Abstract
The detection of nucleobases, the informational subunits of DNA and RNA, in several meteorites suggests that these compounds of biological interest were formed via astrophysical, abiotic processes. This hypothesis is in agreement with recent laboratory studies of irradiation of pyrimidine in H2O-rich ices with vacuum UV photons emitted by an H2-discharge lamp in the 6.9–11.3 eV (110–180 nm) range at low temperature, shown to lead to the abiotic formation of several compounds including the nucleobases uracil, cytosine, and thymine. In this work, we irradiated H2O:pyrimidine ice mixtures under astrophysically relevant conditions (14 K, ≤10−9 torr) with high-energy UV photons provided by a synchrotron source in three different ranges: the 0th order light (4.1–49.6 eV, 25–300 nm), the He i line (21.2 eV, 58.4 nm), and the He ii line (40.8 eV, 30.4 nm). The photodestruction of pyrimidine was monitored with IR spectroscopy, and the samples recovered at room temperature were analyzed with liquid and gas chromatographies. Uracil and its precursor 4(3H)-pyrimidone were found in all samples, with absolute and relative abundances varying significantly from one sample to another. These results support a scenario in which compounds of biological interest can be formed and survive in environments subjected to high-energy UV radiation fields. Key Words: Pyrimidine—Nucleobases—Interstellar ices—Cometary ices—High-energy photons—Molecular processes—Prebiotic chemistry. Astrobiology 14, 119–131.
1. Introduction
Pyrimidine (C4H4N2) is a key molecule of prebiotic and biological interest, as it is the structural backbone of three of the nucleobases, the subunits carrying the genetic information, used in ribonucleic (RNA) and deoxyribonucleic (DNA) acids. Pyrimidine-based biological nucleobases include uracil, cytosine, and thymine, while the two other biological nucleobases adenine and guanine are based on another N-heterocyclic molecule, namely, purine (C5H4N4). The molecular structures of all these compounds can be found elsewhere (Nuevo et al., 2009).
Pyrimidine and several other small N-heterocycles such as purine and pyridine have been searched for in the gas phase of the interstellar medium (ISM) for a long time, though none of these compounds have been detected to date (Simon and Simon, 1973; Kuan et al., 2003, 2004; Charnley et al., 2005; Brünken et al., 2006). In contrast, several purine-based and a few pyrimidine-based compounds including biological nucleobases as well as a number of their derivatives have been found in carbonaceous chondrites such as Murchison, Murray, Orgueil, and Lonewolf Nunataks 94102 (Hayatsu, 1964; Folsome et al., 1971, 1973; Hayatsu et al., 1975; van der Velden and Schwartz, 1977; Stoks and Schwartz, 1979, 1981; Callahan et al., 2011). Isotopic analysis of these meteoritic compounds is consistent with an extraterrestrial origin (Martins et al., 2008), suggesting the existence of abiotic chemical pathways for their formation in astrophysical environments.
Therefore, it is plausible that pure and substituted N-heterocycles are present in the solid phase, condensed on the surface of cold, icy grains in the dense ISM (Sandford et al., 2004; Bernstein et al., 2005), in a similar way to what is observed for polycyclic aromatic hydrocarbons (PAHs) and polycyclic aromatic nitrogen heterocycles (PANHs) (Allamandola et al., 1989; Puget and Léger, 1989; Roelfsema et al., 1996). N-Heterocycles, PAHs, and PANHs are believed to form via similar routes, from the polymerization of small molecules such as acetylene (C2H2) and nitrogen-bearing species such as cyanic (HCN) and isocyanic (HNC) acids (Frenklach and Feigelson, 1989; Ricca et al., 2001).
In addition, it is worth mentioning that nucleobases have been experimentally shown to form from high-energy proton bombardment (2.5–3.0 MeV) of different combinations of carbon sources (CO, CO2, and/or CH4) mixed with H2O and N2 in the gas phase at 297 K (Kobayashi and Tsuji, 1997; Yamanashi et al., 2001; Miyakawa et al., 2002). Although more applicable to denser, hotter environments such as planetary atmospheres, these experiments led to the formation of uracil, cytosine, thymine, and, in a few cases, guanine and adenine in measurable quantities.
More recently, laboratory experiments in which pyrimidine mixed with pure H2O ice was irradiated with UV photons emitted by an H2-discharge lamp at low temperature (<25 K) showed that several photo-products derived from pyrimidine can be formed, such as the nucleobase uracil and its precursor 4(3H)-pyrimidone (Nuevo et al., 2009). These two compounds, which are the most abundant photo-products among the singly [4(3H)-pyrimidone] and doubly oxidized (uracil) variants of pyrimidine in laboratory samples, have been reported in Murchison, Murray, and Orgueil meteorites (Folsome et al., 1971, 1973; Lawless et al., 1972; Stoks and Schwartz, 1979). 4(3H)-Pyrimidone and uracil were also shown to be the most stable and favorable singly and doubly oxidized products of pyrimidine, respectively, from ab initio quantum computations, which also indicated that the presence of H2O as a matrix is essential for the formation of products, as it plays a role of catalyst for proton abstraction (Bera et al., 2010).
However, no studies about the effect of the photon energy on H2O:pyrimidine ice mixtures have ever been performed, although icy mantles on cold grains may experience different types of UV radiation in distinct astrophysical environments, such as the diffuse ISM, dense molecular clouds, and the solar nebula. Indeed, young stars emit energetic photons up to the extreme ultraviolet (EUV) range, which covers the spectral range between 11.7 eV (106 nm) and soft X rays (e.g., Wu et al., 2002). Stellar EUV emission is usually due to several atomic line transitions (Judge and Pietarila, 2004; Peter et al., 2006) and is believed to affect circumstellar environments including proto-planetary nebulae, even though the intensity of such EUV radiation is estimated to be 2–3 orders of magnitude lower than that of vacuum UV radiation (Rees, 1989; Meier, 1991).
The only studies of irradiation of pyrimidine with high-energy photons were conducted recently and focused on the irradiation of pure pyrimidine in the gas phase, as well as other pure compounds of astrobiological interest such as uracil, with the use of soft X rays at 150 eV (Pilling et al., 2011) and 399 eV (Mendoza et al., 2013) to study the photo-destruction and photo-desorption of small N-heterocycles. The results of these studies suggest that the irradiation of pyrimidine with soft X rays leads mainly to the photodestruction of pyrimidine and the subsequent formation of smaller compounds rather than the conversion of pyrimidine into larger pyrimidine-based compounds.
