Abstract
Background
Sarcoidosis is an idiopathic, granulomatous disease for which molecular and immunologic studies have shown an association between it and mycobacterial antigens. Microbial antigens can reduce expression of the tyrosine kinase Lck, which has been associated with sarcoidosis severity. Here we investigate the efficacy of Concomitant Levofloxacin, Ethambutol, Azithromycin, and Rifampin (the CLEAR regimen) for treatment of chronic, pulmonary sarcoidosis.
Methods
Fifteen chronic, pulmonary sarcoidosis patients with forced vital capacities (FVC) between 45–80% of predicted were enrolled in this open-label trial. The primary efficacy endpoint was change in absolute FVC from baseline to completion of therapy. Secondary endpoints were change in functional capacity measured by Six Minute Walk Distance (6MWD) and quality of life assessment measured by St. George’s Respiratory Questionnaire (SGRQ).
Results
Of 15 patients enrolled, 11 completed 4 weeks of therapy, and 8 completed 8 weeks of therapy. The CLEAR regimen was associated with an increase in FVC of 0.23 liters at 4 weeks and 0.42 liters at 8 weeks (P=0.0098 and 0.016, respectively). The 6MWD increased by 87 meters from baseline to 8 weeks (p=0.0078). The mean score of the validated SGRQ was improved at 8 weeks over baseline (p=0.023). Normalized expression of Lck and NF-κB was observed in those with clinical improvement.
Conclusions
The CLEAR regimen is associated with improved absolute FVC, as well as increased functional capacity and quality-of-life in selected chronic pulmonary sarcoidosis patients. Larger, randomized, controlled trials are needed to confirm these findings and to identify patients most likely to benefit from therapy.
Keywords: Sarcoidosis, Clinical trial, antibiotics
Introduction
Sarcoidosis is a granulomatous disease that involves the lung in 90–95% of affected subjects. From 1988 to 2007, the age-adjusted, sarcoidosis-related mortality rate increased 50.5% in women and 30.1% in men (1). The observation of increasing mortality, as well as the limited efficacy of some current sarcoidosis therapies, emphasizes the necessity of new therapeutic options. Although ~1/3 of patients never require therapy (2), for those requiring therapy, current therapeutic options demonstrate limited efficacy on factors associated with sarcoidosis mortality, such as forced vital capacity (FVC) (3). Current treatments focus on limiting the inflammatory response, with corticosteroids serving as the mainstay of therapy. However, trials evaluating the effects of corticosteroids on lung function have yielded mixed results (4,5). Disease-modifying anti-rheumatic drugs (DMARDS), and more recently tumor necrosis factor (TNF) antagonists, have been used for corticosteroid-refractory patients (6). Among non-steroid based treatments, only infliximab has been shown in a randomized, placebo-controlled study to improve lung function (7).
A limitation to identification of effective therapeutics for sarcoidosis pathogenesis is the lack of understanding of mediators of sarcoidosis progression. Reduced expression of Lck and NF-κB is associated with sarcoidosis severity (8). The immunological response to microbial virulence factors is associated with changes in transcription factor expression (9). Mycobacterial virulence factors, such as ManLam, have been shown to interfere with T cell receptor signaling by inhibiting phosphorylation of the tyrosine kinase, Lck (10). Molecular and immunologic evidence from independent laboratories have strengthened the association of mycobacterial antigens with sarcoidosis pathogenesis. Proteins of pathogenic mycobacteria, such as katG and superoxide dismutase A (sodA), are present more frequently in sarcoidosis granulomas than granulomatous control specimens (11,12). Signals consistent with early secreted antigenic target-6 (ESAT-6) are present in sarcoidosis granulomas by mass spectrometry analysis (13) It has been reported that it is the interaction of secreted proteins, such as ESAT-6, with host epithelium that serve as the nidus for granuloma formation (14,15). Peripheral blood mononuclear cells (PBMCs) and diagnostic bronchoalveolar lavage (BAL) fluid from patients with sarcoidosis have been shown to exhibit antigen-specific Th1 cytokine responses against mycobacterial virulence factors, demonstrating that mycobacterial antigens are targets of the sarcoidosis adaptive immune response (16,17). The magnitude of the Th1 response parallels that seen in active mycobacterial infection (18). Effective antimicrobial therapy in patients with mycobacterial infection leads to clearance of these virulence factors and resolution of granulomatous inflammation; immune responses against microbial antigens disappear as the disease resolves (19). A growing body of literature supports the immunomodulatory effects of antimicrobial therapy, such as quinolones increasing IL-2 production in monocytes and macrolides decreasing neutrophils chemotaxis (20). There are case reports of improvement of cutaneous sarcoidosis lesions with antibiotic therapy, such as tetracyclines (21,22). We hypothesize that antimycobacterial therapy will lead to immune regulation of key mediators associated with sarcoidosis pathogenesis, such as Lck.
Also present within sarcoidosis granulomas are genes encoding mycobacterial products that can be targeted by antimycobacterial therapy such as rpoB (rifampin) (23) and DNA gyrase A (levofloxacin) (24). Building upon the observation of these genes within sarcoidosis granulomas, we designed an antibiotic regimen consisting of agents effective in resolution of pulmonary granulomatous inflammation secondary to mycobacterial antigens (25,26). In this study, called the CLEAR (Concomitant Levofloxacin, Ethambutol, Azithromycin, and Rifampin) Trial, we sought to evaluate the safety and efficacy of eight weeks of the CLEAR regimen on chronic pulmonary sarcoidosis subjects with moderate to severe disease.
