Abstract
The cognitive deficits seen in schizophrenia patients are likely related to abnormal glutamatergic and cholinergic neurotransmission in the prefrontal cortex. We hypothesized that these impairments may be secondary to increased levels of the astrocyte-derived metabolite kynurenic acid (KYNA), which inhibits α7 nicotinic acetylcholine receptors (α7AChR) and may thereby reduce glutamate release. Using in vivo microdialysis in unanesthetized rats, we show here that nanomolar concentrations of KYNA, infused directly or produced in situ from its bioprecursor kynurenine, significantly decrease extracellular glutamate levels in the prefrontal cortex. This effect was prevented by the systemic administration of galantamine (3 mg/kg) but not by donepezil (2 mg/kg), indicating that KYNA blocks the allosteric potentiating site of the α7AChR, which recognizes galantamine but not donepezil as an agonist. In separate rats, reduction of prefrontal KYNA formation by (S)-4-ethylsulfonyl benzoylalanine, a specific inhibitor of KYNA synthesis, caused a significant elevation in extracellular glutamate levels. Jointly, our results demonstrate that fluctuations in endogenous KYNA formation bidirectionally influence cortical glutamate concentrations. These findings suggest that selective attenuation of cerebral KYNA production, by increasing glutamatergic tone, might improve cognitive function in individuals with schizophrenia.
Keywords: Cognition, Donepezil, Galantamine, Kynurenine, Microdialysis, Prefrontal cortex
Introduction
The cognitive deficits seen in individuals with schizophrenia (SZ) are now recognized as a core domain of the disease (Keefe et al. 2007). Several of these impairments affect executive functions (i.e., attention, cognitive flexibility), are mediated by the prefrontal cortex (PFC; Kerns et al. 2008; Moghaddam and Homayoun 2008), and may be causally related to abnormal glutamatergic and cholinergic neurotransmission within the PFC (Sarter et al. 2005; Lewis and Moghaddam 2006).
Dysregulation of prefrontal α7 nicotinic acetylcholine receptors (α7nAChRs) might be central to these behavioral and chemical abnormalities. Thus, α7nAChR protein levels are reduced in the PFC of individuals with SZ (Guan et al. 1999), and the α7nAChR gene and a SZ endophenotype (disrupted P50 evoked response to repeated auditory stimuli) are linked to the same locus and associated with disease transmission (Leonard and Freedman 2006). Moreover, specific cognitive improvements in SZ patients can be achieved by galantamine (Schubert et al. 2006; Buchanan et al. 2008), probably by activating the allosteric potentiating ligand (APL) site of the α7nAChR (Samochocki et al. 2003). Notably, α7nAChRs in the mammalian brain are frequently localized presynaptically on glutamatergic nerve terminals, where they regulate the release of glutamate (Albuquerque et al. 2009).
The endogenous metabolite kynurenic acid (KYNA) may play a substantive role in these prefrontal mechanisms and deficits. Initially described as a broad spectrum antagonist of ionotropic glutamate receptors (Perkins and Stone 1982), KYNA was later shown to block the glycine co-agonist (“glycineB”) site of the N-methyl-d-aspartate receptor with much higher potency (IC50 in the absence of glycine, ~10 µM; Kessler et al. 1989). However, KYNA is unlikely to inhibit this site under physiological conditions (IC50 in the presence of glycine, ~230 µM; Hilmas et al. 2001). Rather, endogenous KYNA appears to function as a preferential α7nAChR antagonist (Hilmas et al. 2001), targeting a site which closely resembles the APL site that is activated by galantamine (Lopes et al. 2007). Interestingly and unrelated to antipsychotic medication, KYNA levels are abnormally high in the brain and cerebrospinal fluid of SZ patients (Erhardt et al. 2001; Schwarcz et al. 2001).
The de novo synthesis of KYNA in the mammalian brain is catalyzed by the irreversible transamination of its bioprecursor kynurenine in astrocytes. Of two distinct astrocytic kynurenine aminotransferases (KAT I and KAT II), KAT II is the dominant isozyme in the rat brain (Guidetti et al. 1997). Newly formed KYNA is rapidly released into the extracellular milieu and eventually removed from the brain by probenecid-sensitive, passive efflux (Moroni et al. 1988; Turski et al. 1989). Recently, we reported the synthesis and biological characterization of (S)-4-ethylsulfonyl benzoylalanine (S-ESBA), the first potent and selective KAT II inhibitor (Pellicciari et al. 2006). Using locally administered KYNA, kynurenine or S-ESBA as tools, we now investigated the effects of fluctuations in brain KYNA levels on the extracellular concentrations of glutamate in the rat PFC. These studies, which were conducted using in vivo microdialysis in unanesthetized rats, also examined if α7nAChRs serve as functional targets of KYNA in the brain.