Therefore, to assess how high-energy UV photons may affect the formation of pyrimidine-based compounds from pyrimidine in pure H2O in the solid phase under astrophysical conditions, we performed a series of laboratory experiments of photo-irradiation of three H2O:pyrimidine ice mixtures at 14 K with three different UV/EUV beams provided by a synchrotron light beam, as follows: (i) the 0th order beam light, covering the broad 4.1–49.6 eV (25–300 nm) range, (ii) the He i line transition (21.2 eV, 58.4 nm), and (iii) the He ii line transition (40.8 eV, 30.4 nm). In all these experiments, the relative proportions between H2O and pyrimidine were monitored to stay in the 7:1 to 10:1 range, so that ice matrix effects were dominated by H2O.
After irradiation, each sample was allowed to warm up to room temperature naturally. Each resulting residue was analyzed with high-performance liquid chromatography (HPLC) and gas chromatography coupled with mass spectrometry (GC-MS) to search for the presence of several pyrimidine derivatives, with a focus on the nucleobase uracil and its precursor 4(3H)-pyrimidone as well as photo-products resulting from the photo-dissociation of pyrimidine. After measuring the photo-stability of pyrimidine in H2O ice when irradiated with these three different UV/EUV sources, we estimated the production yields for a few identified photo-products in order to compare them with each other as well as with previous experimental studies of irradiation of similar ice mixtures with UV photons emitted by an H2-discharge lamp. Finally, all the results obtained are discussed from an astrobiological point of view.
2. Experimental Methods
2.1. UV/EUV photo-irradiation of ices at low temperature
Experiments were carried out in an ultrahigh-vacuum (UHV) chamber built in the Department of Physics of the National Central University in Jhongli, Taiwan. This UHV chamber is evacuated by a turbo-molecular pump (KYKY FF-160/620ZE, pumping capacity: 600 L s−1), which is backed up by a dry pump (Alcatel Drytel 31) and a non-evaporative getter pump (SASE GP 50), and equipped with a closed-cycle helium cryostat (CTI-M350) (Chen et al., 2014). The pressure inside the UHV chamber, which is monitored by a Granville-Phillips 370 Stabil-Ion gauge, can reach down to 1×10−10 torr. The vacuum chamber also includes an oxygen-free copper cold finger with a sample holder, which is protected by a radiation shield, and can be cooled down to 14 K. CaF2 windows (PIKE Technologies, research grade), which are transparent in the mid-IR range (5000–815 cm−1), were used as substrate for these experiments. All the parts of the cold finger are connected with 99.99% pure indium to ensure a perfect thermal conductivity. Finally, a silicon diode sensor (LakeShore DT-670-SD) was used to monitor the temperature at the sample location with a 0.1 K accuracy from 14 to 400 K with the use of a LakeShore 331S temperature controller equipped with a tunable heater.
Gas mixtures were prepared in a gas line system that consisted of four stainless-steel bottles of the same volume (300 mL) and was used to determine the relative proportions between the mixture components via their partial pressures. Pressures in the gas line are measured with an MKS Baratron 622A gauge in the 0–10 torr range with a 0.25% accuracy. The gas line is evacuated by a turbo-molecular pump (Oerlikon Leybold TurboVac 151, pumping capacity: 145 L s−1) that is backed up by an oil-sealed mechanical pump (Alcatel 2012A, pumping capacity: 450 L min−1) equipped with an oil trap (molecular sieve type 13X). The background pressure in the gas line is routinely lower than 1×10−7 torr. When not used to mix gases, the gas line is continuously baked at 120°C to avoid any organic contamination.
Samples in the UHV chamber are monitored by a mid-infrared Fourier-transform spectrometer (ABB FTLA-2000-104) equipped with a mercury-cadmium-telluride detector. For this study, IR spectra were acquired with resolutions of 1 cm−1 (background spectra and final spectra of residues) and 4 cm−1 (intermediate spectra during irradiation), and averaged over 512 and 128 scans, respectively. The IR beam path was built under vacuum to avoid any contamination from atmospheric CO2 and H2O.
The UHV chamber was directly connected to the high-flux beamline BL03A of the National Synchrotron Radiation Research Center in Hsinchu, Taiwan, which provides a range of monochromatic and white light beams. For this particular study, we used three different energy beam configurations: the 0th order beam light (4.1–49.6 eV, 25–300 nm), the He i line (21.2 eV, 58.4 nm), and the He ii line (40.8 eV, 30.4 nm). The fluxes of these different beam configurations at the sample location, measured in situ with a calibrated 88% transmittance nickel mesh (Chen et al., 2014), are given in Table 1.
Table 1.
Summary of the Experimental Parameters for the Three Samples Prepared at the National Synchrotron Radiation Research Center
| Sample | Photon energy (eV) | Photon wavelength (nm) | H2O:Pyrimidine ratio | Irradiation time (h) | Photon flux (cm−2 s−1) |
|---|---|---|---|---|---|
| 0th order light | 4.1–49.6 | 25–300 | 7:1 | 20 | 3.08×1016 |
| He i line | 21.2 | 58.4 | 9:1 | 32 | 6.46×1013 |
| He ii line | 40.8 | 30.4 | 7:1 | 32 | 5.55×1013 |
Samples were simultaneously deposited and irradiated for either 20 h (0th order light experiment) or 32 h (He i and He ii line experiments) under similar temperature and pressure conditions (∼14 K, ∼1.0×10−9 torr).
Each experiment was carried out by using one beamline configuration for the irradiation of one H2O:pyrimidine ice mixture with relative proportions ranging from 7:1 to 9:1 (Table 1). Each ice mixture was deposited on the CaF2 window, cooled to 14 K, and simultaneously irradiated for 20 h (0th order beam experiment) or 32 h (He i and He ii line experiments) with a pressure in the vacuum chamber of ∼1×10−9 torr. Deposition rates of pyrimidine and H2O from their respective vapors were adjusted so that their relative abundance in the ice mixtures remained in the 7:1–10:1 range. These abundance ratios between H2O and pyrimidine were verified by IR spectroscopy by using the bands at ∼3300 cm−1 (H2O) and ∼1406 cm−1 (pyrimidine), with integrated band strengths of 1.7×10−16 and 6.7×10−18 cm molecule−1, respectively (Hudgins et al., 1993; Nuevo et al., 2009). In such ice mixtures, H2O is significantly more abundant than pyrimidine to ensure that ice matrix effects are dominated by H2O as it is observed in interstellar and circumstellar environments.