Methods
Protocol Development and Oversight
This was an open-label, Phase Ib, single-center study evaluating the safety and efficacy of a four-drug anti-mycobacterial regimen on chronic pulmonary sarcoidosis outcome. The study protocol was approved by the Vanderbilt University Medical Center Institutional Review Board by Health Sciences Committee 1 (#100552), and all patients were provided written informed consent prior to enrolling in the study. The data and safety monitoring board for this study reviewed data throughout the study and performed the single planned interim analysis for safety and efficacy after 50% of the patients had completed the 8-week regimen. Dr. Wonder Drake had full access to all the data after study completion and assumes full responsibility for the integrity of the data and the accuracy of the data analysis.
Study Population and Randomization
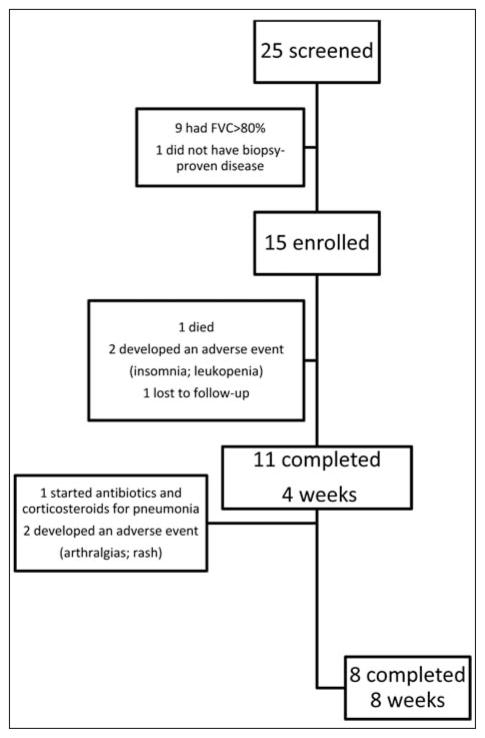
Participants were recruited from outpatient pulmonary clinics at Vanderbilt University Medical Center and the surrounding community between July 14 and August 24, 2010. All patients met American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and Other Granulomatous Diseases (ATS/ERS/WASOG) criteria for diagnosis of sarcoidosis (27) and had negative histologic and microbiologic assessments for tuberculosis, evidence of parenchymal disease on chest radiograph (Scadding stage II, III, or IV), and a forced vital capacity (FVC) between 45–80% at the time of screening. None of the patients were PPD positive. We implemented very strong exclusion criteria to avoid occult infection or superinfection as a possibility for benefit from antibiotic therapy. These exclusion criteria were as follows: 1) Subject has been hospitalized for infection or received IV antibiotics within the previous 2 months prior to baseline; 2) Subject has a history of tuberculosis at anytime or close contact with a person with active tuberculosis within the previous 6 months, or persistent or active infections requiring hospitalization or treatment with IV antibiotics, IV antiretrovirals, or IV antifungals within 30 days of baseline, OR oral antibiotics, antivirals, or antifungals for purpose of treating infection, within 14 days of baseline; 3) Subject has an active infection requiring systemic antibiotics at time of screening; 4) Subject has a history of listeriosis, treated or untreated tuberculosis, exposure to individuals with tuberculosis. To ensure only patients with stable disease were enrolled, those who had any adjustment in their sarcoidosis treatment regimen within six months prior to or during enrollment were excluded. Of the 25 patients screened, one did not have biopsy-proven disease, and others not enrolled failed to meet the criteria of a screening FVC of <80% (Figure 1).
Fig. 1.
Enrollment and Outcomes for Pulmonary Sarcoidosis subjects
Treatment
Patients meeting inclusion criteria received eight weeks of oral antibiotic therapy; antibiotic dosages were based upon ATS/Infectious Diseases Society of America Guidelines (ATS/IDSA) for treatment of atypical mycobacterial pulmonary infection (28).
The 8-week regimen consisted of levofloxacin 750 mg on day 1, then 500 mg once daily; ethambutol 15mg/kg/d to a maximum of 1200 mg once daily, azithromycin 500 mg on day 1, then 250 mg once daily; and either rifampin 10mg/kg/d to a maximum of 600 mg once daily or rifabutin 300 mg once daily. Rifabutin was substituted for rifampin in patients taking medications substantially metabolized via the cytochrome P450 system, such as prednisone.
Clinical Assessments
The primary endpoint was improvement in absolute FVC from baseline to completion of therapy. Spirometry testing was performed using a standardized calibrated laptop spirometer, Flowscreen II USA Spirometer (VIASYS Healthcare Inc., Yorba Linda, CA).
The volume accuracy of the spirometer was checked daily using a three liter calibration syringe. The subjects were instructed on proper technique prior to initiation of spirometry; spirometry was not recorded unless proper technique was visualized (29). Each subject was given at least three attempts and the greatest measurement for absolute FVC and Forced Expiratory Volume (FEV1) at baseline, four week, and eight week assessments was recorded. Assessments of Six Minute Walk distance (6MWD) and St. George Respiratory Questionnaire (SGRQ) were conducted as previously described (30).