Materials and Methods
Animals
A total of 22 adult, male Sprague–Dawley rats (220–260 g) were used in the experiments. The animals were maintained on a 12:12-h light/dark cycle in a temperature- and humidity-controlled, Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility and had free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Microdialysis
Rats were anesthetized with chloral hydrate (360 mg/kg, i.p.) and unilaterally implanted with a microdialysis guide cannula (0.38 mm o.d.; 3.0 mm membrane tip) into the medial PFC (A=3.2 mm in front of bregma, L=0.6 mm from the midline, V=1.0 mm below dura). The guide was fixed to the skull using stainless steel screws and dental acrylic, and the surgical site was swabbed with lidocaine (5%).
On the next day, a microdialysis probe (CMA/10, membrane length 3 mm, Carnegie Medicin, Stockholm, Sweden) was inserted through the guide cannula and connected to a microperfusion pump set to a speed of 1 µl/min. The freely moving animals were continuously perfused with Ringer solution, pH 6.7, containing (in millimolar): NaCl, 144; KCl, 4.8; MgSO4, 1.2; and CaCl2, 1.7. After the establishment of a stable baseline, KYNA (100 nM), kynurenine (2.5 µM) or S-ESBA (3 mM) was applied by reverse dialysis for 2 h. Subsequently, perfusion with Ringer solution continued for 4 h. Data were not adjusted for recovery from the microdialysis probe.
Analysis of KYNA and Glutamate
KYNA and glutamate were determined in the same microdialysate by high-performance liquid chromatography (HPLC) with fluorescence detection. To measure KYNA, 10 µL of the microdialysate were diluted with 5 µL of 0.1 M HCl, and 10 µL of the mixture was injected onto a 3-µm C18 reverse phase column (80×4.6 mm, ESA, Bedford, MA, USA). KYNA was isocratically eluted at a flow rate of 1 mL/min, using a mobile phase containing 200 mM zinc acetate and 5% acetonitrile, pH 6.2. In the eluate, KYNA was detected fluorometrically, using an excitation wavelength of 344 nm and an emission wavelength of 398 nm (fluorescence detector, Perkin-Elmer Series 200). The retention time of KYNA was approximately 5.0 min (Swartz et al. 1990).
Glutamate was measured in 8 µL of the microdialysate, as described by Shank et al. (1993). Briefly, o-phthalaldehyde/β-mercaptoethanol was added to each sample (2:1, v/v) to yield a fluorescent derivative. The mixture was applied to a reverse phase HPLC column (C18, 5 µm; 250×4.6 mm; Thermo Electron Corporation, Waltham, MA, USA) and eluted with a gradient composed of two mobile phases ((a) 20 mM sodium acetate, 7.5% acetonitrile, pH 6.1; (b) 30% acetonitrile, 30% methanol) set at a gradient elution program of 15 min at a flow rate of 1 mL/min. In the eluate, glutamate was detected fluorometrically (excitation wavelength 390 nm; emission wavelength 460 nm; Perkin-Elmer Series 200). The retention time of glutamate was approximately 4.0 min.
Chemicals
l-Kynurenine (sulfate) and galantamine were purchased from Sigma (St. Louis, MO, USA). All other biochemicals were of the highest purity available and were obtained from various commercial suppliers. Donepezil (hydrochloride) was purchased from A & A Pharmachem Inc. (Ottawa, Ontario, Canada).
