Abstract
Purpose
In view of the previous reports demonstrating the positive outcome of bevacizumab in metastatic breast cancer, we aimed at comparing the role of bevacizumab-based metronomic combination with taxane (paclitaxel) versus a different taxane (docetaxel)-based regimen in addition to carboplatin as initial treatment for metastatic Her-2-negative breast cancer.
Patients and methods
This is a randomized Phase III study comparing the progression-free survival (PFS) and safety in Her-2-negative female patients with initial diagnosis of metastatic breast cancer with World Health Organization performance status of 0–II. Forty-one patients were randomized from September 2008 to July 2009 to receive either; (1) bevacizumab 5 mg/kg day 1 and day 15, carboplatin area under the curve (AUC)-2 day 1, day 8, and day 15, and paclitaxel 60 mg/m2 day 1, day 8, and day 15 (arm-I); or (2) carboplatin AUC-5 day 1, docetaxel 75 mg/m2 day 1 (arm-II). The Kaplan–Meier method was used for estimating survival; log-rank test for comparing survival curves. The primary end point was PFS, and secondary end points were overall survival (OS) and safety.
Results
PFS was 10 months in arm I versus 10.2 months in arm II (P = 0.9). The OS rate was similar in both arms: 37.6 months for arm I versus 37.4 months for arm II (P = 0.92). The toxicity revealed higher incidence of hypertension and proteinuria in arm I; however, with higher incidence of grade III–IV neutropenia and neutropenic fever in arm II. No treatment-related mortality was recorded.
Conclusion
Bevacizumab/carboplatin/paclitaxel and carboplatin/docetaxel show comparable PFS and OS with different toxicity profiles.
Keywords: taxanes, breast cancer, bevacizumab, Her-2 negative
Introduction
Breast cancer is the most common malignancy affecting women and the second cause of cancer death in the United States following bronchogenic carcinoma.1 A minority of patients initially present with metastatic breast cancer (MBC); however, it is estimated that 20%–30% of patients with early-stage disease will ultimately progress to metastatic disease.2 In this setting, including anthracyclines, taxanes and antimetabolites are the most preferred agents, but a single standard of care has not been identified.3 Actually, anthracycline-based regimens are commonly preferred in the treatment of metastatic disease, but due to cardiotoxicity, they have a limited role in patients previously exposed to anthracyclines in the adjuvant setting, hence the importance of developing new non-anthracycline regimens for the treatment of metastatic disease.
Platinum complexes are active in a wide range of solid tumors.4 Although both cisplatin and carboplatin have shown activity in breast cancer, carboplatin appears as a more appropriate choice for treatment of metastatic disease, due to less severe non-hematologic toxicities.
Although no large Phase III trials are ongoing to compare carboplatin/paclitaxel combination therapy with paclitaxel alone for MBC, several Phase II studies have shown that combination therapy with carboplatin and paclitaxel is active and reasonably well tolerated as first-line treatment of patients with MBC.5 Weekly paclitaxel is active in patients with MBC, especially patients treated previously in the adjuvant or metastatic setting with anthracyclines, and it has a mild toxicity profile.6 Moreover, some studies, such as the CALGB 9840 study, also demonstrated statistically significant improvements in response rate and time to progression, with a trend for improved survival using weekly instead of every 3 weeks (q3w) paclitaxel as first-line therapy for metastatic disease.7 Bevacizumab was previously tested in the setting of initial treatment of MBC; Eastern Cooperative Oncology Group (ECOG)-2100 randomized 680 previously untreated patients with MBC to paclitaxel 90 mg/m2 weekly, 3 out of 4 weeks, with or without bevacizumab 10 mg/kg given every 2 weeks. The combination yielded a superior response rate of 29.9% compared with 13.9% for single paclitaxel alone. Although progression-free survival (PFS) was superior to the combination (11.4 months compared with 6.11 months for paclitaxel; P ≤ 0.0001), there was no difference in overall survival (OS) (28.4 months for the combination versus 25.2 months for paclitaxel alone).8 On the basis of this study, the Food and Drug Administration (FDA) gave the preliminary approval for the use of bevacizumab for MBC, and that was when our study was launched, prior to the withdrawal of bevacizumab by the FDA. Two subsequent studies, RIBBON 19 and AVADO confirmed that the addition of bevacizumab improved response rate and PFS in MBC, but not OS. However, the absolute increases in PFS in RIBBON 1 and AVADO10 were not of the same magnitude as in ECOG-2100 (5.5 months in ECOG-2100 versus 0.9 in AVADO and 1.2 months for RIBBON I).11 As a result, ODAC (Oncology Drug Advisory Committee of the FDA) voted 12 to 1 to revoke the approval of bevacizumab for the treatment of MBC. This issue is still under review.12 However, in Europe, bevacizumab remains approved, only as a first-line therapy in combination with paclitaxel or capecitabine.