Cytokine Expression Profile
Baseline and ESAT-6 Th1/Th2 cytokine profiling was performed and quantified as previously described (19). Briefly, PBMC’s were isolated from whole-blood through Ficoll-Hypaque separation. Cytometric bead analysis (BD Biosciences, San Diego, CA) was used to measure extracellular cytokine production in unstimulated cells or cells stimulated with ESAT-6 peptide and soluble anti-CD28 antibody (1 μg/mL, BD Biosciences, San Diego, CA) for 24 hours at 37 degrees Celsius under 5% CO2.
Western Blot analysis
CD4+ T cells were activated by cross-linking with plate-bound anti-CD3 and soluble anti-CD28 antibody. At indicated time points, cells were collected and washed with a 1:100 dilution of phosphatase inhibitor cocktail B (Santa Cruz Biotechnology, Santa Cruz, CA) in ice-cold PBS. Cells were then lysed using a buffer solution consisting of 50 μL 2-β-mercaptoethanol, either 940 or 930 μL of Laemmli sample buffer (Biorad, Hercules, CA), and 10 μL of either phosphatase inhibitor cocktail B or 10 μL each of cocktails A and B. Proteins from the whole-cell lysates were separated by standard SDS-page gel and probed with monoclonal antibodies against Y394-Lck, and NF-κB (both 1:1000 dilution, Santa Cruz Biotechnology) and β-actin (1:2000 dilution, Sigma-Aldrich, St. Louis, MO). Detection was performed using Odyssey infrared imaging system (Licor Biosciences, Lincoln, NE) using proprietary secondary antibodies. Quantification was performed using software supplied by the manufacturer.
Sample size and statistical analysis
Given the pilot nature of this study, and no a priori data on potential effect of this antimycobacterial regimen on lung patients, we chose a sample size of 15 persons, comparing the absolute FVC of the cohort at baseline to the absolute FVC of the cohort after completion of therapy.
The subjects were to be analyzed using an intention to treat analysis. If we are unable to obtain endpoint data at the time of study withdrawal on all withdrawing subjects, the data will be analyzed per protocol.
As a result, the data was analyzed using per protocol analysis. Data are presented as means plus-minus standard deviations, unless otherwise stated. Continuous variables were compared between baseline and week 4 or week 8 using the signed rank test. For the binary endpoint, the proportion and its 95% exact confidence interval are reported. All tests are two-tailed. Statistical analyses were performed using the statistical package SAS for Windows (Version 9, Cary, NC) and the statistical software R (www.r-project.org).
Results
Characteristics of the Study Patients
The mean age of the patients was 54 years. 73% were females, and 53% were Caucasian. Approximately 47% were on immunosuppressants at the time of study enrollment. The mean time since histologic diagnosis was 10 years (Table 1).
Table 1.
Data are presented as No. (%) unless otherwise indicated.
| Baseline Characteristics of the 15 Chronic Pulmonary Study Subjects
| |
|---|---|
| Age - yr | |
| Mean | 54 |
| Range | 43–65 |
|
| |
| Sex - number (%) | |
| Male | 4 (36) |
| Female | 11 (73) |
|
| |
| Race - number (%) | |
| African American | 7 (47) |
| Caucasian | 8 (53) |
|
| |
| Years since histologic diagnosis | |
| Mean | 10 |
| Range | 1–32 |
|
| |
| Concominant therapy - no. (%) | 7 (47) |
|
| |
| Corticosteroids only | 3 (20) |
| Immunomodulator only | 1 (7) |
| Corticosteroids + immunomodulator | 3 (20) |
| Corticosteroid dose (mg prednisone equivalent/day) | |
| Mean | 13 |
| Range | 3.75–30 |
|
| |
| Scadding CXR Stage 0/I/II/III/IV | 1/1/8/3/2 |
Efficacy
Of the 15 patients enrolled, 11 completed 4 weeks of the CLEAR regimen and eight completed 8 weeks (Figure 1). We used the last-value-carry-forward rule to impute the missing values, i.e. assuming no change from the last available observation for the missing values should the subjects have stayed in the study. With the imputation in the intention-to-treat analysis, the baseline absolute FVC was 2.43±0.81 (n=15); the Week 8 absolute FVC was 2.67±1.13 (n=15), reflecting an increase of 0.24 liter (p=0.028, paired t-test). Of those that completed 8 weeks of therapy, the FVC improved from a baseline of 2.61+1.01 to an average of 3.03±1.43, reflecting an increase of 0.42 liters (p=0.016) (Table 2A). Four of the eight subjects who completed therapy experienced >10% improvement in absolute FVC, ranging from 14–31% (Figure 2). Of the 11 patients who completed four weeks of therapy, the absolute FVC improved from a baseline of 2.47±0.89 to an average of 2.70±1.05, reflecting an average volume increase of 0.23 liters (p=0.0098) (Table 2B).
Table 2A.