Results
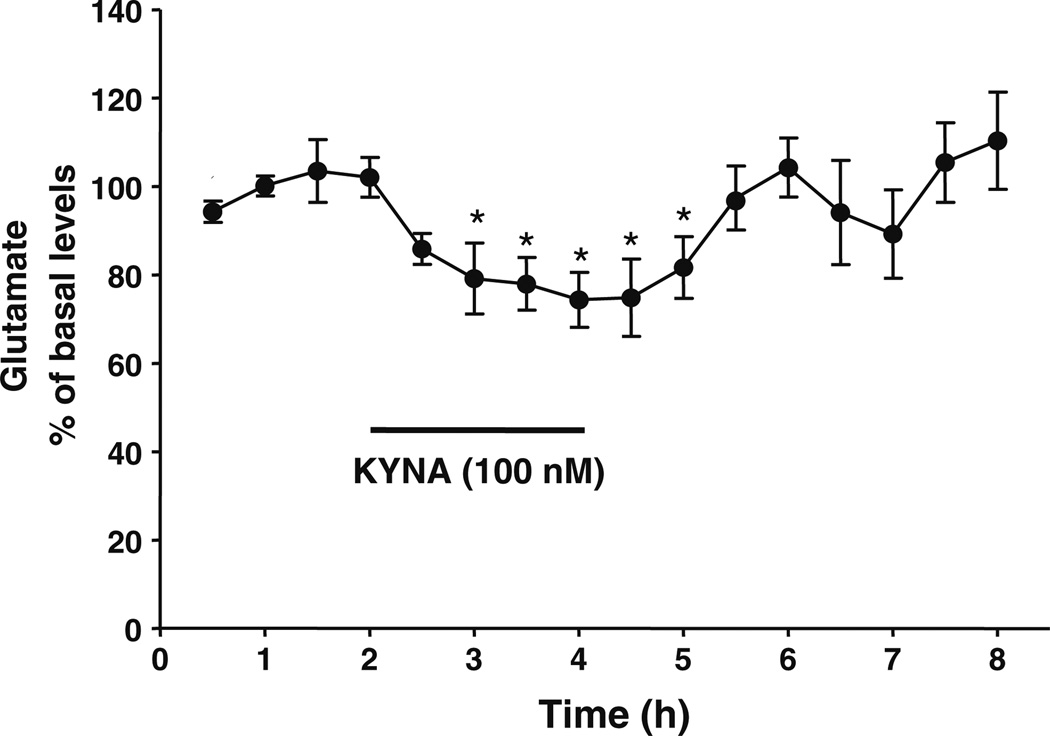
The basal extracellular levels of KYNA and glutamate in the PFC were 2.5±0.2 nM and 1.9±0.1 µM (n=18 and 22, respectively). Local perfusion of KYNA (100 nM) by reverse dialysis caused a significant 26% decrease in extracellular glutamate levels (n=4). This reduction was transient, and glutamate levels promptly reverted to baseline values following the removal of KYNA from the perfusion solution (Fig. 1).
Figure 1.
Effect of KYNA, applied by reverse dialysis (bar), on extracellular levels of glutamate in the PFC. Data are the mean ± standard error of the mean (n=4). *p<0.05 vs. the baseline (one-way ANOVA)
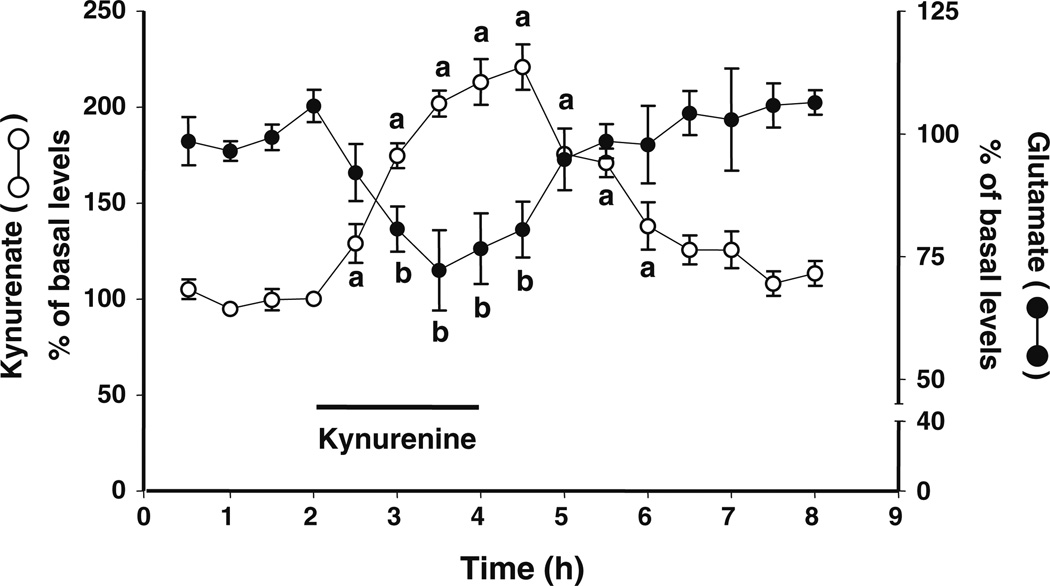
Reverse dialysis of kynurenine (2.5 µM) resulted in an increase in extracellular KYNA, reaching a maximum of 220% of baseline values. In the same microdialysates, extracellular levels of glutamate were reduced, reaching a nadir of −28% compared to baseline levels. Both KYNA and glutamate levels gradually reverted to control values after kynurenine was removed from the perfusion solution (n=4; Fig. 2).