2.2. IR analysis of the samples
Before each long experiment (20 and 32 h) of simultaneous deposition of H2O:pyrimidine ices and irradiation with UV photons, about 1000 monolayers (ML) of each starting ice mixture was deposited onto the substrate (1 ML=1015 molecules cm−2; e.g., Öberg et al., 2007, 2009). These 1000 ML ices were subsequently irradiated by increasing intervals from 0 to 130 min for the 0th order beam and the He ii line experiments and from 0 to 160 min for the He i line experiment. Infrared spectra were recorded after each irradiation interval with a 4 cm−1 resolution, and the column density of pyrimidine was monitored via its IR band near 1406 cm−1 by using an integrated band strength of 6.7×10−18 cm molecule−1 (Hudgins et al., 1993; Nuevo et al., 2009, 2012).
During each long (20 and 32 h) experiment, the chemical evolution of the samples was monitored by collecting IR spectra every 4 h with a 4 cm−1 resolution. Finally, IR spectra with a 1 cm−1 resolution were collected for each final ice sample (after irradiation, 14 K) and for each residue at room temperature after warm-up.
2.3. HPLC and GC-MS analysis of residues at room temperature
Residues recovered at room temperature were extracted from their CaF2 substrates with small amounts of deionized H2O (Merck, Spectrum grade), and the extracts were kept in clean vials. Since the volumes of water used to extract the residues were not the same for each of them, the vials with the dissolved residues were then dried under vacuum overnight (15–16 h) and redissolved in 500 μL of H2O each (Millipore Direct-Q UV 3, purified to 18.2 MΩ cm).
The resulting dissolved samples were first analyzed with HPLC. They were injected into an Agilent 1100 Series device and separated in a Phenomenex Luna 5μ Phenyl-Hexyl column (size: 250 mm×4.60 mm, inner diameter: 5 μm), with a volume of 5 μL for each run. Separated compounds were detected by a diode-array UV detector that recorded signals simultaneously at five wavelengths (220, 245, 256, 280, and 300 nm). In this particular study, we concentrated on the chromatograms obtained at 256 nm, which provided the best signals for the peaks of pyrimidine and its oxidized derivatives. The method used (solvent gradients and pH 5 ammonium formate buffer) are described in Nuevo et al. (2009). Identification of the peaks in the sample chromatograms was performed by comparing both their retention times in the 256 nm chromatograms and their UV spectra with commercial standards, dissolved to 10−3 M in H2O (Millipore, 18.2 MΩ cm resistivity) and injected by using the same method as the samples.
The same samples were then analyzed with GC-MS. For this, 100 μL of each sample were transferred into small prebaked (500°C) vials and dried under vacuum in a desiccator for 2 h. Once dry, 50 μL of a 3:1:1 mixture of N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide (MTBSTFA) with 1% of tert-butyldimethylchlorosilane (tBDMCS) (Restek), dimethylformamide (Pierce, silylation grade solvent), and pyrene (Sigma-Aldrich, analytical standard, 100 ng μL−1 dissolved in cyclohexane) were added to each residue. These mixtures were then stirred and heated to 100°C for 1 h to convert all compounds containing α-hydrogen moieties (mainly OH groups in this particular study) into their tert-butyldimethylsilyl (tBDMS) derivatives (MacKenzie et al., 1987; Casal et al., 2004; Schummer et al., 2009).
Separation of the samples was carried out with a Thermo Trace gas chromatograph coupled with a DSQ II mass spectrometer with a splitless injection, a Restek Rxi-5ms column (length: 30 m, inner diameter: 0.25 mm, film thickness: 0.50 μm), an injector temperature of 250°C, and a helium (carrier gas; Airgas, ultrapure) flow of 1.3 mL min−1. The temperature gradient used for these separations is described in detail elsewhere (Nuevo et al., 2009). Masses were recorded in the 50–550 amu range, and data analysis was performed with the Xcalibur software (Thermo Finnigan). Identification of the peaks in sample chromatograms was performed by comparing both their retention times and mass spectra with the same standards as used for HPLC analysis, which were derivatized the same way as the samples.
In the samples produced in this study, we mainly searched for pyrimidine itself, 2,2′-bipyrimidine, as well as oxidized variants of pyrimidine, including 2-hydroxypyrimidine (singly oxidized), 4(3H)-pyrimidone (singly oxidized), pyrimidine N-oxide (singly oxidized), uracil (doubly oxidized), 4,6-dihydroxypyrimidine (doubly oxidized), barbituric acid (triply oxidized), and isobarbituric acid (triply oxidized). We also searched for small aliphatic compounds, including urea and the amino acids glycine, alanine, N-ethylglycine, and N-formylglycine. The purity, origin, molecular structures, molecular weights, expected retention times in the HPLC and GC-MS devices, as well as the molecular weights of the derivatized compounds for all these standards can be found elsewhere (Materese et al., 2013).