Data are presented as mean +/− SD. Ranges are listed as (lowest value, highest value).
| Changes in Prespecified Primary and Secondary Lung Outcomes at 8 weeks (8 subjects) | ||||
|---|---|---|---|---|
|
| ||||
| Absolute FVC | Absolute Difference | |||
| Pulmonary Function | Baseline | 8 week | Week 8 | P value |
|
| ||||
| Forced Vital Capacity | 2.61+1.01 | 3.03+1.43 | 0.42 (0.041, 0.799) | p=0.016 |
|
| ||||
| Forced Expiratory Volume (FEV1) | 1.72±0.68 | 1.97±0.87 | 0.24 (−0.00015, 0.49) | p=0.078 |
|
| ||||
| Dyspnea | ||||
|
| ||||
| Six Minute Walk Distance (meter) | 352.0+70.3 | 438.8+97.5 | 86.8 (35.7, 137.8) | p=0.0078 |
|
| ||||
| Borg Dyspnea Index after walking | 4.13±0.99 | 1.63±1.41 | −2.5 (−4.05, −0.95) | p=0.016 |
|
| ||||
| Quality of Life | ||||
|
| ||||
| Saint George Respiratory Questionnaire | ||||
| Total Score | 51.38+17.96 | 37.19+11.36 | −14.19 (−24.33,−4.05) | p=0.023 |
|
| ||||
| Symptoms Score | 56.55±20.31 | 37.61±11.76 | −18.94 (−39.37,1.48) | p=0.078 |
|
| ||||
| Activity Score | 66.67±19.72 | 55.26±26.96 | −11.41 (−22.36,−0.46) | p=0.016 |
|
| ||||
| Impacts Score | 40.72±20.73 | 26.65±11.15 | −14.07 (−29.46,1.33) | p=0.078 |
|
| ||||
| X-Ray (Muers) | 0.58+0.93 | 0.56+0.79 | −0.021 | p=1.0 |
|
| ||||
| Laboratory | ||||
| CBC | 8.23+3.36 | 6.83+4.09 | −1.55 | p=0.16 |
|
| ||||
| ALT | 21.00+6.12 | 30.45+30.77 | 8.45 (−10.66,27.57) | p=0.70 |
FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; 6MWD = 6 minute walk distance; SGRQ = St. George’s Respiratory Questionnaire; WBC = white blood cell; ALT = alanine aminotransferase
Fig. 2.
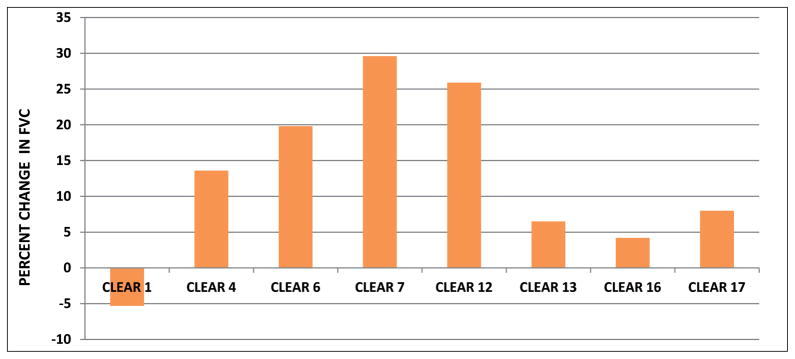
Percent change in forced vital capacity following completion of 8 weeks of CLEAR regimen. FVC was measured at baseline and following completion of 8 weeks of CLEAR regimen. Four of the eight subjects demonstrated improvement in FVC of >10%.
Table 2B.
Data are presented as mean +/− SD. Ranges are listed as (lowest value, highest value).
| Changes in Pulmonary Function at 4 weeks (11 subjects) | ||||
|---|---|---|---|---|
| Absolute FVC | Absolute Difference | |||
| Pulmonary Function | Baseline | 4 week | Week 4 | P value |
| Forced Vital Capacity | 2.47+0.89 | 2.70+1.05 | 0.23 (0.085, 0.371) | p=0.0098 |
| Forced Expiratory Volume (FEV1) | 1.65±0.62 | 1.80±0.54 | 0.16 (−0.066, 0.377) | p=0.25 |
FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second
The improvement in FVC at completion of therapy was associated with improvements in Six Minute Walk Distance (6MWD), as well as perception of dyspnea. Two patients were unable to participate in the 6MWD due to arthritis and congestive heart failure, respectively. The 6MWD increased from a baseline of 352.0+70.3 meters to 438.8+97.5 meters (p=0.0078), reflecting an increase of ~87 meters.
The improvement in 6MWD was associated with a reduction in the perception of dyspnea after completion of the walk, as measured by the Borg dyspnea score.
The Borg score decreased by an average of 2.5 points (p=0.016) (Table 2A). In addition, there was improved quality of life, as measured by the Saint George’s Respiratory Questionnaire (SGRQ).
The study participants reported a significant decrease in the impact of sarcoidosis on activities of daily living (p=0.016). Improvement in symptom frequency and severity, as well as social functioning was also noted but not significant (p=0.078, both) (Table 2A). There was not a significant difference in the Muer R score after completion of therapy (Table 2A).