Figure 2.
Effect of a local perfusion of kynurenine (2.5 µM; bar) on extracellular levels of KYNA and glutamate in the PFC. The two analytes were measured in the same microdialysates. Data are the mean ± standard error of the mean of four animals. a, b p<0.05 compared to the respective baseline values (one-way ANOVA)
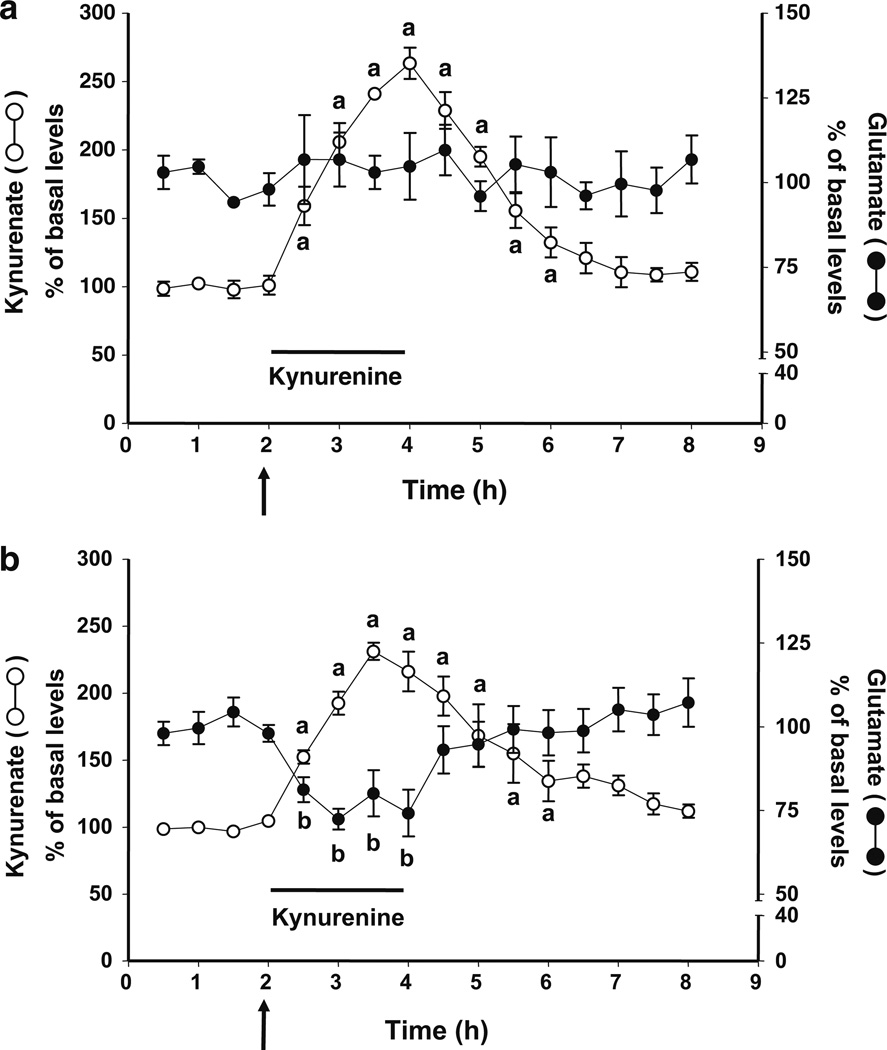
As illustrated in Fig. 3a, systemic administration of galantamine (3 mg/kg, i.p.) prevented the kynurenine-induced decrease in extracellular glutamate without, however, affecting the de novo production of KYNA (n=4). In contrast, a peripheral injection of donepezil (2 mg/kg, i.p.) did not affect either the increase in extracellular KYNA or the reduction in extracellular glutamate caused by the intracortical perfusion of kynurenine (n=6; Fig. 3b).
Figure 3.
