Abstract
Aim
Favipiravir and oseltamivir are antiviral compounds used for the treatment of influenza infections. We have aimed to investigate the efficacy of the compounds in combination to treat influenza H1N1 virus infections in mice.
Materials & methods
Mice infected with pandemic influenza A/California/04/2009 (H1N1pdm) virus or an oseltamivir-resistant (H275Y neuraminidase mutation) influenza A/Mississippi/ 3/2001 (H1N1) virus were treated orally with inhibitors twice a day for 5 days starting 4 h after infection.
Results
Complete protection from death was afforded by favipiravir treatments of 100 mg/kg/day, but lower doses were less effective. Combinations of oseltamivir (1 and 3 mg/kg/day) with favipiravir (3, 10 and 30 mg/kg/day) resulted in a synergistic improvement in survival rates against H1N1pdm infections. Significant reductions in lung virus titers also occurred. Against the H275Y virus infection, oseltamivir alone was only 30% protective from death at 100 mg/kg/day, but combinations of the two compounds produced a synergistic improvement in survival rate.
Conclusion
The utility of treating H1N1 influenza virus infections with oseltamivir and favipiravir in combination has been established.
Keywords: antiviral, drug combination, drug resistance, favipiravir, H1N1, influenza, mice, oseltamivir, T-705, treatment
The threat of severe infections requiring hospitalization caused by the emerging pandemic influenza A (H1N1pdm) virus created a significant concern in 2009 and underscores the need for new and effective antivirals. Especially needed are agents directed at a different parts of the influenza replication cycle besides the M2 ion channel and viral neuraminidase, which are targeted by currently approved drugs. The H1N1pdm virus that emerged in 2009 is resistant to the drugs amantadine and rimantadine [1], as are the majority of currently circulating H3N2 viruses [2,3] and most highly pathogenic H5N1 avian viruses [4]. However, the 2009 pandemic virus was sensitive to the neuraminidase inhibitors oseltamivir and zanamivir [5–7]. This is in contrast to the 2007 Brisbane-like seasonal H1N1 viruses in circulation, which just prior to that time were resistant to the viral neuraminidase-inhibiting drug oseltamivir [8–10]. Fortunately this shift in circulating H1N1 virus strains has preserved the utility of oseltamivir for influenza treatment, at least until new drug-resistant viruses emerge.
The first line of defense against influenza is vaccination. Antiviral therapies are important as a second line of defense, especially when an antigenically divergent influenza virus variant emerges during an ongoing influenza season or after vaccine production has occurred. Oseltamivir and zanamivir, which are influenza viral neuraminidase inhibitors, are approved drugs for the treatment of influenza virus infections [11], although zanamivir is not presently in use. Within the last several years, favipiravir (also referred to as T-705) has been identified as a potential new drug candidate that has been shown to be effective against influenza virus infections in cell culture and in mice [12–15]. Clinical trials with favipiravir are ongoing in Japan. The compound undergoes intracellular metabolism from a heterocycle to a nucleoside triphosphate analog that inhibits influenza virus’ RNA polymerase [16]. Favipiravir inhibits wild-type and oseltamivir-resistant isolates of the novel 2009 H1N1pdm virus [17]. Since favipiravir has a different mechanism of action than the adamantanes or neuraminidase inhibitors, it may be useful in treating the currently identified drug-resistant influenza virus strains.
Numerous drug combination experiments have been conducted in mice infected with influenza viruses (for a review up to 2010, see [18]) [19,20]. Recently, human studies have been conducted using oseltamivir with zanamivir [21,22], as well as the triple combination of oseltamivir, amantadine and ribavirin [23]. The studies were too small in scope to assess whether the combinations were superior to oseltamivir alone. The expectation of combination treatments is that they may prevent or delay the emergence of drug-resistant viruses, and be more efficacious in ameliorating symptoms of infection. We reported that favipiravir and oseltamivir were synergistic in the treatment of certain seasonal H1N1 and H3N2 virus infections, as well as a low-pathogenic H5N1 virus infection in mice [15]. Low doses of each inhibitor, when combined, produced an improvement in the numbers of survivors. More recently we demonstrated that the combination of favipiravir and peramivir was more effective than either compound alone in protecting mice from death following H1N1pdm virus infections in mice [20]. In the present investigation, combinations of favipiravir and oseltamivir were evaluated for efficacy against 2009 influenza A H1N1pdm virus infections in mice. In addition, these inhibitors were also tested in a newly developed oseltamivir-resistant (H275Y, N1 numbering) virus infection model in mice [24].
In order to conduct evaluations to detect synergy in mouse models, it is necessary that suboptimal doses of each inhibitor be tested. No meaningful conclusions can be derived if all of the animals survive the infection. By performing studies in this manner, we are not implying that the compounds should be used in the clinic at suboptimal doses, since the goal is a maximum achievable benefit at the prescribed full doses.
Materials & methods
Viruses & cells
The influenza A/California/04/2009 (H1N1-pdm) strain was originally obtained from Elena Govorkova (St Jude Children’s Research Hospital, TN, USA). The virus was adapted for mice by Natalia Ilyushina at the same institution by a published procedure [25]. In our laboratory the virus was amplified in MDCK cells (purchased from the American Type Culture Collection, VA, USA) to prepare stocks for mouse experiments. The virus pool was pretitrated in mice prior to performing these studies to determine an appropriate challenge dose.
Influenza A/Mississippi/3/2001 (H1N1) containing an H275Y mutation in the neuraminidase gene was obtained from the Neuraminidase Inhibitor Surveillance Network (Melbourne, Australia). The virus was passaged seven times in mice to increase its virulence, then once in MDCK cells. The virus was later titrated in BALB/c mice for lethality. The mouse-adapted virus was genetically analyzed and confirmed to be a homogeneous population of H275Y-containing virus [24]. It is >1000-fold more resistant to oseltamivir carboxylate in cell culture than influenza A/California/04/2009 (H1N1pdm) virus, which is similar to the resistance found in an influenza A/Hong Kong/2369/2009 (H1N1pdm) H275Y virus [24].