Carboplatin/docetaxel doublet has been also extensively studied in the setting of initial treatment of MBC. Data from Phase II trials has shown that the combination of carboplatin and docetaxel is active in the first-line treatment of MBC. The North Central Cancer Treatment Group (NCCTG) investigated the role of carboplatin/docetaxel regimen in patients irrespective of Human Epidermal growth factor Receptor-2 (Her-2) status.13 The overall response rate reached 58%, the median PFS time was 9.8 months, and the 1-year survival rate was 72%. Therefore, it was concluded that the combination of docetaxel and carboplatin showed activity in the first-line setting for MBC and that the toxicities of this regimen were acceptable.
In our developing country, the comparison between both regimens, bevacizumab/carboplatin/paclitaxel and carboplatin/docetaxel, was appealing due to the problematic financial burden of metronomic anti-angiogenic regimens adding to the cost of more frequent hospitalization days. We assumed that in view that weekly paclitaxel is more active than three weekly regimen in patients with MBC,6 yet, three weekly docetaxel is more active than weekly schedule, hence, was the treatment design. Besides, as anti-angiogenic treatment bevacizumab is better combined with paclitaxel, as in the ECOG-2100 study, rather than docetaxel, as in the AVADO trial.8,10 The bevacizumab was combined to paclitaxel (arm I), but with lower doses than usual (5 mg/kg every 2 weeks) to reduce expected major side effects and risks, which were contributing factors for its FDA withdrawal. This study was designed prior to the FDA withdrawal of bevacizumab in the setting of MBC; yet, even after its withdrawal this issue remains still under review, and in Europe, bevacizumab remains approved as a first-line therapy in combination with paclitaxel or capecitabine. Therefore, the study was considered logical to reach a comparative analysis of PFS and safety outcomes of both regimens.
Patients and methods
This is a randomized study comparing PFS and toxicity profiles of bevacizumab/carboplatin/paclitaxel versus carboplatin/docetaxel in 41 females (age ≥18 years) with HER-2-negative MBC with World Health Organization performance status (WHO PS) of 0 to II, who presented to Dar El-Fouad Hospital during the period from September 2008 to July 2009.
Forty-one patients were randomized in a 1:1 ratio to:
-
Arm I: Bevacizumab 5 mg/kg → (Day [D]1 + D15).
Carboplatin area under the curve (AUC)-2 →(D1 + D8 + D15).
Paclitaxel 60 mg/m2 → (D1 + D8 + D15).
To be recycled every 28 days.
-
Arm II: Carboplatin AUC-5 → D1.
Docetaxel 75 mg/m2 → D1.
To be recycled every 21 days.
Pretreatment assessment included medical history, physical examination, complete blood count/platelet, routine biochemical profile including CA15-3 assay. Metastatic workup included isotopic bone scan and computed tomography scans of the chest, abdomen, and pelvis. Patients with impaired hematological, hepatic, renal functions; or with central nervous system disease at presentation were excluded.
Clinical examination blood count, liver enzymes, and serum creatinine were performed on a 3-weekly basis, while CA15-3 was performed on a 6-weekly basis. Radiological images were performed following the third and sixth cycles of chemotherapy and by the end of the treatment course. Bone-only disease was evaluated by bone scan after 6 cycles.
Steroidal premedication started 24 hours prior to docetaxel and continued for 3 days, in addition to vigorous hydration and antiemetic for arm II. Twenty patients were assigned to arm I (bevacizumab/carboplatin/paclitaxel), and 21 patients were assigned to arm II (carboplatin/docetaxel).
The Kaplan–Meier estimator was used for estimating survival, and log-rank test was used for comparing survival curves. The primary end point of the study was PFS, while secondary end points were OS and safety.
Results
A total of 41 patients were randomly assigned (20 patients to bevacizumab/carboplatin/paclitaxel and 21 patients to carboplatin/docetaxel). The base line patients and disease characteristics are show in Table 1.
Table 1.