Galantamine (3 mg/kg; a) but not donepezil (2 mg/kg; b) blocks the reduction in extracellular glutamate caused by the local perfusion of kynurenine (2.5 µM; bar) in the PFC. Neither of the two drugs, injected i.p. (arrows), affected the de novo formation of KYNA from kynurenine. KYNA and glutamate were measured in the same microdialysates. Data are the mean ± standard error of the mean of four (a) and six (b) animals, respectively. a, b p<0.05 compared to the respective baseline values (one-way ANOVA)
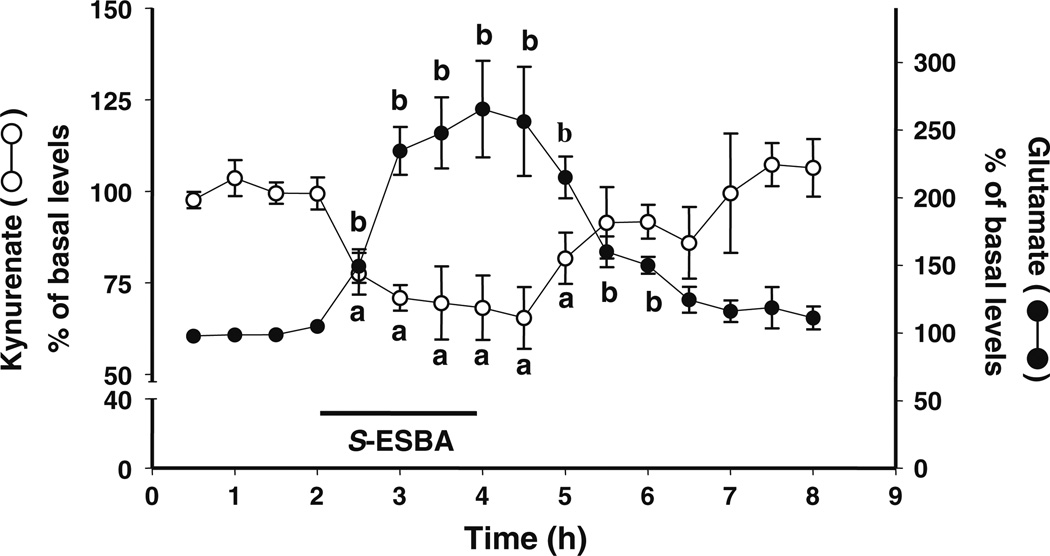
Intracortical perfusion of S-ESBA (3 mM) resulted in a significant 35% reduction in extracellular KYNA, which was accompanied by a 244% elevation of extracellular glutamate levels. The levels of both analytes gradually reverted to control values after the KAT II inhibitor was removed from the perfusion solution (n=4; Fig. 4).
Figure 4.
Effect of a local perfusion of S-ESBA (3 mM; bar) on extracellular levels of KYNA and glutamate in the PFC. The two analytes were measured in the same microdialysates. Data are the mean ± standard error of the mean of four animals. a, b p<0.05 compared to the respective baseline values (one-way ANOVA)
Discussion
The present results demonstrate that nanomolar (i.e., endogenous) concentrations of KYNA exert bidirectional control over extracellular glutamate levels in the rat PFC and that these in vivo effects are mediated by α7nAChRs. More specifically, our experiments indicate that astrocytes, by generating and releasing KYNA, play a pivotal role in the modulation of extracellular glutamate via the allosteric potentiating site of the α7nAChR. Since both glutamate and α7nAChRs are linked to normal and impaired cognition involving the PFC (Hashimoto et al. 2008; Zahr et al. 2008), our study implies that fluctuations in endogenous KYNA may play a significant role in prefrontally mediated cognitive processes.
In subcortical regions, KYNA inhibition of α7nAChRs leads to a reduction in extracellular glutamate levels (Carpenedo et al. 2001; Rassoulpour et al. 2005; Grilli et al. 2006). We show here that KYNA, applied directly into the parenchyma or produced locally by perfusing physiological concentrations of kynurenine, also decreases glutamate levels in the PFC. Throughout the forebrain, this effect is presumably initiated mainly by the blockade of presynaptic α7nAChRs on glutamatergic nerve terminals (Gioanni et al. 1999; Marchi et al. 2002; Rousseau et al. 2005; Dickinson et al. 2008). However, α7nAChRs are also situated on postsynaptic, somatodendritic structures and on nonglutamatergic nerve endings (Csillik et al. 1998; Alkondon et al. 2000; Krenz et al. 2001; Albuquerque et al. 2009), so that it is conceivable that the inhibition of α7nAChRs receptors by KYNA reduces glutamate levels indirectly—either locally or through a distributed system involving brain areas with reciprocal links to the PFC (Del Arco and Mora 2005, 2008; Biton et al. 2007; Couey et al. 2007). Jointly, these mechanisms, which clearly need to be elaborated in greater detail, probably account for the fact that even modest increases in cortical KYNA levels also influence extracellular dopamine and acetylcholine levels in the PFC (Wu et al. 2006; Zmarowski et al. 2009).
