J Behav Med. 1996;19:577–586. doi: 10.1007/BF01904905. [DOI] [PubMed] [Google Scholar]
- Bartley EJ, Rhudy JL, Williams AE. Experimental assessment of affective processing in fibromyalgia. J Pain. 2009;10:1151–1160. doi: 10.1016/j.jpain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Hautzinger M, editors. Beck– Depressions– Inventar (BDI); Testhandbuch (Autoren: Beck AT, Steer RA; Bearbeitung Der Dt. Ausgabe: Hautzinger M) Bern: Huber; 1994. [Google Scholar]
- Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Chan C, Lovibond P. Expectancy bias in trait anxiety. J Abnorm Psychol. 1996;105:637–647. doi: 10.1037//0021-843x.105.4.637. [DOI] [PubMed] [Google Scholar]
- Clifford JW. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, King TE, Meagher MW, Grau JW. Shock-induced hyperalgesia: III. Role of the bed nucleus of the stria terminalis and amygdaloid nuclei. Behav Neurosci. 2000;114:561–573. [PubMed] [Google Scholar]
- Dettmer N, Chrostek M. Gedanken zur Dokumentation bei der generalisierten Tendomyopathie (GTM) In: Müller W, editor. Generalisierte Tendomyopathie (Fibromyalgie) Darmstadt: Steinkopff; 1991. pp. 63–70. [Google Scholar]
- Dillmann U, Nilges P, Saile H, Gerbershagen HU. Assessing disability in chronic pain patients. Schmerz. 1994;8:100–110. doi: 10.1007/BF02530415. [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Schulz R, Grüsser SM, Mucha RF. Pavlovian conditioning of opioid and nonopioid pain inhibitory mechanisms in humans. Eur J Pain. 2002;6:395–402. doi: 10.1016/s1090-3801(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Flor H, Grösser SM. Conditioned stress-induced analgesia in humans. Eur J Pain. 1999;3:317–324. doi: 10.1053/eujp.1999.0137. [DOI] [PubMed] [Google Scholar]
- Flor H, Turk DC. Psychophysiology of chronic pain: Do chronic pain patients exhibit symptom-specific psychophysiological responses? Psychol Bull. 1989;105:215–259. doi: 10.1037/0033-2909.105.2.215. [DOI] [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Porges SW. Accuracy of the LifeShirt® (Vivometrics) in the detection of cardiac rhythms. Biol Psychol. 2007;75:300–305. doi: 10.1016/j.biopsycho.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Lesions of the amygdala block conditional hypoalgesia on the tail flick test. Brain Res. 1993;612:253–257. doi: 10.1016/0006-8993(93)91669-j. [DOI] [PubMed] [Google Scholar]
- Karen GR, Malvin NJ, Sangeetha N, Joseph ES, Rollin MG. Psychiatric comorbidities in a community sample of women with fibromyalgia. Pain. 2006;124:117–125. doi: 10.1016/j.pain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Land C, Riccio DC. D-cycloserine: Effects on long-term retention of a conditioned response and on memory for contextual attributes. Neurobiol Learn Mem. 1999;72:158–168. doi: 10.1006/nlme.1998.3897. [DOI] [PubMed] [Google Scholar]
- Laux L, Vossel G. Theoretical and methodological issues in achievement related stress and anxiety research. In: Krohne HW, Laux L, editors. Achievement, Stress, and Anxiety. Washington: Hemisphere; 1982. pp. 3–18. [Google Scholar]
- Leeuw M, Goossens M, Linton S, Crombez G, Boersma K, Vlaeyen J. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- Lesley MA. Strategies for managing fibromyalgia. Am J Med. 2009;122:S31–S43. doi: 10.1016/j.amjmed.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, Pine DS, Grillon C. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behav Res Ther. 2009;47:111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok EYC, Mok CC, Cheng CW, Cheung EFC. Prevalence and determinants of psychiatric disorders in patients with rheumatoid arthritis. Psychosomatics. 2010;51:338–3388. doi: 10.1176/appi.psy.51.4.338. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. J Exp Psychol Anim Behav Process. 2002;28:3–26. [PubMed] [Google Scholar]
- Lutz J, Jäger L, DeQuervain D, Till K, Frank P, Martina W, Beyer A, Stahl R, Zirngibl B, Morhard D, Reiser M, Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: A diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58:3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Selective processing of threat cues in anxiety states. Behav Res Ther. 1985;23:563–569. doi: 10.1016/0005-7967(85)90104-4. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: Effects of affective picture modulation. Psychosom Med. 2001;63:79–90. doi: 10.1097/00006842-200101000-00010. [DOI] [PubMed] [Google Scholar]
- Meulders A, Vansteenwegen D, Vlaeyen JW. Women, but not men, report increasingly more pain during repeated (un)predictable painful electrocutaneous stimulation: Evidence for mediation by fear of pain. Pain. 2012;153:1030–1041. doi: 10.1016/j.pain.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Neeck G. Pathogenic mechanisms of fibromyalgia. Ageing Res Rev. 2002;1:243–255. doi: 10.1016/s1568-1637(01)00004-6. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. The Neurosci. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Odling Smee F. Background stimuli and the inter-stimulus interval during Pavlovian conditioning. Q J Exp Psychol. 1975;27:387–392. doi: 10.1080/14640747508400498. [DOI] [PubMed] [Google Scholar]
- Park SH, Bang SM, Nam E, Cho EK, Shin DB, Lee JH, Ahn JY. A randomized double-blind placebo-controlled study of low-dose intravenous lorazepam to reduce procedural pain during bone marrow aspiration and biopsy. Pain Medicine. 2008;9:249–252. doi: 10.1111/j.1526-4637.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins J, Nicholas P, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. Fear and anxiety: Divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- Schett G, Firestein GS. Mr Outside and Mr Inside: Classic and alternative views on the pathogenesis of rheumatoid arthritis. Ann Rheum Dis. 2010;69:787–789. doi: 10.1136/ard.2009.121657. [DOI] [PubMed] [Google Scholar]
- Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–184. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Staud R. Biology and therapy of fibromyalgia: Pain in fibromyalgia syndrome. Arthritis Res Ther. 2006;8:208. doi: 10.1186/ar1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait RC, Chibnall JT, Krause S. The Pain Disability Index: Psychometric properties. Pain. 1990;40:171–182. doi: 10.1016/0304-3959(90)90068-O. [DOI] [PubMed] [Google Scholar]
- Thieme K, Flor H, Turk DC. Psychological pain treatment in fibromyalgia syndrome: Efficacy of operant behavioural and cognitive behavioural treatments. Arthritis Res Ther. 2006;8:R121. doi: 10.1186/ar2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- Wolfe F. The relation between tender points and fibromyalgia symptom variables: Evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis. 1997;56:268–271. doi: 10.1136/ard.56.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Micha A, Clark PG, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell JI, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]