Test compounds
Oseltamivir phosphate (unformulated, and referred to simply as oseltamivir in this article; when formulated in capsules with excipients it is the drug Tamiflu®) was obtained from Roche (CA, USA). The calculated mg/kg/day doses of oseltamivir reported in all the tables and figures are those of the free base (active) form of the drug and not of oseltamivir phosphate. Favipiravir was obtained from Toyama Chemical Company (Tokyo, Japan). Compounds were shipped to us by the sponsors at ambient temperature and stored in like manner. Favipiravir (at 2× concentration) was prepared in sterile 0.4% carboxymethylcellulose (CMC) in water for oral (by gavage) treatment of mice, whereas oseltamivir (at 2× concentrations) was dissolved in water. The compounds in solution were combined with each other, with 0.4% CMC, or with water to achieve the 1× final concentrations required for the specified mg/kg/day doses. The resulting amount of CMC per solution was 0.2% in water. The placebo control for the studies was 0.2% CMC. By this manner of drug preparation, each animal was treated only once per time point with 0.1 ml. Compounds in solution or suspension were stored at 4°C between dosing.
Animals
Female 18–20-g (6–7 weeks of age) BALB/c mice were obtained from Charles River Laboratories (MA, USA) for this study. They were maintained on standard rodent chow and tap water ad libitum. Antiviral experiments were initiated 48 h after receipt of the mice.
General animal experimental design
Mice were anesthetized by intraperitoneal injection of ketamine/xylazine (50/5 mg/kg) followed by infection intranasally with a 90-µl suspension of influenza virus. The infection inoculum of approximately 104.3 50% cell culture infectious doses/mouse equated to three 50% mouse lethal challenge doses. Groups of mice were administered favipiravir, oseltamivir or placebo by oral gavage twice on day 0 beginning 4 h after virus exposure, and then twice per day (at 12-h intervals) for 4 more days after virus exposure to mice. The mice were individually weighed prior to treatment, followed by weighing every other day thereafter for a 21-day period. Mice whose body-weights fell below 30% of initial weight or animals that were moribund upon inspection were humanely euthanized and included in mortality counts. Ten mice of each group (10–20 placebos, as indicated in the Tables 1–3) were followed for survival up to day 21.
Table 1.
Effects of oral gavage administration of favipiravir and oseltamivir, either alone or in combination, on the survival of mice inoculated with pandemic influenza A/California/04/2009 (H1N1pdm) virus.
| Compound (mg/kg/day) | Oseltamivir (3) | Oseltamivir (1) | Oseltamivir (0.3) | Oseltamivir (0.1) | Oseltamivir (0) |
|---|---|---|---|---|---|
| Favipiravir (30) | 10/10*** (>21) | 6/10** (9.3 ± 2.6) | 4/10* (7.8 ± 1.2) | 4/10* (8.0 ± 0.9) | 1/10 (7.4 ± 1.3) |
| Favipiravir (10) | 9/10*** (9.0) | 3/10* (8.4 ± 1.3) | 2/10 (8.1 ± 1.9) | 1/10 (7.8 ± 0.8) | 0/10 (6.9 ± 0.9) |
| Favipiravir (3) | 8/10*** (9.5 ± 2.1) | 3/10* (9.7 ± 2.0) | 2/9 (8.9 ± 2.5) | 1/10 (8.0 ± 1.1) | 0/10 (8.0 ± 1.6) |
| Favipiravir (1) | 7/10*** (8.7 ± 1.2) | 3/10* (8.4 ± 1.7) | 4/10* (6.7 ± 1.2) | 1/10 (7.6 ± 1.1) | 0/10 (6.7 ± 1.1) |
| Favipiravir (0) | 5/10** (7.8 ± 0.8) | 4/10* (7.2 ± 0.8) | 4/10* (7.2 ± 1.3) | 1/10 (7.0 ± 0.9) | 1/20 (6.5 ± 0.6) |
Results are demonstrated as survivors/total (mean day of death of mice that died during the 21-day observation period ± standard deviation). The mean day of death test is statistically analyzed only for mice that die. Mean without standard deviation data means only one mouse died. >21 indicates that all of the mice lived through the end of the experiment.
p < 0.05;
p < 0.01;
p < 0.001, compared with placebo.
Table 3.
Effects of oral gavage administration of favipiravir and oseltamivir, either alone or in combination, on the survival of mice inoculated with influenza A/Mississippi/03/2001 (H1N1) H274Y (oseltamivir-resistant) virus.
| Compound (mg/kg/day) | Oseltamivir (100) | Oseltamivir (50) | Oseltamivir (25) | Oseltamivir (0) |
|---|---|---|---|---|
| Favipiravir (100) | –† | –† | –† | 10/10** |
| Favipiravir (50) | 10/10** | 10/10** | 10/10** | 10/10** |
| Favipiravir (25) | 9/10** (10.0) | 10/10** | 10/10** | 6/10** (9.25 ± 2.9) |
| Favipiravir (12.5) | 6/10** (11.5 ± 1.3) | 10/10** | 2/10 (10.0 ± 0.8) | 0/10 (10.0 ± 2.9) |
| Favipiravir (0) | 3/10* (11.7 ± 1.4) | 0/9 (9.4 ± 1.6) | 0/10 (9.4 ± 0.5) | 0/10 (8.7 ± 0.9) |
Results are demonstrated as survivors/total (mean day of death of mice that died during the 21-day observation period ± standard deviation). Mean without standard deviation data means only one mouse died.
p < 0.01;
p < 0.001, compared with placebo.