Regardless of the precise cellular or regional localization of the targeted receptors, our experiments with galantamine and donepezil demonstrated unambiguously that the KYNA-induced reduction in extracellular glutamate in the PFC is indeed α7nAChR dependent. Thus, systemic administration of galantamine, which acts as an agonist at a site that is very similar or identical to the APL site of the α7nAChR that is inhibited by KYNA (Samochocki et al. 2003; Lopes et al. 2007), totally prevented the effect of KYNA. In contrast, no such neutralizing effect was observed after the peripheral application of a similar dose of donepezil, which is a substantially more potent acetylcholinesterase inhibitor than galantamine (Geerts et al. 2005). Moreover, donepezil does not function as an APL site agonist either in vitro (Samochocki et al. 2003) or in vivo (Schilström et al. 2007). These results, together with pharmacokinetic considerations (Geerts et al. 2005), suggest that the effect of galantamine shown here was in all likelihood due to selective α7nAChR stimulation rather than a nonspecific activation of cholinergic receptors by elevated acetylcholine levels.
As expected, intracortical perfusion of the KAT II inhibitor S-ESBA for 2 h transiently decreased the extracellular levels of KYNA, confirming that astrocytes, which contain KAT II almost exclusively (Guidetti et al. 2007), continuously produce KYNA from its endogenous bioprecursor kynurenine in vivo and steadily release newly formed KYNA into the extracellular milieu. In the same microdialysates, glutamate levels were elevated compared to baseline values, indicating a causal relationship between a reduction in KYNA synthesis and increased glutamate release. These results were the inverse of the effects of KYNA or kynurenine (see above and also Konradsson-Geuken et al. 2009), suggesting that astrocyte-derived KYNA, probably primarily by controlling the activation of presynaptic α7nAChRs, tonically modulates the release of glutamate in the PFC. Notably, as in the case of KYNA elevations, the effects of KYNA synthesis inhibition are not limited to the glutamatergic system since intracortical perfusion of S-ESBA also causes significant increases in extracellular dopamine and acetylcholine concentrations (Wu et al. 2006; Zmarowski et al. 2009). Moreover, similar bidirectional consequences of fluctuations in KYNA production are seen in subcortical brain areas such the striatum (Amori et al. 2009) and the hippocampus (Wu et al. 2007) and may therefore represent a more general, novel mechanism by which astrocytes influence neurotransmission in the mammalian brain.
The functional consequences of KYNA’s neurochemical effects in the PFC are especially interesting in light of the fact that prefrontal KYNA levels are abnormally high in SZ (Schwarcz et al. 2001) and that debilitating polymorphisms in the regulatory region of the α7nAChR gene are associated with the disease (Stephens et al. 2009). Thus, enhanced inhibition of already dysfunctional α7nAChRs by KYNA and subsequent impairment of glutamatergic (and/or cholinergic and dopaminergic) neurotransmission within the PFC may be causally related to several of the cognitive deficits seen in individuals with SZ. This concept is supported by pharmacological studies in experimental model systems, where manipulations of α7nAChRs have been shown to predictably influence—exacerbate or ameliorate—disease-relevant, prefrontally mediated phenomena such as working memory (Chan et al. 2007; Chess et al. 2007), selective attention (Pichat et al. 2007), and cognitive flexibility (Zmarowski et al. 2008). Together, these data encouraged clinical investigators to use nicotinic agents such as galantamine or the partial α7nAChR agonist DMXB-A as adjunctive treatments in SZ, and first studies have revealed promising, selective cognitive improvements in patients (Olincy et al. 2006; Schubert et al. 2006; Buchanan et al. 2008). Based on our present results, it is tempting to speculate that these beneficial effects of α7nAChR stimulation are related to a secondary normalization of glutamatergic function, which is suspected to correct cognitive deficits in individuals with SZ (Buchanan et al. 2007). Notably, these considerations are also remarkably compatible with the increasingly propagated hypothesis that an activated immune system, resulting in impaired α7nAChR function and increased KYNA formation, plays a role in the pathophysiology of SZ (Wang et al. 2003; Müller and Schwarz 2007; Schwarcz and Hunter 2007; Holtze et al. 2008).