3. Results
3.1. Photo-destruction of pyrimidine in H2O ice (IR spectroscopy)
3.1.1. Photo-destruction half-lives of pyrimidine
Infrared spectra were recorded for the H2O:pyrimidine ice mixtures (1000 ML) subjected to the three different UV beams (0th order beam light, He i line, and He ii line). From these spectra, we derived the column densities of pyrimidine in each ice sample by integrating its IR band at ∼1406 cm−1 in the spectra. The column densities obtained were then plotted as a function of the irradiation time. As a first approach, these curves could be fitted with first-order exponential decay functions of the form:
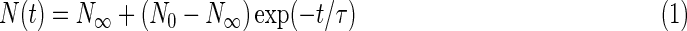 |
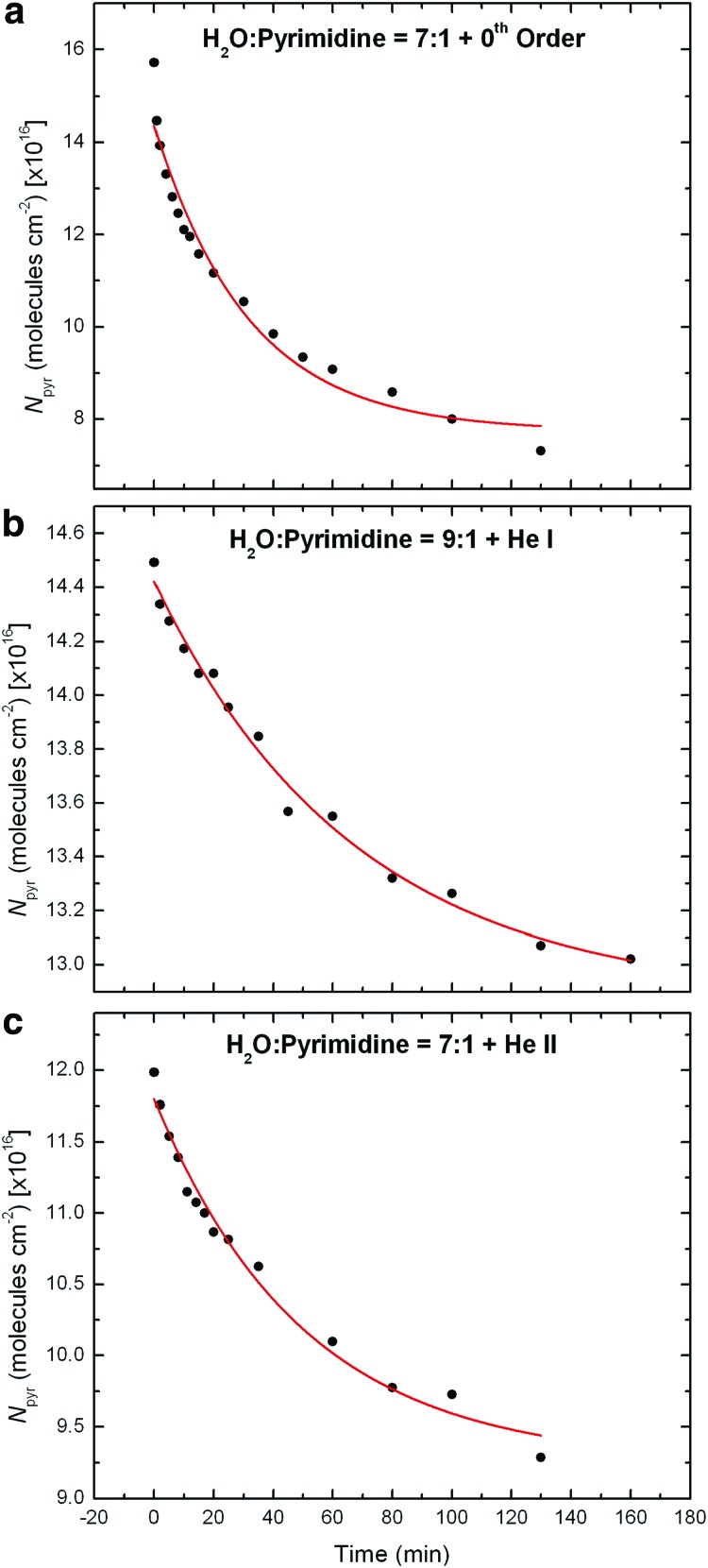
where N(t) is the column density of pyrimidine (in molecules cm−2) as a function of the time t, N0 the column density of pyrimidine at t=0, N∞ the column density of pyrimidine at t=∞ (asymptotic thickness of the ice film), and τ the characteristic time (exponential decay factor). The fits of the experimental data with such first-order exponential decay functions for all three samples (0th order beam light, He i line, and He ii line), which were obtained with the Fityk software1, are shown in Fig. 1.
FIG. 1.
Experimental data showing the decreases in column density of pyrimidine (Npyr) derived from the ∼1406 cm−1 bands in the IR spectra measured for the three samples irradiated with (a) the 0th order beam light, (b) the He i line, and (c) the He ii line, as well as the first-order exponential decay fits of these photo-destruction curves of pyrimidine following Eq. 1. The half-lives derived from these curves are given in Table 2.
Half-lives (t1/2) correspond to the time needed for half of the pyrimidine to be destroyed, that is, the time when its column density reaches a value of N1/2=(N0+N∞)/2, and are given by t1/2=τ ln 2. The half-lives obtained for pyrimidine in each experiment are summarized in Table 2. These first-order exponential decays (Eq. 1) provide general information about the photo-stability of pyrimidine when embedded in an H2O ice matrix and subjected to UV/EUV photons of different energies.
Table 2.
Comparison between the UV/EUV Photo-Induced Half-Lives of Pyrimidine in Pure H2O Ice When Subjected to the 0th Order Light, the He i Line, and the He ii Line
| Experiment/UV source | Photon energy (eV) | Half-lives (min) |
|---|---|---|
| Synchrotron 0th order light | 4.1–49.6 | 22 |
| Synchrotron He i line | 21.2 | 47 |
| Synchrotron He ii line | 40.8 | 35 |
| H2-discharge lampa | 6.9–11.3 | 38 |
| Pyrimidine in argon (1:750) with H2-discharge lampb | 6.9–11.3 | 0.93 |
These half-lives are compared with that of an H2O:pyrimidine=20:1 ice mixture irradiated with an H2-discharge UV lamp (Nuevo et al., 2009) and with that of pyrimidine in an argon matrix (Peeters et al., 2005) with a similar H2-discharge lamp.
Data from Nuevo et al. (2009).
Data from Peeters et al. (2005).
The half-lives measured for pyrimidine for these three samples are of the same order of magnitude as that derived for an H2O:pyrimidine=20:1 ice mixture irradiated with an H2-discharge lamp (38 min; Nuevo et al., 2009). The shortest half-life was measured for the residue irradiated with the 0th order light (22 min), while the half-lives derived for the residues irradiated with the He i and He ii lines were found to be 47 and 35 min, respectively (Table 2).