Mechanism of Action
It was recently reported that reduced levels of nuclear transcription factor NF-kappaB (p50, p65, and p105) and the tyrosine kinase p56Lck were associated with severe and progressive sarcoidosis. To determine the mechanism by which the CLEAR regimen was mediating improvement in chronic pulmonary sarcoidosis, we postulated that improvement in expression of NF-κB and Lck would be apparent. We conducted Western blot analysis for activated Lck and NF-κB at baseline and following completion of the CLEAR regimen. There was increased protein expression of activated Lck and NF-κB (Figure 3). The Lck and NF-κB expression levels approached that of healthy controls at baseline and following TCR stimulation, after completion of the CLEAR regimen. Because the changes in Lck and NF-κB were observed in peripheral cells, they may not reflect cellular responses in localized sites of sarcoidosis involvement, such as the lung.
Fig. 3.
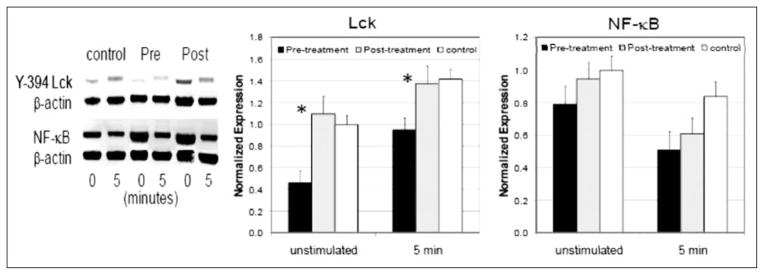
Anti-mycobacterial therapy increases activated Lck and NF-κB expression. Y394-Lck and NF-κB levels were measured from whole-cell lysates generated from 1 × 106 CD4+ T cells unstimulated or stimulated for 5 minutes with plate-bound anti-CD3 and soluble anti-CD28 antibodies before and after completion of anti-mycobacterial therapy. Protein expression was normalized to β-actin levels. (*=p<0.05). Data are presented as the mean normalized expression ± SD from five sarcoidosis subjects and three healthy controls.
Furthermore, we compared baseline responses against mycobacterial ESAT-6 to responses among sarcoidosis subjects for whom PBMC were available. We noted no improvement in FVC among sarcoidosis subjects who lacked ESAT-6 antigen-specific responses at study entry, but marked improvements in those that did demonstrate ESAT-6 antigen-specific responses (Table 3).
Table 3.
Change in Absolute FVC = change from baseline to 8 weeks of therapy.
| Correlation of baseline responses to ESAT-6 and improvement in absolute FVC | ||
|---|---|---|
| Subject | ESAT-6 Recognition | Change in Absolute FVC |
| Clear Lung 1 | − | −5.3 |
| Clear Lung 4 | + | 13.6 |
| Clear Lung 6 | + | 19.8 |
| Clear Lung 7 | + | 30.1 |
| Clear Lung 12 | + | 25.9 |
| Clear Lung 13 | − | 6.5 |
| Clear Lung 15 | − | 4.3 |
| Clear Lung 16 | + | 5.9 |
ESAT-6 = early secreted antigen 6; FVC = forced vital capacity
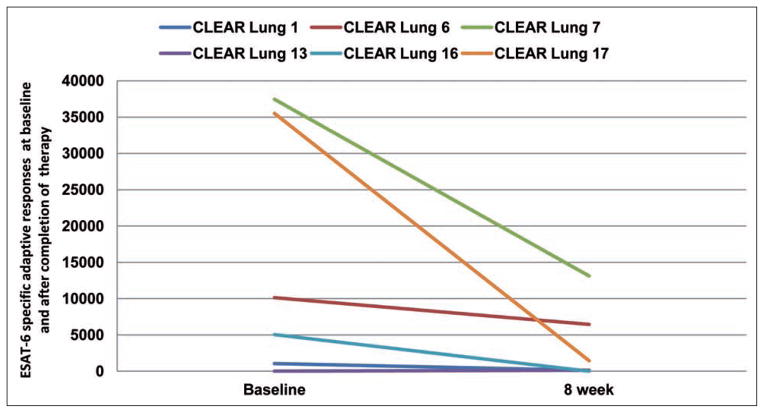
The patients with clinical improvement in absolute FVC following the CLEAR regimen also had marked reductions in IFN-γ production upon ESAT-6 stimulation (Figure 4). Because the immune response against mycobacterial antigens such as ESAT-6 can serve as a nidus for granuloma formation (14,15), we postulate that these declining responses reflect a decline in ESAT-6 antigen burden.
Fig. 4.
Reductions in immune responses against ESAT-6. Analogous to reports of declining immunoreactivity against microbial antigens following antifungal and antituberculous treatment, reductions in adaptive immune responses against ESAT-6 was noted post completion of the CLEAR regimen. Data was collected on the six subjects for whom PBMC were available.
Adverse Events
Seven of the 15 patients enrolled experienced an AE (Table 4), four of which led to study withdrawal. One AE was the development of leucopenia and mild hepatitis. The patient had a baseline WBC count of 5.5 WBC/mm3 that decreased to 1.5 WBC/mm3, and an increase of AST and ALT to twice the upper limit of normal within 18 days of taking the CLEAR regimen; all laboratories normalized with cessation of therapy.
Table 4.