Establishment of a functional link between enhanced KYNA levels in the PFC and cognitive defects would support the hypothesis that attenuation of cerebral KYNA formation, for example by selective inhibition of KAT II, might improve cognitive abilities in patients afflicted with SZ (Schwarcz and Pellicciari 2002). Ongoing studies in our laboratories are therefore designed to investigate if experimental manipulations of KYNA levels in the PFC and beyond produce salient behavioral effects, which may provide insights into the pathophysiology and treatment of cognitive deficits in SZ.
Acknowledgment
This work was supported by USPHS grant NS25296.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proceedings of the XIII International Symposium on Cholinergic Mechanisms
Contributor Information
Hui-Qiu Wu, Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, P.O. Box 21247, Baltimore, MD 21228, USA.
Edna F. R. Pereira, Department of Pharmacology and Experimental Therapeutics, University of Maryland School of Medicine, Baltimore, MD 21201, USA
John P. Bruno, Department of Psychology and Neuroscience, The Ohio State University, Columbus, OH 43210, USA
Roberto Pellicciari, Dipartimento di Chimica e Tecnologia del Farmaco, Università di Perugia, Perugia, Italy.
Edson X. Albuquerque, Department of Pharmacology and Experimental Therapeutics, University of Maryland School of Medicine, Baltimore, MD 21201, USA
Robert Schwarcz, Email: rschwarc@mprc.umaryland.edu, Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, P.O. Box 21247, Baltimore, MD 21228, USA.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiological Reviews. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: A mechanism for inhibition and disinhibition of neuronal networks. Journal of Neuroscience. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amori L, Wu H-Q, Marinozzi M, Pellicciari R, Guidetti P, Schwarcz R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton B, Bergis OE, Galli F, et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology. 2007;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Conley RR, Dickinson D, et al. Galantamine for the treatment of cognitive impairments in people with schizophrenia. American Journal of Psychiatry. 2008;165:82–89. doi: 10.1176/appi.ajp.2007.07050724. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophrenia Bulletin. 2007;33:1120–1130. doi: 10.1093/schbul/sbm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenedo R, Pittaluga A, Cozzi A, et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. European Journal of Neuroscience. 2001;13:2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- Chan WK, Wong PT, Sheu F. Frontal cortical α7 and α4β2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology. 2007;52:1641–1649. doi: 10.1016/j.neuropharm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophrenia Bulletin. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, et al. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Csillik B, Nemcsók J, Boncz I, Knyihár-Csillik E. Nitric oxide synthase and the acetylcholine receptor in the prefrontal cortex: Metasynaptic organization of the brain. Neurobiology (Budapest) 1998;6:383–404. [PubMed] [Google Scholar]
- Del Arco A, Mora F. Glutamate-dopamine in vivo interaction in the prefrontal cortex modulates the release of dopamine and acetylcholine in the nucleus accumbens of the awake rat. Journal of Neural Transmission. 2005;112:97–109. doi: 10.1007/s00702-004-0172-5. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: In vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacology, Biochemistry and Behavior. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Molecular Pharmacology. 2008;74:348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neuroscience Letters. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Research. 2005;1033:186–193. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepousé C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: Increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. European Journal of Neuroscience. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Grilli M, Raiteri L, Patti L, et al. Modulation of the function of presynaptic alpha7 and non-alpha7 nicotinic receptors by the tryptophan metabolites, 5-hydroxyindole and kynurenate in mouse brain. British Journal of Pharmacology. 2006;149:724–732. doi: 10.1038/sj.bjp.0706914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. NeuroReport. 1999;10:1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. Journal of Neuroscience Research. 1997;50:457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ishima T, Fujita Y, et al. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the novel selective alpha7 nicotinic receptor agonist SSR180711. Biological Psychiatry. 2008;63:92–97. doi: 10.1016/j.biopsych.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. Journal of Neuroscience. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtze M, Asp L, Schwieler L, Engberg G, Karlsson H. Induction of the kynurenine pathway by neurotropic influenza A virus infection. Journal of Neuroscience Research. 2008;86:3674–3683. doi: 10.1002/jnr.21799. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Archives of General Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-d-aspartic acid receptors: Characterization and identification of a new class of antagonists. Journal of Neurochemistry. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Konradsson-Geuken Å, Gash CR, Alexander K, et al. Second-by-second analysis of alpha7 nicotinic receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63:1069–1082. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz I, Kalkan D, Wevers A, et al. Parvalbumin-containing interneurons of the human cerebral cortex express nicotinic acetylcholine receptor proteins. Journal of Chemical Neuroanatomy. 2001;21:239–246. doi: 10.1016/s0891-0618(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Leonard S, Freedman R. Genetics of chromosome 15q13–q14 in schizophrenia. Biological Psychiatry. 2006;60:115–122. doi: 10.1016/j.biopsych.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: Convergence of gamma-aminobutyric acid and glutamate alterations. Archives of Neurology. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lopes C, Pereira EFR, Wu H-Q, et al. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha7* nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics. 2007;322:48–58. doi: 10.1124/jpet.107.123109. [DOI] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. Journal of Neurochemistry. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Homayoun H. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Russi P, Lombardi G, Beni M, Carlà V. Presence of kynurenic acid in the mammalian brain. Journal of Neurochemistry. 1988;51:177–180. doi: 10.1111/j.1471-4159.1988.tb04852.x. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. The immunological basis of glutamatergic disturbance in schizophrenia: Towards an integrated view. Journal of Neural Transmission. Supplementum. 2007;72:269–280. doi: 10.1007/978-3-211-73574-9_33. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Archives of General Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Rizzo R, Costantino G, et al. Modulators of the kynurenine pathway of tryptophan metabolism: Synthesis and preliminary biological evaluation of (S)-4-(ethylsulfonyl) benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II) inhibitor. ChemMedChem. 2006;1:528–531. doi: 10.1002/cmdc.200500095. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Research. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Pichat P, Bergis OE, Terranova JP, et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007;32:17–34. doi: 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- Rassoulpour A, Wu H-Q, Ferré S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. Journal of Neurochemistry. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Rousseau SJ, Jones IW, Pullar IA, Wonnacott S. Presynaptic alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate [3H]d-aspartate release from rat frontal cortex in vitro. Neuropharmacology. 2005;49:59–72. doi: 10.1016/j.neuropharm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Höffle A, Fehrenbacher A, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. Journal of Pharmacology and Experimental Therapeutics. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophrenia Bulletin. 2005;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Schilström B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32:43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biological Psychiatry. 2006;60:530–533. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophrenia Bulletin. 2007;33:652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. Journal of Pharmacology and Experimental Therapeutics. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biological Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Shank RP, Leo GC, Zielke HR. Cerebral metabolic compartmentation as revealed by nuclear magnetic resonance analysis of D-[1-13C]glucose metabolism. Journal of Neurochemistry. 1993;61:315–323. doi: 10.1111/j.1471-4159.1993.tb03570.x. [DOI] [PubMed] [Google Scholar]
- Stephens SH, Logel J, Barton A, et al. Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophrenia Research. 2009;109:102–112. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: An endogenous antagonist of excitatory amino acid receptors. Journal of Neuroscience. 1990;10:2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski WA, Gramsbergen JB, Traitler H, Schwarcz R. Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. Journal of Neurochemistry. 1989;52:1629–1636. doi: 10.1111/j.1471-4159.1989.tb09218.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wu H-Q, Pellicciari R, Schwarcz R. Bidirectional regulation of extracellular dopamine by endogenous kynurenic acid in the rat medial prefrontal cortex. Society for Neuroscience Abstracts. 2006;32:624.3. [Google Scholar]
- Wu H-Q, Marinozzi M, Pellicciari R, Schwarcz R. Endogenous kynurenic acid modulates extracellular glutamate levels in the rat hippocampus. Society for Neuroscience Abstracts. 2007;32:500.15. [Google Scholar]
- Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV. Low striatal glutamate levels underlie cognitive decline in the elderly: Evidence from in vivo molecular spectroscopy. Cerebral Cortex. 2008;18:2241–2250. doi: 10.1093/cercor/bhm250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmarowski A, Wu H-Q, Schwarcz R, Bruno JP. Elevations in brain kynurenic acid impair cognitive flexibility in rats: relevance to schizophrenia. Society for Neuroscience Abstracts. 2008;33:657.11. [Google Scholar]
- Zmarowski A, Wu H-Q, Brooks JM, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. European Journal of Neuroscience. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]