Therefore, when pyrimidine is embedded in H2O ice and irradiated with the broad 4.1–49.6 eV 0th order beam light, its photo-destruction half-life is significantly shorter (about half) than when it is irradiated with the H2-discharge lamp. This indicates that the presence of high-energy (E>12 eV) photons significantly increases the destruction and/or alteration of pyrimidine and therefore decreases its photodestruction half-life, compared with low-energy (E<12 eV) photons. However, not all high-energy photons have a similar effect on pyrimidine, since the half-life of pyrimidine in H2O irradiated with He ii photons (40.8 eV) is comparable to that measured when it is irradiated with the H2-discharge lamp. The longest half-life for pyrimidine in H2O ice was measured for the He i line experiment (47 min), which suggests that He i photons (21.2 eV) are not absorbed by pyrimidine and/or H2O molecules as efficiently as photons of other energies.
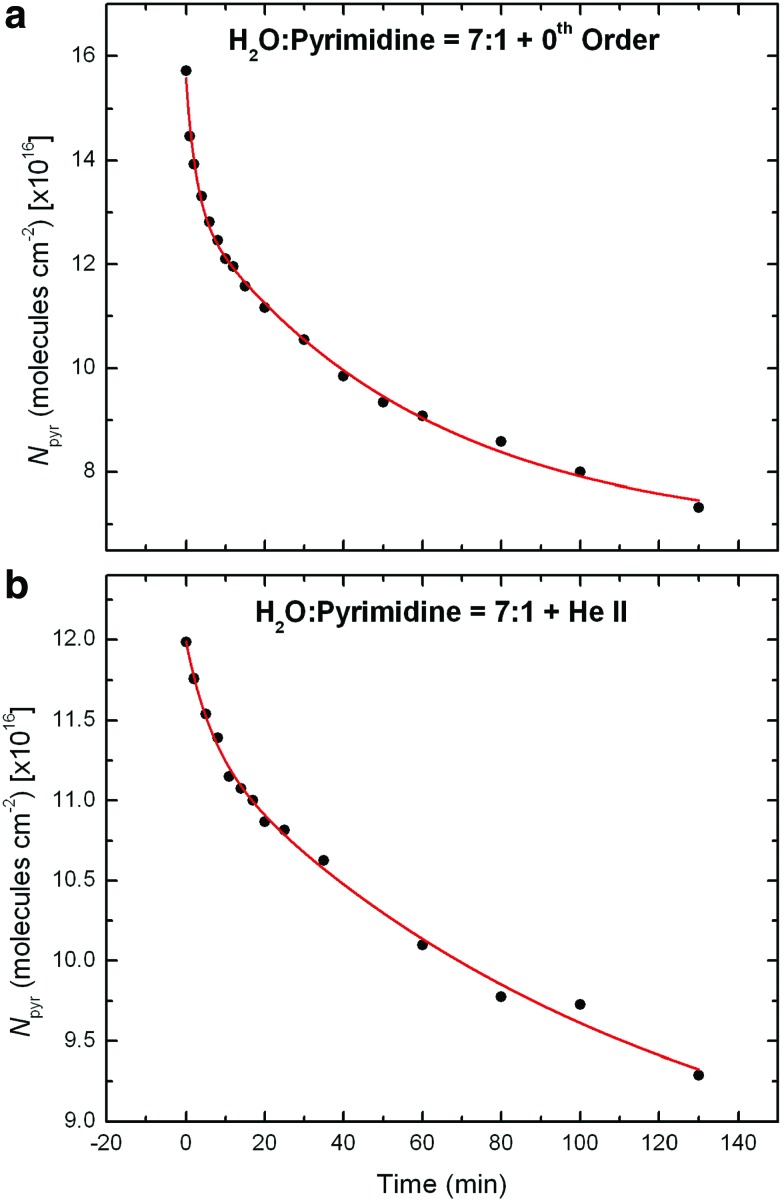
The evolution of the column density of pyrimidine for the 0th order light and He ii line experiments can actually be better fitted with a more complex function of the form:
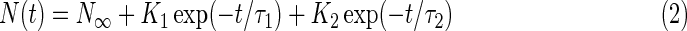 |
where K1 and K2 are two independent pre-exponential factors and τ1 and τ2 two independent characteristic times (exponential decay factors). Such a function can be seen as the sum of two first-order exponential decays (Eq. 1), usually with one characteristic time (arbitrarily, τ1) significantly shorter than the other one (τ2), converging to a common asymptotic value N∞. The fits of the experimental data with the functions described by Eq. 2 for the samples irradiated with the 0th order beam light and the He ii line, which were obtained with the Fityk software, are shown in Fig. 2. They clearly indicate that the photo-destruction curves of pyrimidine for these two experiments are better fitted with such two-component exponential decay functions, with the fitting parameters summarized in Table 3. These results suggest that the photo-destruction and/or photo-conversion of pyrimidine in these two experiments take place via two distinct processes that have yet to be identified.
FIG. 2.
Experimental data showing the decreases in column density of pyrimidine (Npyr) derived from the ∼1406 cm−1 bands in the IR spectra measured for the samples irradiated with (a) the 0th order beam light and (b) the He ii line, as well as the two-component first-order exponential decay fits of these photo-destruction curves of pyrimidine following Eq. 2. The resulting fitting parameters for these curves are given in Table 3.
Table 3.
Exponential Parameters (N∞, K1, τ1, K2, and τ2) Obtained for the Best Fits of the Pyrimidine Photo-Destruction Curves with the Two-Component Exponential Function Given in Eq. 2 for the H2O:Pyrimidine Ice Samples Irradiated with the 0th Order Beam Light and the He ii Line
| H2O:Pyrimidine ice samples | ||
|---|---|---|
| Exponential parameters from Eq. 2 | 0thorder | He ii |
| N∞ (molecules cm−2) | 6.88×1016 | 8.36×1016 |
| K1 (molecules cm−2) | 2.52×1016 | 0.63×1016 |
| τ1 (min) | 2.62 | 6.54 |
| K2 (molecules cm−2) | 6.29×1016 | 3.00×1016 |
| τ2 (min) | 58.79 | 114.30 |
One possible explanation is that the photo-depletion of pyrimidine occurs via two distinct channels, one channel being much slower than the other one. Irradiations with the 0th order light, the He i line, and the He ii line can, in theory, all dissociate H2O into OH radicals together with O and H atoms, as well as their respective ionized forms, since the ionization energy of both H and O is about 13.6 eV (Itikawa et al., 1989). All these species would therefore become available in the ice to react with pyrimidine in its neutral, radical, and/or cationic forms (Bera et al., 2010) and open new pathways for the photo-destruction and photo-conversion of pyrimidine.