Not determined.
We performed these studies (as other investigators do) with the assumption that infected animals that die after the treatment period do so as a result of influenza virus infection and not from treatment trauma or from an unidentified cause. Untreated animals usually begin to die from influenza-like illness starting at approximately day 6 of the infection (note the earliest mean day of death values in Tables 1 & 2 [realizing that the data are from treated mice]). Oral gavage treatments sometimes result in mortality. In these studies, we excluded two mice that died during the treatment period and indicate this in the manuscript, resulting in initial group sizes of nine animals each in two instances (see Tables 1 & 3).
Table 2.
Follow-up experiment demonstrating the effects of oral gavage administration of favipiravir and oseltamivir, either alone or in combination, on the survival of mice inoculated with pandemic influenza A/California/04/2009 (H1N1pdm) virus.
| Compound (mg/kg/day) | Oseltamivir (3) | Oseltamivir (1) | Oseltamivir (0) |
|---|---|---|---|
| Favipiravir (100) | 10/10*** (>21) | 10/10*** (>21) | 10/10*** (>21) |
| Favipiravir (30) | 9/10*** (9.0) | 10/10*** (>21) | 4/10* (9.0 ± 2.9) |
| Favipiravir (10) | 10/10*** (>21) | 9/10*** (10.0) | 3/10* (8.1 ± 1.3) |
| Favipiravir (0) | 8/10*** (6.5 ± 0.7) | 6/10** (8.3 ± 1.0) | 0/10 (6.8 ± 0.9) |
Results are demonstrated as survivors/total (mean day of death of mice that died during the 21-day observation period ± standard deviation). The mean day of death test is statistically analyzed only for mice that die. Mean without standard deviation data means only one mouse died. >21 indicates that all of the mice lived through the end of the experiment.
p < 0.05;
p < 0.01;
p < 0.001, compared with placebo.
It should be indicated that we performed many experiments with these or related viruses with either oseltamivir or favipiravir monotherapy prior to these studies, and had an understanding of doses that would be appropriate (i.e., suboptimal in terms of preventing mortality) for the drug combination experiments reported here. Regardless of this preparation, this did not preclude variability seen from experiment to experiment. After the first study with H1N1pdm virus reported here, it was deemed important to conduct a follow-up experiment using different doses of each compound in order to support the results of the first experiment.
Test compound toxicity determination
Three additional animals were treated with each drug using the treatment schedules described above. However, these animals were not exposed to virus and constituted toxicity controls for each drug and treatment regimen. Toxicity was evaluated by visual observation, in terms of weight loss and visually apparent adverse events, which could include ruffling of fur, lethargy, paralysis, incontinence, repetitive circular motion and aggression. No hematology, blood chemistry or histopathology analyses were performed.
Lung virus titer determinations
The lungs from five additional mice per group were removed from sacrificed animals on days 3 and 6. The lungs were weighed then frozen at −80°C. Later, thawed lungs were homogenized in cell culture medium and assayed in 96-well microplates of MDCK cells for infectious virus by an end point dilution method using four micro-wells per titrated dilution [26,27]. Virus titers were converted to 50% cell culture-infectious doses per gram of lung tissue (50% cell culture infectious doses/g).
Statistical analyses
Survival curves were plotted by the Kaplan–Meier method and analyzed by the Mantel–Cox log-rank test. These analyses revealed significant differences among the treatment groups. Therefore, pairwise comparisons of survivor curves (placebo vs treatment) were subsequently analyzed by the Gehan–Breslow–Wilcoxon test, and the relative significance was adjusted to a Bonferroni-corrected significance threshold for the number of treatment comparisons done. Significant lung virus titer differences were evaluated by two-way ANOVA with Dunnett’s multiple comparisons test. Analyses were made using Prism® software (GraphPad Inc., CA, USA).
Drug–drug interactions were analyzed by the 3D model of Prichard and Shipman [28], using the MacSynergy II software program (kindly provided by Mark Prichard, University of Alabama at Birmingham, AL, USA) at 95% CIs. Descriptions of antagonistic, additive or synergistic interactions using this computer model have been described for data represented as percentages [29]. Briefly, values calculated as 0–25, 25–50, 50–100 and >100 µm2 unit% in either a positive or negative direction using the software are defined as insignificant synergy or antagonism (indifference), minor synergy or antagonism, moderate synergy or antagonism, or strong synergy or antagonism, respectively. The reported values represent the net volume of synergy (total volume of synergy minus total volume of antagonism) for each set of data. The volume is essentially a compilation of the percentages above or below the baseline (see Figures 1–3). The expected value for each combination, assuming no interaction, is zero (i.e., no percentage of survival increase either above or below baseline).