Baseline patients and disease characteristics for randomly assigned patients
| Bevacizumab/carboplatin/paclitaxel | Carboplatin/docetaxel | P-value | |
|---|---|---|---|
| Age (median) | 50.6 years | 52.33 years | 0.59 |
| – Range | 32–69 years | 37–69 years | |
| WHO PS | |||
| – I | 17/20 (85%) | 18/20 (85.7%) | 0.66 |
| – II | 03/20 (15%) | 03/21 (14.3%) | |
| ER/PR status | |||
| – Negative | 07/20 (35%) | 06/21 (28.6%) | 0.66 |
| – Positive | 13/20 (65%) | 15/21 (71.4%) | |
| Location of disease | |||
| – Bone only | 03/20 (15%) | 05/21 (23.8%) | 0.697 |
| – Visceral | 17/20 (85%) | 16/21 (76.2%) | |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; WHO PS, World Health Organization performance status.
The median PFS for patients randomly assigned to (bevacizumab/carboplatin/paclitaxel) was 10 months and almost equivalent to that of (carboplatin/docetaxel) 10.2 months (P = 0.9). Figure 1 shows the Kaplan–Meier curve for PFS for both arms.
Figure 1.
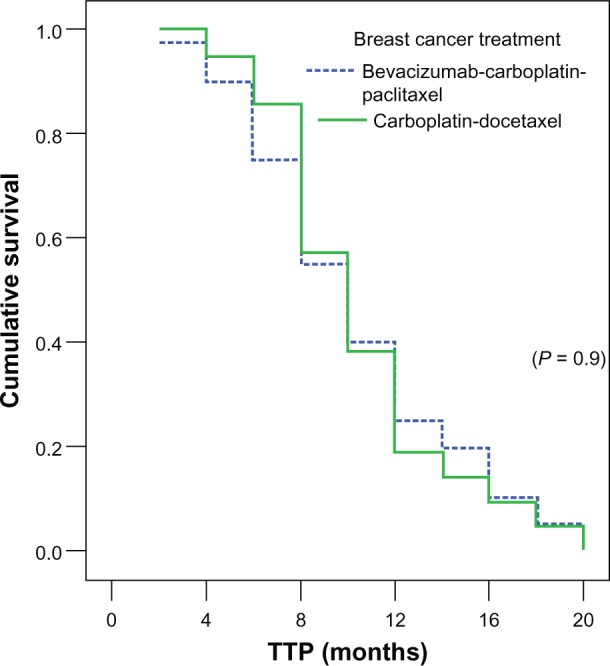
Median TTP in both arms.
Abbreviation: TTP, time to progression.
WHO PS was a strong prognostic factor for PFS; patients with PS 0–I had a PFS of 11 months, whereas patients with PS II had a PFS of 6 months (P = 0.035), as shown in Figure 2.
Figure 2.

WHO PS correlation with TTP in the whole study group.
Abbreviations: TTP, time to progression; WHO PS, World Health Organization performance status.
It was also obvious that in the setting of Her-2 negative status, estrogen receptor/progesterone receptor positive achieved better PFS of 12 months than triple negative, achieving median PFS of 8 months (P = 0.01) (Figure 3).
Figure 3.

Steroid hormone correlation with TTP in the whole study group.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; TTP, time to progression.
Also, PFS was worse in patients with visceral metastasis (Figure 4), (10 months versus 12 months in patients with bone-only disease), but did not reach statistical significance (P = 0.872), and the median OS was numerically longer (Figure 5) in the bone-only disease patients, (39.5 months versus 37.5 months in the visceral metastasis arm; P = 0.28).
Figure 4.

Bone/visceral metastasis correlation with TTP in the whole study group.
Abbreviations: TTP, time to progression; visc, visceral.
Figure 5.

Bone/visceral metastasis correlation with median OS in the whole study group.
Abbreviation: OS, overall survival; visc, visceral.
The study did not show any difference in the median OS (Figure 6) for arm I (bevacizumab/carboplatin/paclitaxel), which was 37.6 months versus 37.4 months for (carboplatin/docetaxel) (P = 0.92).
Figure 6.

Median OS in both arms.
Abbreviation: OS, overall survival.
Subgroup analysis of patients less than 50 years (Figure 7) showed OS of 36.5 months, where patients over 50 years showed OS of 39.5 months.
Figure 7.

Age correlation with median OS in the whole study group.
Abbreviation: OS, overall survival.