However, in the case of the irradiation with the He i line (58.4 nm, 21.2 eV), the contribution of O, H, and OH ions is expected to be significantly weaker than in the 0th order and the He ii experiments, because the photo-ionization cross sections for O, H, and OH at 58.4 nm are much smaller than at 30.4 nm (Stephens and McKoy, 1987; Itikawa et al., 1989). This may explain why the photo-destruction curve of pyrimidine in this experiment follows a one-component first-order exponential decay (Eq. 1 and Fig. 1). In contrast, the presence of higher-energy photons in the 0th order and He ii experiments will increase the population of ionized species susceptible to react with pyrimidine. The presence of these species in the ice will increase the number of reaction pathways for the photo-depletion of pyrimidine, in particular those involving O+, H+, and OH+, as supported by the two-component exponential decay fits for the photo-destruction of pyrimidine in these two experiments (Eq. 2 and Fig. 2). However, because of the small abundances of these ions compared with other species present in the ices, these pathways are expected to provide significantly smaller conversion yields for pyrimidine. This is in agreement with the fact that, in the two-component exponential decays describing the half-lives of pyrimidine in the 0th order and He ii experiments, one of the characteristic times is always significantly longer than the other one (Table 3) and may correspond to pathways involving less abundant species such as O+, H+, and OH+. It is also interesting to note that the characteristic times τ1 and τ2 derived for the 0th order light experiments are about twice as short as those derived for the He ii experiment, which is consistent with the half-lives measured for these two experiments from the one-component exponential decays (Table 2).
These results may also explain the one-component exponential decay measured for pyrimidine when it is mixed in pure H2O ice and subjected to the UV radiation emitted by a regular H2-discharge lamp (Nuevo et al., 2009; Table 2). Indeed, such a light source cannot ionize O, H, or OH, as the experimental ionization energy of OH was measured to be 13.017 eV (Barr et al., 1999; Ruscic et al., 2002). Consequently, the photo-conversion rate of pyrimidine reacting with O+, H+, and OH+ is expected to be much lower than that of pyrimidine directly photo-destroyed, so that such a pathway can be considered as negligible in experiments in which H2O:pyrimidine ices are irradiated with an H2-discharge lamp.
As a final remark, all the half-lives measured for pyrimidine in the present study are much longer than that measured for pyrimidine in an argon matrix (Table 2), which is assumed to simulate pyrimidine in the gas phase (Peeters et al., 2005). This indicates that when embedded in an H2O ice matrix, pyrimidine is always significantly more stable to UV/EUV radiation than in the gas phase, regardless of the light source employed. Extrapolating the half-lives found in our laboratory experiments to different astrophysical environments similarly to what was done previously for an H2O:pyrimidine=20:1 ice mixture (Nuevo et al., 2009) and for pyrimidine in argon (Peeters et al., 2005) irradiated with an H2-discharge lamp is difficult here, mainly because the light sources employed in the present study have very different energy ranges. Indeed, the 0th order beam light provides photons with energies in the very broad 4.1–49.6 eV range, while the He i and He ii lines are both monochromatic and thus very narrow.
Nonetheless, EUV radiation is present in astrophysical environments and more particularly in the Solar System (Judge and Pietarila, 2004; Peter et al., 2006), so that pyrimidine in H2O-rich ice—for example, at the surface of a comet or an icy planet or satellite—is expected to receive a non-negligible dose of high-energy photons, contributing to its partial photo-destruction and/or photochemical alteration. However, the fact that pyrimidine is shielded by a thick layer of ices and minerals in the ISM and the Solar System will allow it to survive longer times to harsh radiation, as supported by the detection of pyrimidine derivatives and other N-heterocycles in meteorites (Hayatsu, 1964, 1975; Folsome et al., 1971, 1973; van der Velden and Schwartz, 1977; Stoks and Schwartz, 1979, 1981; Callahan et al., 2011).
3.1.2. Formation of photo-products
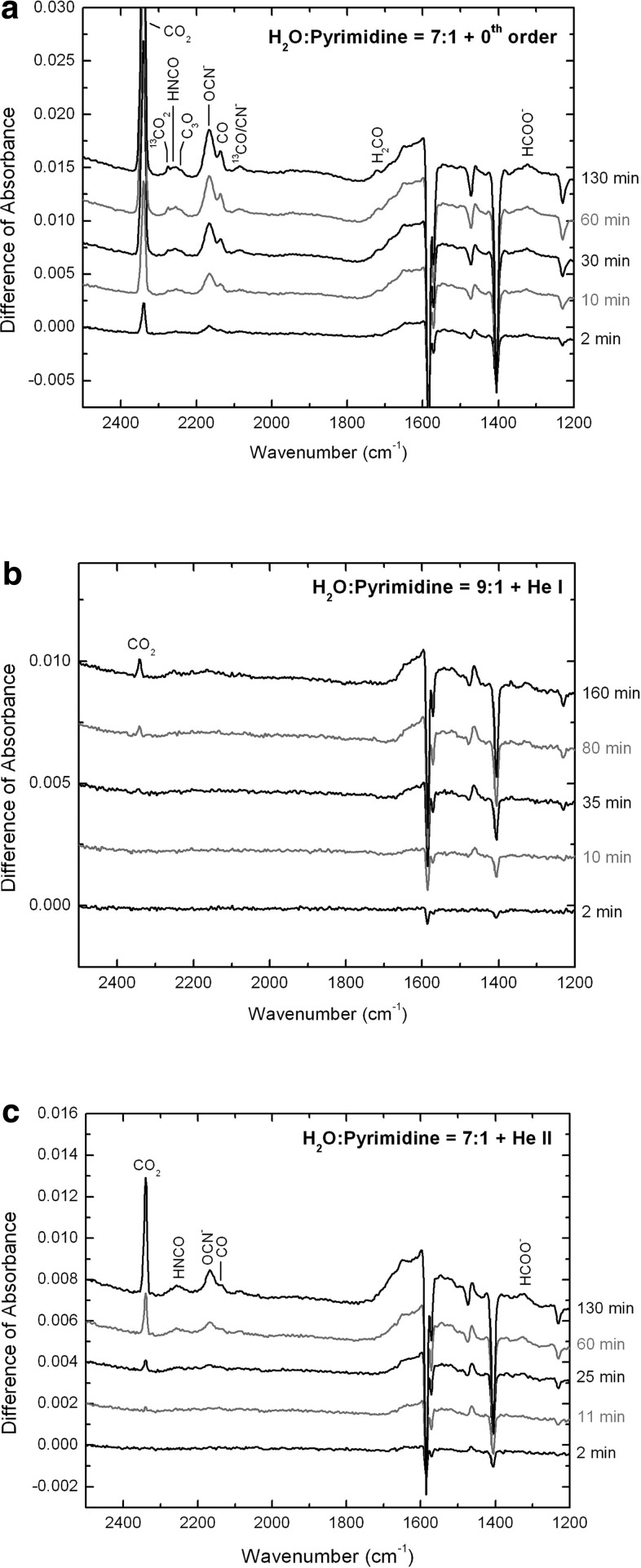