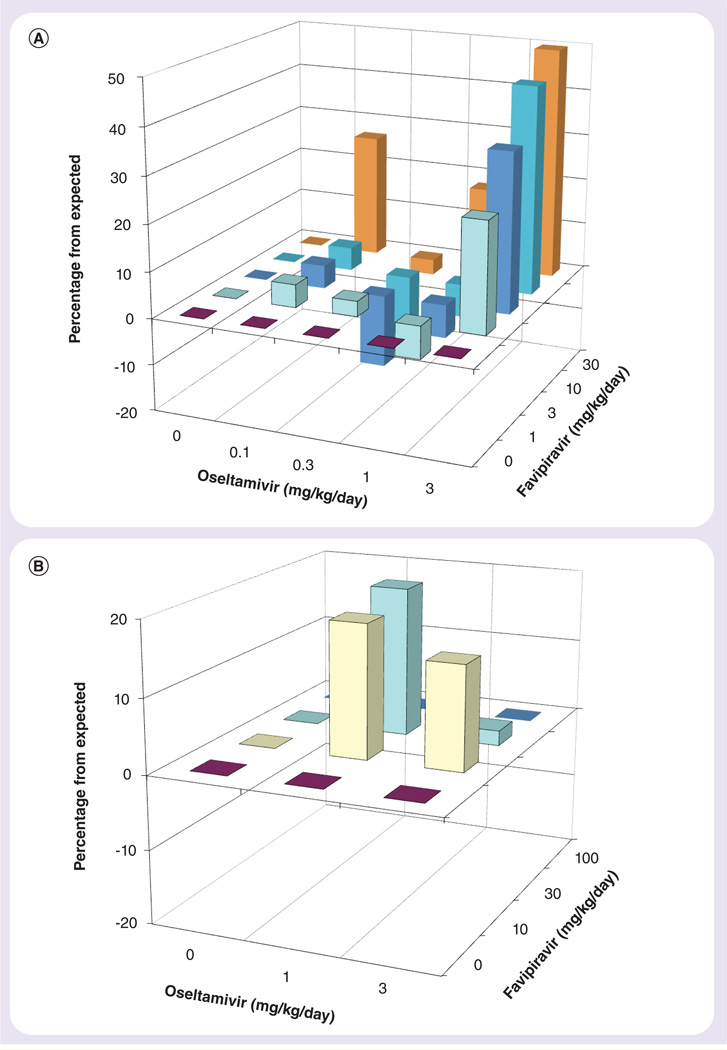
Figure 1. Synergy plot of the interaction between favipiravir and oseltamivir on survival from an influenza A/California/04/2009 (H1N1pdm) virus infection in mice.
The volumes of synergy for (A & B) are 139 and 58, respectively, using data from Tables 1 & 2, respectively.
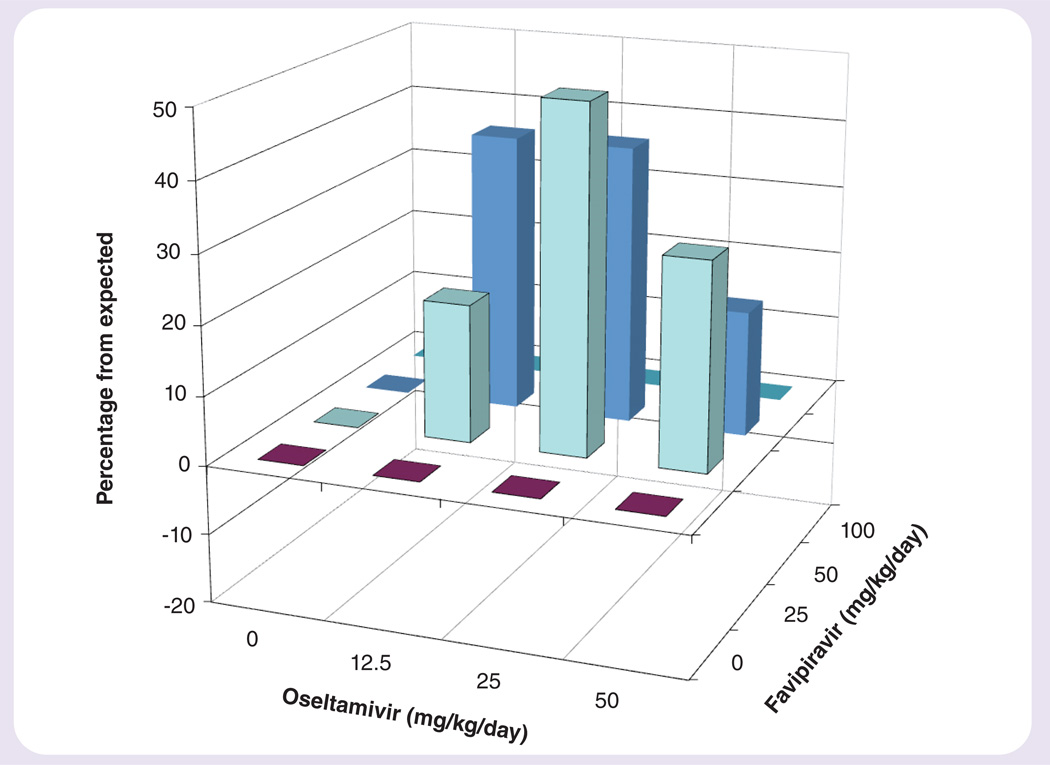
Figure 3. Synergy plot of the interaction of favipiravir and oseltamivir on survival from an influenza A/Mississippi/3/2001 (H1N1) H275Y (oseltamivir-resistant) virus infection in mice.
The volume of synergy for the interaction is 248 using data from Table 3.
Results
Toxicity evaluations
The compounds were evaluated in combination for possible toxic interactions, as manifested by weight loss and other adverse reactions such as diarrhea, ruffled fur, tremors, and so on. At the highest concentration of each compound used in combination, there was no evidence of toxicity. The animals in treated and placebo groups were similar in appearance and in bodyweight (data not shown).
Treatment of H1N1pdm virus infections in mice
Various low doses of favipiravir and oseltamivir were combined to determine the efficacy of combination therapy against an influenza A/California/04/2009 (H1N1pdm) virus infection in mice (Table 1). In this experiment, 95% of mice in the placebo control group succumbed to the infection. Favipiravir treatments alone were ineffective at all doses tested (1–30 mg/kg/day) in this experiment. Oseltamivir doses of 0.3, 1 and 3 mg/kg/day provided partial protection from death due to virus infection. Increases in the numbers of survivors over monotherapy (i.e., oseltamivir or favipiravir alone) were evident for the 3-mg/kg/day dose of oseltamivir combined with 1–30 mg/kg/day of favipiravir, and for oseltamivir at 1 mg/kg/day combined with 30 mg/kg/day of favipiravir. An analysis of drug interactions using MacSynergy II software is shown in Figure 1A. The region of synergy is primarily clustered at the highest dose of each inhibitor. The volume of synergy for the experiment was 139, which is indicative of strong synergy.