Adverse event profileAdverse Events of Cutaneous and Pulmonary Sarcoidosis Participants
| Adverse Event | Therapy Days Completed | |
|---|---|---|
| CLEAR LUNG 3 | Insomnia; hypotension | 11 |
| CLEAR LUNG 6 | Headache | 56 |
| CLEAR LUNG 12 | Headache | 56 |
| CLEAR LUNG 17 | Arthralgias | 56 |
| CLEAR LUNG 23 | Leucopenia | 18 |
| CLEAR LUNG 24 | Arthralgias | 34 |
| CLEAR LUNG 25 | Rash | 28 |
The patient refused to return for us to obtain study endpoint data. One patient developed insomnia and transient hypotension; another had worsening of baseline arthralgias; and one developed rash one month into therapy. One patient was withdrawn from the study due to the necessity of administering antibiotics by her physician. Six weeks into the regimen, this subject developed productive cough, lower lobe infiltrate and leukocytosis that responded to empiric vancomycin and piperacillin-tazobactam; high-dose systemic corticosteroids were added to treat possible recurrence of sarcoidosis. Although no etiology was identified, she clinically improved on this regimen. One subject had a pre-existing diagnosis of ischemic cardiomyopathy, who 18 days into the CLEAR regimen, developed cardiogenic shock that led to her death. The patient had expired before we were notified. We were unable to obtain primary endpoint data. Review of both cases by the DSMB did not feel that neither the pneumonia nor the death could be attributed to study drug. Another subject denied any adverse events but did not maintain any study visits after study enrollment.
Review of baseline and week 8 ALT and WBC of the subjects as a cohort did not reveal any significant differences (Table 2A).
Discussion
In the current study, we demonstrate that administration of antimycobacterial therapy results in improvement in absolute FVC, functional capacity and perception of dyspnea among chronic pulmonary sarcoidosis subjects. The molecular basis for the improvement was normalized expression of the tyrosine kinase Lck, and NF-κB; reduced expression of both had been previously associated with sarcoidosis severity (31). It is unclear if peripheral anergy represents specific sites of sarcoidosis involvement such as the lung. The observed clinical improvement was accompanied by an increase in absolute FVC that exceeds that reported of any current sarcoidosis therapeutic options (Table 2A).
An improvement in percent of predicted FVC was 2.5% in sarcoidosis subjects treated for 24 weeks with infliximab (7), or with corticosteroid therapy alone for acute pulmonary sarcoidosis (32, 33). Improvement in vital capacity has been noted in children administered steroid therapy (34). We noted an average increase in the absolute FVC of 0.42 liters, reflecting a mean percent increase of 16% above baseline. Also, a major limitation of this study is that no placebo control was included; the improvement observed could not be separated from spontaneous improvement. Due to the limitation of the lack of inclusion of a placebo control, this trial is a Phase 1b clinical trial that successfully establishes proof of concept with serum biomarkers and potential clinical outcome benefit in sarcoidosis.
In addition to enhancement of absolute FVC, we observed an increase in 6MWD; this increase was associated with a reduction in the perception of dyspnea after completion of the walk, as measured by the Borg dyspnea score (Table 2A). In addition, there was improved quality of life; the study participants reported a significant decrease in the impact of sarcoidosis on activities of daily living, as well as improvement in symptom frequency and severity. These findings suggest that the improvement in FVC carried clinical significance and resulted in positive improvements in the patient’s daily life. We were able to obtain PBMC on only 8 of the enrolled CLEAR subjects. Improvement correlated with the presence of systemic immune responses against mycobacterial ESAT-6 at the time of study entry. We noted no improvement among sarcoidosis subjects who did not possess these Th-1 responses at baseline, and significant improvement among those who did (Table 3). Prior reports have noted that the quantity of mycobacterial ESAT-6-specific immune response decreases as tuberculosis subjects resolve their disease during treatment (35). It has also been demonstrated that immune responses against mycobacterial antigens decrease as sarcoidosis subjects resolve their disease (36), (personal observation). Detection of immune responses against mycobacterial antigens may aid in stratifying sarcoidosis subjects who would improve on the CLEAR regimen. The lack of ESAT-6 antigen specific responses and failure to improve on the CLEAR regimen may reflect a subject for whom mycobacterial antigens do not contribute to their pathogenesis, but rather an alternative cause. For those subjects, who do possess antimycobacterial immune responses, it is possible that effective antimycobacterial therapy leads to improved host immunity which aids clearance of pathogenic antigens. Further investigation into the mechanism is warranted. Future studies will involve inclusion of cognate antigens, such as tetanus, CMV, EBV, and influenza, at baseline and after completion of therapy to assess if improved T cell function is noted after completion of the CLEAR regimen in pulmonary sarcoidosis patients.
The CLEAR regimen did not lead to radiographic improvement as measured by Muer R score. The 8 week regimen is considered an initiation phase for treatment of mycobacterial lung disease and may not provide adequate time for diminution of radiographic evidence of inflammation. Radiographic resolution may likely require a continuation phase of 4–6 months; four months of antituberculous therapy has been demonstrated to resolve noncaseating granulomas induced by M. tuberculosis (37). Radiographic changes may never occur if the findings on chest radiograph are predominately due to scarring and fibrosis. In several randomized, controlled studies evaluating the effects of systemic corticosteroids on sarcoidosis, chest radiographs were noted to show significant improvement at follow-up dates ranging from three months to two years, but not at subsequent follow-up beyond two years (2). Alternatively, chest computer tomography radiographs may aid in detecting parenchymal and nodal changes, following therapy. This will be a consideration for future studies.