The photo-destruction of pyrimidine is further witnessed by the emergence of new bands in the IR spectra recorded during irradiation, most of them corresponding to products formed after rupture of the pyrimidine ring (Fig. 3). A few examples of these are bands appearing at about 2340, 2262, 2168, 2136, 2085, and 1320 cm−1 in the IR spectra of the ices irradiated with the 0th order light and the He ii line (Fig. 3a and 3c, respectively). These peaks are assigned to CO2 (Sandford and Allamandola, 1990), HNCO (Moore and Hudson, 2003), OCN− (Schutte and Greenberg, 1997; Demyk et al., 1998; Bernstein et al., 2000), CO (Sandford et al., 1988), CN− (overlapping with CO in most cases; Moore and Hudson, 2003; Gerakines et al., 2004), and HCOO− (van Broekhuizen et al., 2004), respectively. Since pyrimidine is the only source of carbon in our ice mixtures, these species can only be formed from the fragmentation of pyrimidine and subsequent oxidation of these fragments. These peaks appear to be more intense in the case of the 0th order light sample compared to those for the He ii line sample, which suggests again a more efficient photo-destruction of pyrimidine when the broad-range 4.1–49.6 eV beam is employed, and are in agreement with the half-lives (Eq. 1) and characteristic times (Eq. 2) derived for pyrimidine in those experiments (Tables 2 and 3).
FIG. 3.
Infrared spectra (4000–850 cm−1) showing the differences of absorbance (relative to the spectra before irradiation) for the three H2O:pyrimidine ice samples (1000 ML) irradiated with (a) the 0th order beam light for 2, 10, 30, 60, and 130 min; (b) the He i line for 2, 10, 35, 80, and 160 min; and (c) the He ii line for 2, 11, 25, 60, and 130 min. The identified photo-products are indicated on the spectra according to the positions of their respective bands.
The IR spectrum of the 0th order light ice also shows additional peaks near 2240 cm−1, which can be assigned to C3O (Gerakines et al., 1996) or the CN radical (Wu et al., 2012), and near 1720 cm−1, assigned to H2CO (Gerakines et al., 1996), as well as weak peaks corresponding to 13CO2 and 13CO at about 2275 and 2087 cm−1, respectively (Fig. 3a).
In contrast, the IR spectra of the ice irradiated with the He i line only shows a weak band for CO2 (Fig. 3b), which indicates a less efficient photo-destruction of pyrimidine when this light source is employed. This result is consistent with the longer photo-destruction half-life of pyrimidine in the corresponding irradiation experiment (Table 2).
Finally, a few minor bands, sometimes barely rising above the noise level, could be seen around 2135 (overlapping with CO), 1650, 1515, 1335, 1110, 1040, and/or 1020 cm−1 in all three IR spectra (Fig. 3). These bands may be due to the presence of other species formed from fragments of pyrimidine, such as CH2CHNC, CH3CH=NH, 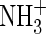 , C2H3CN+, CH3CH=NH, H2CNO, and C2H5CN (Shimanouchi, 1972; Stolkin et al.,
1977; Hashiguchi et al.,
1984; Delwiche et al.,
1993; McCluskey and Frei, 1993; Thompson and Jacox, 2001; Hudson and Moore, 2004; Danger et al.,
2011).
, C2H3CN+, CH3CH=NH, H2CNO, and C2H5CN (Shimanouchi, 1972; Stolkin et al.,
1977; Hashiguchi et al.,
1984; Delwiche et al.,
1993; McCluskey and Frei, 1993; Thompson and Jacox, 2001; Hudson and Moore, 2004; Danger et al.,
2011).
During these short irradiations of 1000 ML of H2O:pyrimidine ices, the amount of pyrimidine was continuously decreasing either because of its photo-destruction or its photo-conversion into other products. This was, however, not the case for the long experiments, during which H2O:pyrimidine mixtures were continuously deposited on the CaF2 substrate, which increased the amounts of photoproducts formed in the residues, both from the destruction and conversion of pyrimidine, including pyrimidine derivatives that cannot be detected with IR spectroscopy because of their small abundances. Their presence in our residues was therefore sought with the use of HPLC and GC-MS techniques.
3.2. Composition of the residues (HPLC/GC-MS)
High-performance liquid chromatography and gas chromatography coupled with mass spectrometry analyses provided independent, complementary information about the chemical composition of the residues recovered at room temperature after their UV/EUV irradiation at 14 K. Since this study was focused on the formation of uracil and its precursor 4(3H)-pyrimidone, we mainly searched for the presence of singly and doubly oxidized compounds in the residues and derived the abundances of 4(3H)-pyrimidone and uracil from the HPLC data in order to estimate their production yields.
3.2.1. HPLC analysis
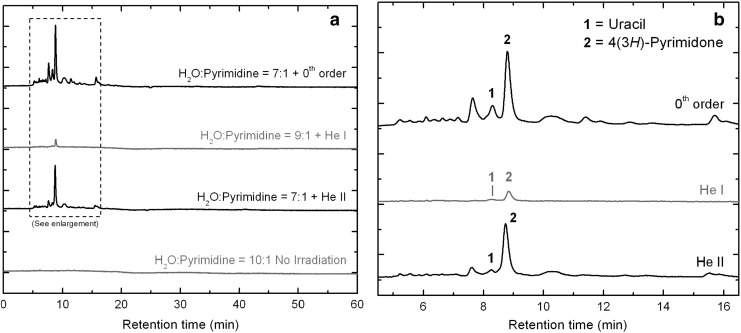
Figure 4 shows the HPLC chromatograms (λ=256 nm channel) of the three residues produced from the irradiation of H2O:pyrimidine ice mixtures with the 0th order beam light, the He i line, and the He ii line (top three traces), compared with the chromatogram of an H2O:pyrimidine=10:1 ice mixture that was deposited for 25 h under similar conditions to the three other samples but not photo-irradiated (blank, bottom trace). In contrast to the chromatogram of the blank sample, which shows no peaks at all, the chromatograms of the three residues produced from the UV/EUV irradiations of pyrimidine in H2O ice show a number of peaks among which only a few could be identified.