A follow-up experiment was performed, in which favipiravir was used up to 100 mg/kg/day (Table 2) to treat an H1N1pdm virus infection. Favipiravir protected 30, 40 and 100% of treated mice from the lethal effects of the virus infection at doses of 10, 30 and 100 mg/kg/day, respectively. In this experiment, the 10- and 30-mg/kg/day doses of favipiravir provided greater protection than what was reported in the first study (see Table 1). Oseltamivir was 60 and 80% protective at doses of 1 and 3 mg/kg/day, respectively. Mortality was observed in all placebo-treated animals. All placebo-treated animals died from infection in the experiment. Combined doses of either 10 or 30 mg/kg/day of favipiravir with 1 or 3 mg/kg/day of oseltamivir increased survival percentages over monotherapy (Table 2). A 3D analysis of the results is presented in Figure 1B. Here, the volume of synergy for the limited number of doses evaluated was 58, which was moderate synergy.
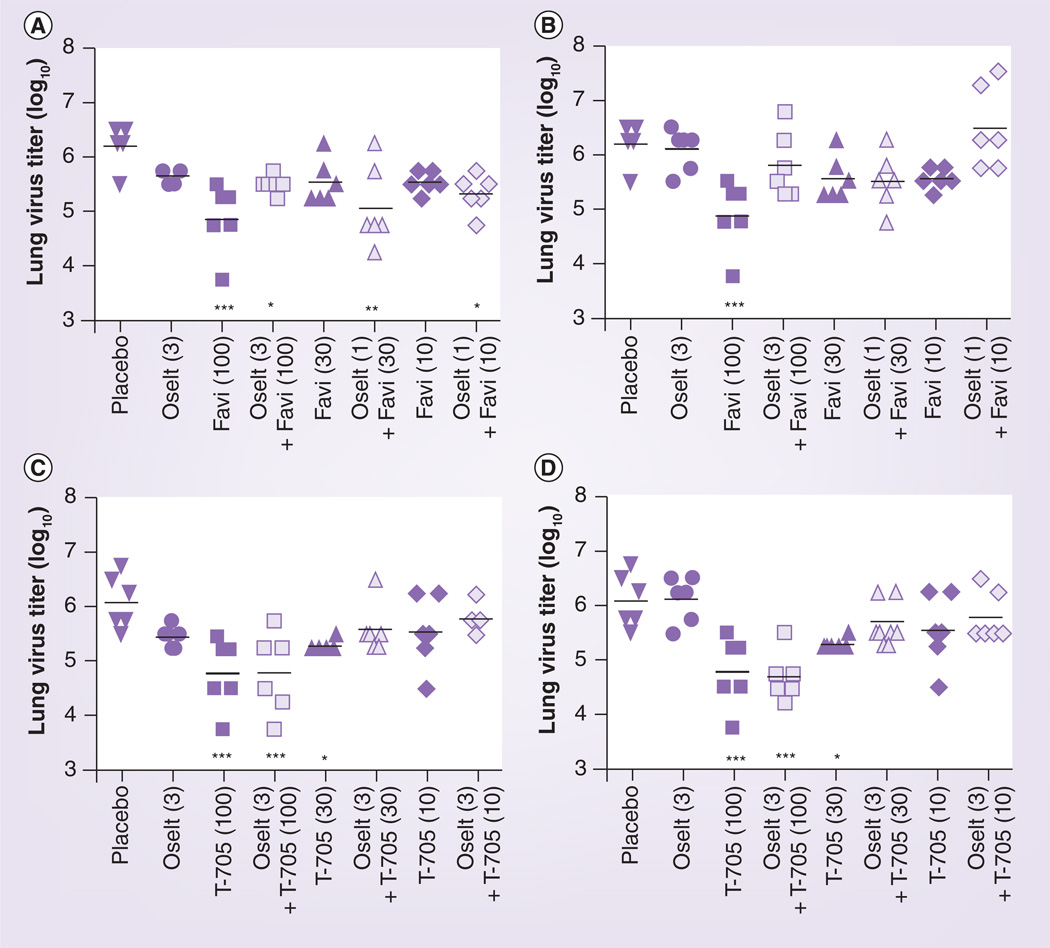
Lung virus titers from H1N1pdm virus-infected mice were determined as part of the experiment reported in Table 2, and these are presented graphically in Figure 2. Significant decreases in lung virus titers were noted in groups treated with favipiravir alone (at 10, 30 or 100 mg/kg/day) or with favipiravir combined with oseltamivir (at 3 mg/kg/day). However, the addition of oseltamivir to any dose of favipiravir did not result in lower viral titers. Analyses of drug interactions for lung infection parameters did not demonstrate significant synergy or antagonism (data not shown). Favipiravir was also combined with 1 mg/kg/day of oseltamivir for purposes of determining virus titers. The results (not shown) were similar to those reported in Figure 2.
Figure 2. Effects of the combination of favipiravir and oseltamivir on lung virus titers (log10 50% cell culture infectious doses/g) during an influenza A/California/04/2009 (H1N1pdm) virus infection in mice.
Oral gavage treatments were administered twice a day for 5 days starting 4 h after virus exposure. Viral titers were determined from lungs harvested on day 3 (A & B) and day 6 (C & D). mg/kg/day doses are given in parentheses. This study ran concurrently with that of Table 2.
*p < 0.05; **p < 0.01; ***p < 0.001, compared with placebo.
Favi: Favipiravir; Oselt: Oseltamivir.