Safety
The incidence of hypertension and proteinuria was higher in arm I, while neutropenia and neutropenic fever as well as neuropathy were higher in arm II. However, deep venous thrombosis (DVT) was slightly higher in arm I, but did not reach statistical significance. No treatment-related mortality was recorded. Table 2 shows the encountered side effects evaluation in both arms.
Table 2.
Toxicity profile in both treatment arms
| Bevacizumab/carboplatin/paclitaxel | Carboplatin/docetaxel | P-value | |
|---|---|---|---|
| Hypertension | 04/20 (20%) | 01/21 (4.8%) | 0.18 |
| Proteinuria | 02/20 (10%) | 00/21 (0%) | 0.23 |
| DVT | 02/20 (10%) | 01/21 (4.8%) | 0.61 |
| Neutropenia | 04/20 (20%) | 07/21 (33.3%) | 0.34 |
| Neutropenic fever | 01/20 (5%) | 04/21 (19%) | 0.34 |
| Neuropathy | 07/20 (35%) | 10/21 (47.6%) | 0.41 |
Abbreviation: DVT, deep vein thrombosis.
Discussion
Due to cumulative cardiotoxicity and their common use in adjuvant chemotherapy, anthracycline-based regimens have a limited role in patients with MBC. Therefore, new nonanthracycline regimens are needed for metastatic disease. A considerable number of Phase II studies support incorporation of carboplatin as a standard agent in the management of patients eligible to receive first-line chemotherapy for MBC. Other Phase II studies of patients whose Her-2 status was unspecified demonstrated that the combination therapy with carboplatin and paclitaxel produced objective response rates of 53%–62%.5,14,15 These rates are substantially higher than those achieved in other Phase II trials of either single-agent carboplatin or paclitaxel.7,17,18 The combination of carboplatin/paclitaxel therapy was active in patients with anthracycline-resistant disease.10 Moreover, other studies pointed to synergy of bevacizumab with weekly paclitaxel over paclitaxel alone in improving PFS.8 Besides, results from Phase II studies also suggest that the combination of carboplatin and docetaxel is effective in the first-line treatment of metastatic disease7,16 and comparable to previously mentioned studies. This study was designed to compare different taxanes schedules; carboplatin/docetaxel supported by the NCCTG-trial,13 with bevacizumab/carboplatin/paclitaxel supported by data from ECOG-21008, and was designed prior to bevacizumab FDA withdrawal.12
In our study, the median PFS for bevacizumab/carboplatin/paclitaxel was 10 months, which was slightly less than the median PFS reported in ECOG-2100 of 11.8 months, while PFS in carboplatin/docetaxel was 10.2 months, which is almost the same as the median PFS reported in the NCCTG-trial of 9.8 months. The median PFS was similar in both arms; 10 versus 10.2 months (P = 0.9). The bevacizumab arm reported more toxicity as regards to hypertension and proteinuria in comparison with the non-bevacizumab arm. It is worth mentioning that in our study hypertension was recorded in 20% and proteinuria in 10% of patients in the bevacizumab arm versus 14.7% and 3.5% respectively in the E-2100 study, an incidence which was slightly higher but comparable with 14.7% for hypertension 3.5% in E-2100 study. The rate of DVT in the bevacizumab arm was 10% higher than that reported in ECOG-2100 at 2.1%, with the difference probably attributable to our smaller sample size; also, there was no statistical significance between both arms in our study concerning DVT. Neuropathy was recorded in 35% of patients versus 23.5% in ECOG-2100. The rate of hematological toxicity and neutropenic fever was higher in the carboplatin/docetaxel arm in our study (P = 0.34). The OS was equivalent in our study in both arms (37.6 months for arm I versus 37.4 months for arm II), which was longer than that reported in ECOG-2100 (26.7 months median survival), probably due to a different patient population and to our smaller sample size. Finally, it was obvious that patients older than 50 years achieved better survival; however, this did not reach statistical significance (P = 0.144), and this limitation could be attributed to the small sample size recruited. Age might have shown significance in a larger sized population.
In conclusion, regimens, bevacizumab/carboplatin/paclitaxel and carboplatin/docetaxel exhibit comparable PFS and OS but different toxicity profiles (hematological toxicity favored the metronomic arm, while hypertension, proteinuria, and DVT did not). Bevacizumab treatment required more hospital admission and more financial budget, which were not justified in this setting in terms of the non-superior clinical value witnessed in our study; however, it will be interesting in the future if different molecular markers will potentially guide the selection of patients who will most likely benefit from anti-angiogenic treatment in MBC.