While the CLEAR regimen did demonstrate improvement in the absolute FVC, one limitation was the high withdrawal rate due to adverse events (Figure 1, Table 4). Four subjects withdrew due to adverse events, although a total of seven subjects experience adverse events (Table 4). The observation of a high withdrawal rate on four drug antimycobacterial regimen has been previously reported; up to half of patient with tuberculosis do not complete their four drug regimen (38).
The side effect profile of the CLEAR regimen was similar in prevalence to that reported among other quinolone-containing regimens for pulmonary tuberculosis (39). Arthralgias, nausea and insomnia are likely due to levofloxacin (40). Rates of leucopenia in four-drug antituberculosis therapy range from 1–6%, and are more common in women (41). The leucopenia is most commonly associated with isoniazid, but can occur with rifampin or ethambutol (41).
Two hypertensive patients reported lower blood pressure on this regimen, which reversed after cessation of the regimen; one patient withdrew because of the lower blood pressure. Intravenous administration of rifampin has been associated with hypotension, and reported to be mediated by rifampin’s effect on the histaminergic systems participating in vascular tension control (42). In addition, we noted that three subjects did not complete the study for causes unrelated to study drugs (pneumonia necessitating antibiotics, death, and one denied adverse events but did not maintain study visits). Additional investigation of optimal patient selection, as well consideration of a regimen with fewer drugs, is warranted.
ESAT-6 has been shown to modulate TCR signaling pathways downstream of ZAP70, thereby inhibiting human T cell IFN- responses (43, 44). Furthermore, the reduction of Lck expression has been shown to be a microbial target to suppress the development of protective immune responses. It is possible that mycobacterial antigens, such as ESAT-6, can repress Lck expression. Another possibility is that persistent TCR stimulation could drive reductions in Lck expression. Normalized Lck expression may reflect reductions in bacillary antigen load, leading to decrease granuloma burden and improvement in absolute FVC (45). Further investigation into this possibility is warranted.
In conclusion, several lines of evidence suggest that mycobacterial antigens contribute to sarcoidosis pathogenesis among some subjects. This proof-of-concept trial suggests that a four-drug antimycobacterial regimen to treat chronic sarcoidosis leads to improvements in absolute FVC, functional capacity, and perception of dyspnea. These findings support conducting a multicenter, placebo-controlled, randomized trial to determine patient selection, optimal dosing, dose duration, and to better delineate the risk-benefit profile.
Acknowledgments
Founding: The study was supported input by NIH 1K01HL103179-01; NIH T32HL0697665-08; The Moma Eliassen Foundation; The Pierce Foundation; UL1 RR-024975
We are grateful to the sarcoidosis patients who participated in this trial.
Abbreviations
- 6MWD
6 minute walk distance
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ATS
American Thoracic Society
- BAL
bronchoalveolar lavage
- CBC
complete blood count
- CLEAR
Concomitant Levofloxacin, Ethambutol, and Rifampin
- DMARD
disease-modifying anti-rheumatic drug
- ESAT-6
early secreted antigen 6
- ERS
European Respiratory Society
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- IDSA
Infectious Disease Society of America
- KATG
catalase-peroxidase PBMC, peripheral blood mononuclear cells
- SGRQ
St. George’s Respiratory Questionnaire
- sodA
superoxide dismutase A
- TNF
tumor necrosis factor
- WASOG
World Association of Sarcoidosis and Other Granulomatous Diseases
- WBC
white blood cell
References
- 1.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related Mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baughman RP, Lynch JP. Difficult treatment issues in sarcoidosis. J Intern Med. 2003;253(1):41–45. doi: 10.1046/j.1365-2796.2003.01076.x. [DOI] [PubMed] [Google Scholar]
- 3.Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled, multicenter study. Chest. 1999;116:424–431. doi: 10.1378/chest.116.2.424. [DOI] [PubMed] [Google Scholar]
- 4.Selroos O, Sellergren TL. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis. 1979;60(4):215–221. [PubMed] [Google Scholar]
- 5.Zaki MH, Lyons HA, Leilop L, et al. Corticosteroid therapy in sarcoidosis. A five-year, controlled follow-up study. N Y State J Med. 1987;87(9):496–499. [PubMed] [Google Scholar]
- 6.Lazar CA, Culver DA. Treatment of sarcoidosis. Semin Respir Crit Care Med. 2010;31(4):501–518. doi: 10.1055/s-0030-1262218. [DOI] [PubMed] [Google Scholar]
- 7.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 8.Lee NS, Barber L, Kanchwala A, et al. Low levels of NF-kappaB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin Vaccine Immunol. 2011;18(2):223–234. doi: 10.1128/CVI.00469-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zea AH, Ochoa MT, Ghosh P, et al. Changes in expression of signal transduction proteins in T lymphocytes of patients with leprosy. Infect Immun. 1998;66(2):499–504. doi: 10.1128/iai.66.2.499-504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahon RN, Sande OJ, Rojas RE, et al. Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell Immunol. 2012;275(1–2):98–105. doi: 10.1016/j.cellimm.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Z, Marzilli L, Greenlee BM, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201(5):755–767. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen SS, Evans W, Carlisle J, et al. Superoxide dismutase A antigens derived from molecular analysis of sarcoidosis granulomas elicit systemic Th-1 immune responses. Respir Res. 2008;9:36. doi: 10.1186/1465-9921-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oswald-Richter KA, Beachboard DC, Seeley EH, et al. Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL. J Clin Immunol. 2012;32(5):1129–1140. doi: 10.1007/s10875-012-9700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkman HE, Pozos TC, Zheng J, et al. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327(5964):466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkman HE, Clay H, Beery D, et al. Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant. PLoS Biol. 2004;2(11):e367. doi: 10.1371/journal.pbio.0020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake WP, Dhason MS, Nadaf M, et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007;75(1):527–530. doi: 10.1128/IAI.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen ES, Wahlstrom J, Song Z, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181(12):8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalvani A, Pathan AA, Durkan H, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357(9273):2017–2021. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 19.Sester U, Fousse M, Dirks J, et al. Whole-blood flow-cytometric analysis of antigen-specific CD4 T-cell cytokine profiles distinguishes active tuberculosis from non-active states. PLoS One. 2011;6(3):e17813. doi: 10.1371/journal.pone.0017813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Infect Dis. 2003;3(6):359–371. doi: 10.1016/s1473-3099(03)00658-3. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt CE, Fabi SG, Kukreja T, et al. Hypopigmented cutaneous sarcoidosis responsive to minocycline. J Drugs Dermatol. 2012;11(3):385–389. [PubMed] [Google Scholar]
- 22.Bachelez H, Senet P, Cadranel J, et al. The use of tetracyclines for the treatment of sarcoidosis. Arch Dermatol. 2001;137(1):69–73. doi: 10.1001/archderm.137.1.69. [DOI] [PubMed] [Google Scholar]
- 23.Drake WP, Pei Z, Pride DT, et al. Molecular analysis of sarcoidosis tissues for mycobacterium species DNA. Emerg Infect Dis. 2002;8(11):1334–1341. doi: 10.3201/eid0811.020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dauendorffer JN, Guillemin I, Aubry A, et al. Identification of mycobacterial species by PCR sequencing of quinolone resistance-determining regions of DNA gyrase genes. J Clin Microbiol. 2003;41(3):1311–1315. doi: 10.1128/JCM.41.3.1311-1315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 27.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149–173. [PubMed] [Google Scholar]
- 28.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 30.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 31.Oswald Richter KA, Richmond BW, Braun NA, Jsom J, Abraham S, Taylor TR, Drake JM, Culver DA, Wilkes DS, Drake WP. Reversal of global CD4+ subset dysfunction is associated with spontaneous clinical resolution of pulmonary sarcoidosis. J Immunol. 2013;190(11):5446–53. doi: 10.4049/jimmunol.1202891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson GJ, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51(3):238–247. doi: 10.1136/thx.51.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Study Group. Chest. 1999;116(2):424–431. doi: 10.1378/chest.116.2.424. [DOI] [PubMed] [Google Scholar]
- 34.Baculard A, Blanc N, Boule M, et al. Pulmonary sarcoidosis in children: a follow-up study. Eur Respir J. 2001;17(4):628–635. doi: 10.1183/09031936.01.17406280. [DOI] [PubMed] [Google Scholar]
- 35.Ewer K, Millington KA, Deeks JJ, et al. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2006;174(7):831–839. doi: 10.1164/rccm.200511-1783OC. [DOI] [PubMed] [Google Scholar]
- 36.Chen ES, Wahlstrom J, Song Z, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181(12):8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84(1–2):29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Munro SA, Lewin SA, Smith HJ, et al. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4(7):e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daley CL. Update in tuberculosis 2009. Am J Respir Crit Care Med. 2010;181(6):550–555. doi: 10.1164/rccm.201001-0140UP. [DOI] [PubMed] [Google Scholar]
- 40.Liu HH. Safety profile of the fluoroquinolones: focus on levofloxacin. Drug Saf. 2010;33(5):353–369. doi: 10.2165/11536360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Cormican LJ, Schey S, Milburn HJ. G-CSF enables completion of tuberculosis therapy associated with iatrogenic neutropenia. Eur Respir J. 2004;23(4):649–650. doi: 10.1183/09031936.04.00053804. [DOI] [PubMed] [Google Scholar]
- 42.Berezhinskaia VV, Dolgova GV, Svinogeeva TP, et al. Mechanism of hypotension induced by rifampicin during intravenous administration. Antibiot Khimioter. 1988;33(10):771–775. [PubMed] [Google Scholar]
- 43.Peng H, Wang X, Barnes PF, et al. The Mycobacterium tuberculosis Early Secreted Antigenic Target of 6 kDa Inhibits T Cell Interferon-{gamma} Production through the p38 Mitogen-activated Protein Kinase Pathway. J Biol Chem. 2011;286(27):24508–24518. doi: 10.1074/jbc.M111.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samten B, Wang X, Barnes PF. Mycobacterium tuberculosis ESX-1 system-secreted protein ESAT-6 but not CFP10 inhibits human T-cell immune responses. Tuberculosis (Edinb) 2009;89 (Suppl 1):S74–S76. doi: 10.1016/S1472-9792(09)70017-4. [DOI] [PubMed] [Google Scholar]
- 45.Millington KA, Innes JA, Hackforth S, et al. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178(8):5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]