Treatment of oseltamivir-resistant H1N1 virus infections in mice
Combinations of oseltamivir and favipiravir were used to treat an influenza A/Mississippi/3/2001 (H1N1; H275Y – viral neuraminidase mutation resulting in oseltamivir resistance) infection in mice (Table 3). The 50- and 100-mg/kg/day doses of favipiravir were 100% protective from death, and lower doses were partially protective. The highest dose of oseltamivir was 30% protective, as has been reported previously [24]. When oseltamivir was combined with favipiravir, the numbers of survivors were greatly increased. Analysis of drug interactions was performed (Figure 3) and a volume of synergy value of 248 was found, indicating strong synergy.
Discussion
Combinations of favipiravir and oseltamivir at suboptimal doses for each compound (i.e., when used individually, and did not provide 100% protection from death) were shown to provide improvements in the survival rate. Based upon the time of initiation of the first treatments relative to virus exposure, the experimental treatments were considered as postexposure prophylaxis. Suboptimal doses were used for research purposes; we are not advocating using them in the clinic. Analyses of the resulting drug–drug interactions indicated that a moderate-to-high degree of synergy was achieved. The improved effect on survival against H1N1pdm virus occurred at doses of each compound that were similar to those previously reported for combinations of favipiravir and oseltamivir against seasonal H1N1 and H3N2 virus infections, and against low pathogenic avian H5N1 virus infections in mice [15]. In the infection of mice with the oseltamivir-resistant influenza A/Mississippi/3/2001 (H1N1) H275Y virus, the dose of oseltamivir had to be increased (relative to the doses used for the H1N1pdm virus infection) to higher levels to provide even a weak (30% survival) benefit, as was done previously [24]. At these oseltamivir levels, the combination of oseltamivir and favipiravir provided a synergistic improvement in survival rate. Two things need to be understood from these data. First, viral resistance to oseltamivir is seldom absolute; it just requires higher doses to achieve an effect. Second, and more importantly, the dose of oseltamivir approved for the clinic may be lower than the required virus-inhibitory concentration for the particular drug-resistant virus. From what has been reported, a dose of approximately 30 mg/kg/day in mice is equivalent to the human dose [19]. Thus, the doses used here were more than triple the approved human dose. There may be no benefit from treatment of H275Y virus infections in humans with oseltamivir when used alone or in combination, unless the dosage is increased. Regarding the present work, this represents the first reports of drug combination studies of oseltamivir and favipiravir against H1N1pdm and oseltamivir-resistant H275Y virus infections in mice. The results with the H1N1pdm virus were similar to our published work with seasonal H1N1 infections in mice [15], indicating that they are confirmatory.
In the two reported studies with pandemic H1N1 virus, variability in response to particular doses affected the cleanness of the results. Because of this, we felt that the second experiment was necessary to confirm the results of the first study. It is always hoped when studies are first planned that the results will be consistent from one experiment to another. Variability in survival rates at particular drug doses, particularly doses that are only partially protective from death, are not uncommon. It is our observation that the H1N1pdm virus infection model is more prone to this problem of inconsistency than seasonal viruses that we have used, such as A/NWS/33 (H1N1) and A/Victoria/3/75 (H3N2) [15]. However, the pandemic H1N1 virus is the one more often favored by researchers due to its recent emergence, and not necessarily for consistency of response in studies.
In this report we used a wild-type (oseltamivirsensitive) pandemic H1N1 and oseltamivir-resistant H274Y seasonal H1N1 virus infections in mice for evaluating drug combination efficacy. It could be argued that better models would be the use of genetically closely matched viruses – one sensitive to oseltamivir and the other resistant. Historically, it has been difficult to develop lethal infections in mice with oseltamivir-resistant viruses [30,31]. It has only been recently that oseltamivir-resistant viruses capable of causing lethal infections in mice have been reported. In the case of the present research, there is no guarantee that the mouse-adapted wild-type influenza A/California/04/2009 (H1N1pdm) virus can be converted to an H275Y virus by genetic manipulation and still retain its virulence in mice. Previously, we reported the adaptation of a pandemic influenza A/Hong Kong/2369/2009 (H1N1pdm) H275Y virus in mice [24]. This virus is closely related to the A/California/04/2009 (H1N1pdm) virus. The A/Hong Kong/2369/2009 (H1N1pdm) H275Y virus infection in mice was weakly treatable with high doses of oseltamivir in a manner very similar to what was found with the A/Mississippi/3/2001 (H1N1) H275Y virus infection in mice [24]. Our prediction is that any future work with genetically closely matched viruses (one sensitive to oseltamivir and the other resistant) will give results similar to those presented here. Regarding our selection of the A/Mississippi/3/2001 (H1N1) H275Y virus for the current studies, infection with this virus gives more consistent mortality from experiment to experiment compared with the A/Hong Kong/2369/2009 (H1N1pdm) H275Y virus [24]. Further work with the A/Mississippi/3/2001 (H1N1) H275Y mouse model will continue as novel inhibitors are discovered and used in combination with oseltamivir.
Lung virus titers were reduced by treatment with favipiravir alone or with favipiravir combined with oseltamivir, relative to placebo treatment. It was not apparent that the combination resulted in further decreases in lung virus titers compared with favipiravir monotherapy. We have reported a similar effect in evaluating combinations of amantadine, oseltamivir and ribavirin [27]. Survival appears to be a more sensitive parameter for assessing differences in drug activity among treatment groups.