Acknowledgments
The authors would like to acknowledge all of the oncology team registrars and nurses at Dar Al Fouad Hospital who have made it possible to conduct this clinical trial. We also acknowledge the invaluable support received from Dr Nelly (head of Statistics Department in National Cancer Institute, Egypt) for her voluntary contribution for statistical analysis.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.American Cancer Society . Cancer Facts and Figures 2003. Atlanta, GA: American Cancer Society; 2003. [Google Scholar]
- 2.Mincey BA, Perez EA. Concise review for clinicians: advances in screening, diagnosis and treatment of breast cancer. Mayo Clin Proc. 2004;79:810–816. doi: 10.4065/79.6.810. [DOI] [PubMed] [Google Scholar]
- 3.Carlson RW, Anderson BO, Bensinger W, et al. NCCN Practice Guidelines in Oncology, Version 2. National Comprehensive Cancer Network; 2002. [Google Scholar]
- 4.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–422. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 5.Fountzilas G, Athanassiades A, Papadimitriou V, et al. Paclitaxel and carboplatin as first-line chemotherapy for advanced breast cancer. Oncology (Huntingt) 1998;12(Suppl 1):45–48. [PubMed] [Google Scholar]
- 6.Perez EA, Vogel CL, Irwin DH, et al. Multicenter Phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2001;19:4216–4223. doi: 10.1200/JCO.2001.19.22.4216. [DOI] [PubMed] [Google Scholar]
- 7.Seidman AD, Berry D, Cirrincione C, et al. Phase III weekly paclitaxel via 1-hr infusion vs. standard 3-hr infusion every third week in the treatment of metastatic breast cancer, with trastuzumab for HER2+ MBC and randomized to trastuzumab for HER2 normal MBC. Proc Am Soc Clin Oncol. 2004;23:6sa. [Google Scholar]
- 8.Miller KD. E2100: a phase III trial of paclitaxel versus paclitaxel/bevacizumab for metastatic breast cancer. Clin Breast Cancer. 2003;3(6):421–422. doi: 10.3816/CBC.2003.n.007. [DOI] [PubMed] [Google Scholar]
- 9.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 10.Pivot X, Schneeweiss A, Verma S, et al. Efficacy and safety of bevacizumab in combination with docetaxel for the first line treatment with elderly patients with locally recurrent or metastatic breast cancer: results from AVADO. Eur J Cancer. 2011;47:2387–2395. doi: 10.1016/j.ejca.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first line treatment of human epidermal growth factor receptor 2 negative metastatic breast cancer. J Clin Oncol. 2010;28(20):3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 12.Ocana A, Amir E, Vera F, et al. Addition of bevacizumab to chemotherapy for treatment of solid tumor; similar results but different conclusions. J Clin Oncol. 2011;29(3):254–256. doi: 10.1200/JCO.2010.32.0275. [DOI] [PubMed] [Google Scholar]
- 13.Fitch RA, Suman VJ, Mailliard JA, et al. N9932: Phase II cooperative group trial of docetaxel (D) and carboplatin (CBDCA) as first-line chemotherapy for metastatic breast cancer (MBA) Proc Am Soc Clin Oncol. 2003;22:23. [Google Scholar]
- 14.Perez EA, Hillman DW, Stella PJ, et al. A Phase II study of paclitaxel plus carboplatin as first-line chemotherapy for women with metastatic breast carcinoma. Cancer. 2000;88:124–131. doi: 10.1002/(sici)1097-0142(20000101)88:1<124::aid-cncr17>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Loesch D, Robert N, Asmar L, et al. Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol. 2002;20:3857–3864. doi: 10.1200/JCO.2002.08.129. [DOI] [PubMed] [Google Scholar]
- 16.Brufsky A, Matin K, Cleary D, et al. A Phase II study of carboplatin and docetaxel as first line chemotherapy for metastatic breast cancer. Proc Am Soc Clin Oncol. 2002;21:52b. [Google Scholar]
- 17.Martin M, Diaz-Rubio E, Casado A, et al. Carboplatin: an active drug in metastatic breast cancer. J Clin Oncol. 1992;10:433–437. doi: 10.1200/JCO.1992.10.3.433. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien ME, Talbot DC, Smith IE. Carboplatin in the treatment of advanced breast cancer: a Phase II study using a pharmacokinetically guided dose schedule. J Clin Oncol. 1993;11:2112–2117. doi: 10.1200/JCO.1993.11.11.2112. [DOI] [PubMed] [Google Scholar]