Combination therapy using antiviral agents may reduce the incidence of drug resistance emergence. Ilyushina and colleagues reported that drug-resistant influenza viruses are less likely to emerge with combination chemotherapy in cell culture [32]. However, it is much more difficult to assess drug resistance development in short-term experiments with animals. Amantadine-resistant viruses have been recovered from normal mice infected with wild-type virus and treated with amantadine [33], but recovery of amantadine-resistant viruses in vivo may be attributable to the fact that influenza viruses rapidly develop resistance to amantadine. Oseltamivir-resistant virus has been recovered from severe combined immunodeficient mice infected with wild-type virus and treated with oseltamivir [34], but not from normal mice. Attempts to select for favipiravir resistance either in cell culture or in mice have not been reported. The low-pathogenic influenza A/Duck/MN/1525/81 (H5N1) virus has been propagated in cell culture in the presence of 5–20 µM of favipiravir for 25 passages without recovering drug-resistant virus [Smee DF, Unpublished Data]. We did not attempt to recover drug-resistant virus in the present in vivo work. We believe that the highly conserved influenza virus RNA polymerase cannot be readily mutated under favipiravir treatment pressure without losing its ability to function efficiently. The recent report that favipiravir induces lethal mutagenesis in influenza H1N1 virus in vitro [35] supports this hypothesis.
Combinations of favipiravir and oseltamivir were found to be effective against these two H1N1 virus infections, with no adverse effects associated with the treatments of the mice. The data support the premise that the combination of favipiravir and oseltamivir may be more effective in treating pandemic influenza A H1N1 virus infections in humans compared with monotherapy. In addition, H275Y-carrying viruses that are resistant to oseltamivir were effectively treated in mice with the combination of oseltamivir and favipiravir. In general, patients with influenza will not know whether they are infected with an oseltamivir-resistant virus or not. Whether patients are infected with oseltamivir-sensitive or oseltamivir-resistant virus (which is usually determined from nasal or throat swabs collected during acute infection but not assessed until after the infection has run its course or else after the individual has expired), treatment with a drug combination such as favipiravir plus oseltamivir should be more beneficial than treatment with oseltamivir alone. These studies provide support for evaluating oseltamivir and favipiravir in combination in humans infected with influenza (particularly in severe cases) once favipiravir has been US FDA approved.
Future perspective
To date there are no FDA-approved drugs for combination use against H1N1 virus infections in humans. The data from many studies indicate that drug combinations are more beneficial than monotherapy. The emergence of drug-resistant viruses against neuraminidase inhibitors will likely be suppressed with the use of other drugs in combination. Once some of the newer antiviral compounds are approved, we envision that physicians may use them in combination for treating severe cases of influenza. Treatment options are limited because the only currently available drugs are oseltamivir and zanamivir.
Executive summary.
Treatment of H1N1pdm virus infections in mice
-
▪
Low doses of oseltamivir combined with favipiravir were synergistically effective in reducing mortality in infected animals, as determined by the 3D MacSynergy method.
-
▪
Certain doses of favipiravir, used alone and in combination, significantly reduced lung virus titers compared with placebo.
-
▪
Combinations of oseltamivir plus favipiravir did not provide a significant reduction in lung virus titers compared with favipiravir by itself.
Treatment of oseltamivir-resistant H1N1 H275Y virus infections in mice
-
▪
Much higher doses of oseltamivir were required to improve response to this infection compared with the H1N1pdm virus infection, as was expected.
-
▪
Combinations of oseltamivir and favipiravir were synergistically effective in reducing mortality in animals infected with the H275Y virus.
Acknowledgments
Y Furuta is an employee of Toyama Chemical Company and is involved in the development of favipiravir as a treatment for human influenza virus infections. This work was supported by contracts N01-AI-30063 (awarded to Southern Research Institute, Birmingham, AL, USA) and HHSN272201000039I from the Virology Branch and the Respiratory Diseases Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, NIH, USA.
Footnotes
Publisher's Disclaimer: Disclaimer
The contents of this article do not necessarily reflect the position or policy of the government and no official endorsement should be inferred. The animal experiments were conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University in the AAALAC-accredited Laboratory Animal Research Center. Work was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. No human subjects were used.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Mossad SB. The resurgence of swine-origin influenza A (H1N1) Cleve. Clin. J. Med. 2009;76(6):337–343. doi: 10.3949/ccjm.76a.09047. [DOI] [PubMed] [Google Scholar]
- 2.Deyde VM, Xu X, Bright RA, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007;196(2):249–257. doi: 10.1086/518936. [DOI] [PubMed] [Google Scholar]
- 3.Hata M, Tsuzuki M, Goto Y, et al. High frequency of amantadine-resistant influenza A (H3N2) viruses in the 2005–2006 season and rapid detection of amantadine-resistant influenza A (H3N2) viruses by MAMA-PCR. Jpn J. Infect. Dis. 2007;60(4):202–204. [PubMed] [Google Scholar]
- 4.Govorkova EA, Baranovich T, Seiler P, et al. Antiviral resistance among highly pathogenic influenza A (H5N1) viruses isolated worldwide in 2002–2012 shows need for continued monitoring. Antiviral Res. 2013;98(2):297–304. doi: 10.1016/j.antiviral.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall RJ, Peacey MP, Ralston JC, et al. Pandemic influenza A(H1N1)v viruses currently circulating in New Zealand are sensitive to oseltamivir. Euro. Surveill. 2009;14(30):19282. doi: 10.2807/ese.14.30.19282-en. [DOI] [PubMed] [Google Scholar]
- 6.Rungrotmongkol T, Intharathep P, Malaisree M, et al. Susceptibility of antiviral drugs against 2009 influenza A (H1N1) virus. Biochem. Biophys. Res. Commun. 2009;385(3):390–394. doi: 10.1016/j.bbrc.2009.05.066. [DOI] [PubMed] [Google Scholar]
- 7.Gubareva LV, Trujillo AA, Okomo-Adhiambo M, et al. Comprehensive assessment of 2009 pandemic influenza A (H1N1) virus drug susceptibility in vitro. Antivir. Ther. 2010;15:1151–1159. doi: 10.3851/IMP1678. [DOI] [PubMed] [Google Scholar]
- 8.Besselaar TG, Naidoo D, Buys A, et al. Widespread oseltamivir resistance in influenza A viruses (H1N1), South Africa. Emerg. Infect. Dis. 2008;14(11):1809–1810. doi: 10.3201/eid1411.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharan NJ, Gubareva LV, Meyer JJ, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301(10):1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- 10.Meijer A, Lackenby A, Hungnes O, et al. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg. Infect. Dis. 2009;15(4):552–560. doi: 10.3201/eid1504.081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michiels B, Van Puyenbroeck K, Verhoeven V, Vermeire E, Coenen S. The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PLoS ONE. 2013;8(4):e60348. doi: 10.1371/journal.pone.0060348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta Y, Takahashi K, Fukuda Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46(4):977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidwell RW, Barnard DL, Day CW, et al. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents Chemother. 2007;51(3):845–851. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiso M, Takahashi K, Sakai-Tagawa Y, et al. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc. Natl Acad. Sci. USA. 2010;107(2):882–887. doi: 10.1073/pnas.0909603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smee DF, Hurst BL, Wong MH, et al. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice. Antimicrob. Agents Chemother. 2010;54(1):126–133. doi: 10.1128/AAC.00933-09. Demonstrates that oseltamivir and favipiravir in combination are synergistically active against seasonal H1N1, H3N2 and low pathogenic avian H5N1 virus infections in mice.
- 16.Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49(3):981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sleeman K, Mishin VP, Deyde VM, Furuta Y, Klimov AI, Gubareva LV. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza And 2009 A(H1N1) viruses. Antimicrob. Agents Chemother. 2010;54(6):2517–2524. doi: 10.1128/AAC.01739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Govorkova EA, Webster RG. Combination chemotherapy for influenza. Viruses. 2010;2:1510–1529. doi: 10.3390/v2081510. Review of drug combination studies conducted up to 2010.
- 19.Nguyen JT, Smee DF, Barnard DL, et al. Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptible and amantadine-resistant influenza A viruses. PLoS ONE. 2012;7(1):e31006. doi: 10.1371/journal.pone.0031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarbet EB, Maekawa M, Furuta Y, Babu YS, Morrey JD, Smee DF. Combinations of favipiravir and peramivir for the treatment of pandemic influenza A/California/04/2009 (H1N1) virus infections in mice. Antiviral Res. 2012;94(1):103–110. doi: 10.1016/j.antiviral.2012.03.001. Reports on the synergistic benefit of favipiravir and peramivir in combination against H1N1pdm virus infections in mice.
- 21.Carrat F, Duval X, Tubach F, et al. Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households. Antivir. Ther. 2012;17(6):1085–1090. doi: 10.3851/IMP2128. [DOI] [PubMed] [Google Scholar]
- 22.Escuret V, Cornu C, Boutitie F, et al. Oseltamivir-zanamivir bitherapy compared with oseltamivir monotherapy in the treatment of pandemic 2009 influenza A(H1N1) virus infections. Antiviral Res. 2012;96(2):130–137. doi: 10.1016/j.antiviral.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Seo S, Englund JA, Nguyen JT, et al. Combination therapy with amantadine, oseltamivir and ribavirin for influenza A infection: safety and pharmacokinetics. Antivir. Ther. 2013;18(3):377–386. doi: 10.3851/IMP2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smee DF, Julander JG, Tarbet EB, Gross M, Nguyen J. Treatment of oseltamivir-resistant influenza A (H1N1) virus infections in mice with antiviral agents. Antiviral Res. 2012;96(1):13–20. doi: 10.1016/j.antiviral.2012.07.002. Shows the efficacies of oseltamivir, zanamivir, amantadine, rimantadine and ribavirin against oseltamivir-resistant influenza A (H1N1) virus infections in mice.
- 25. Ilyushina NA, Khalenkov AM, Seiler JP, et al. Adaptation of pandemic H1N1 influenza viruses in mice. J. Virol. 2010;84(17):8607–8616. doi: 10.1128/JVI.00159-10. Describes the development of the H1N1pdm virus used for the present study.
- 26.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–498. [Google Scholar]
- 27.Smee DF, Hurst BL, Wong MH, Bailey KW, Morrey JD. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob. Agents Chemother. 2009;53(5):2120–2128. doi: 10.1128/AAC.01012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prichard MN, Shipman C., Jr A three-dimensional model to analyze drug–drug interactions. Antiviral Res. 1990;14(4–5):181–205. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 29.Ilyushina NA, Hay A, Yilmaz N, Boon AC, Webster RG, Govorkova EA. Oseltamivirribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob. Agents Chemother. 2008;52(11):3889–3897. doi: 10.1128/AAC.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ives JA, Carr JA, Mendel DB, et al. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antiviral Res. 2002;55(2):307–317. doi: 10.1016/s0166-3542(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 31.Carr J, Ives J, Kelly L, et al. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo. Antiviral Res. 2002;54(2):79–88. doi: 10.1016/s0166-3542(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 32.Ilyushina NA, Bovin NV, Webster RG, Govorkova EA. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res. 2006;70(3):121–131. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Oxford JS, Logan IS, Potter CW. In vivo selection of an influenza A2 strain resistant to amantadine. Nature. 1970;226(5240):82–83. doi: 10.1038/226082a0. [DOI] [PubMed] [Google Scholar]
- 34.Ison MG, Mishin VP, Braciale TJ, Hayden FG, Gubareva LV. Comparative activities of oseltamivir and A-322278 in immunocompetent and immunocompromised murine models of influenza virus infection. J. Infect. Dis. 2006;193(6):765–772. doi: 10.1086/500464. [DOI] [PubMed] [Google Scholar]
- 35.Baranovich T, Wong SS, Armstrong J, et al. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J. Virol. 2013;87(7):3741–3751. doi: 10.1128/JVI.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]