FIG. 4.
(a) HPLC chromatograms (λ=256 nm) of the three residues produced from the UV/EUV irradiations of H2O:pyrimidine ice mixtures with the 0th order beam light (top trace, irradiation time: 20 h), the He i line (top middle trace, 32 h), and the He ii line (bottom middle trace, 32 h). The bottom trace corresponds to an H2O:pyrimidine=10:1 ice mixture deposited on the substrate for 25 h but not photo-irradiated. (b) Enlargement of the chromatograms for the three residues to the range in which most peaks are eluting. The main identified photo-products are uracil (1) and 4(3H)-pyrimidone (2).
The first result that can be mentioned is the absence of pyrimidine in the three irradiated samples (Fig. 4, top three traces). This could be explained by either or both of the following reasons: (i) pyrimidine was fully consumed via photo-destruction and/or photo-conversion into other products, similarly to what was observed for comparable ices irradiated with an H2-discharge UV lamp (Nuevo et al., 2009), and (ii) the remaining, unirradiated pyrimidine sublimed away during the warm-up from 14 K to room temperature.
The chromatogram of the sample irradiated with the 0th order light (top trace) has some resemblance to that of H2O:pyrimidine ice mixtures irradiated with an H2-discharge lamp (Nuevo et al., 2009), which supports an efficient photo-conversion of pyrimidine into other products. In particular, it shows a number of peaks in the short retention time range (Rt≤16 min, Fig. 4b), with the largest intensities among the chromatograms of the three irradiated samples. The peaks for 4(3H)-pyrimidone (Rt=8.78 min) and uracil (8.30 min) could be easily identified by comparison of their retention times and UV spectra with the corresponding standards. Other peaks in this chromatogram could not be identified, either because of the weak signal to noise of their UV spectra or because of the lack of commercial standards.
The HPLC chromatogram of the residue produced from the irradiation of an H2O:pyrimidine=7:1 ice mixture with the He ii line (Fig. 4a, bottom middle trace) looks similar to that of the residue produced with the 0th order light (top trace), with smaller intensities. 4(3H)-Pyrimidone (8.73 min) and uracil (8.25 min) could be clearly identified in this chromatogram, with different relative proportions compared with the 0th order light residue. Finally, the chromatogram of the residue produced from an H2O:pyrimidine=9:1 ice mixture with the He i line (Fig. 4a, top middle trace) shows very few weak peaks. The peak at 8.84 min, assigned to 4(3H)-pyrimidone, is the only obvious peak in the whole chromatogram. The peak assigned to uracil (Rt=8.34 min) is very weak and overlapping with other weak peaks, but could be separated from the others during data analysis.
3.2.2. GC-MS analysis
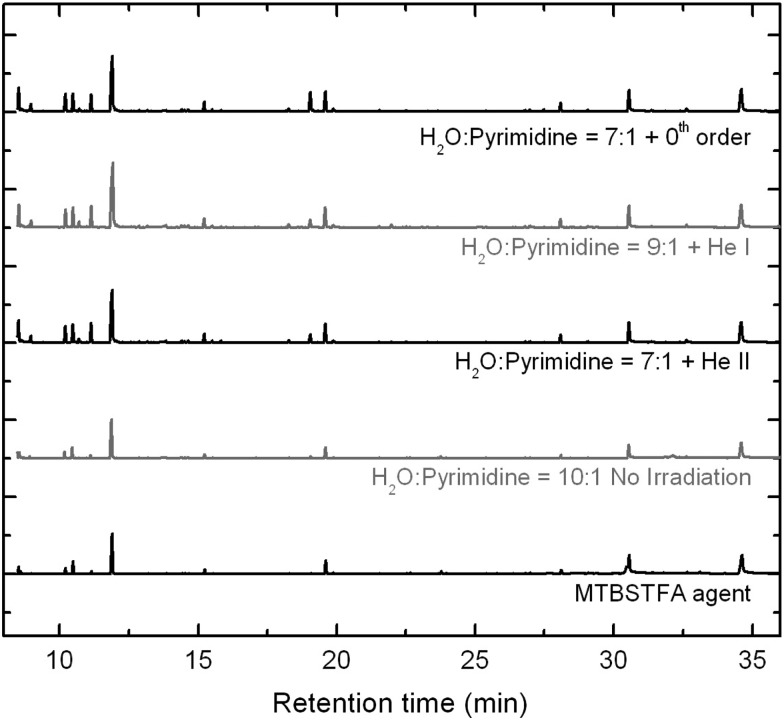
The GC-MS chromatograms of the same three samples (Figs. 5 and 6) confirm the presence of 4(3H)-pyrimidone and uracil in all of them by comparison of both their retention times and mass spectra (Fig. 6). Comparison of the chromatograms of the residues with those of the unirradiated sample (Fig. 5, bottom middle trace) and of the agent (MTBSTFA) which was used to derivatize all samples and all standards (bottom trace), indicates that only a few peaks in the residue chromatograms are due to the presence of photo-products formed from the UV/EUV irradiation of the starting H2O:pyrimidine ices.
FIG. 5.
GC-MS total-ion chromatograms (TICs) of the three residues produced from the UV/EUV irradiations of H2O:pyrimidine ice mixtures with the 0th order beam light (top trace, irradiation time: 20 h), the He i line (top middle trace, 32 h), and the He ii line (middle trace, 32 h). The bottom middle trace corresponds to the TIC of the same H2O:pyrimidine=10:1 ice mixture deposited on the substrate for 25 h but not photo-irradiated, whose HPLC chromatogram is shown in Fig. 4a. The bottom trace corresponds to the injection of the MTBSTFA agent used to derivatize all residues, control samples, and standards in this study.
FIG. 6.
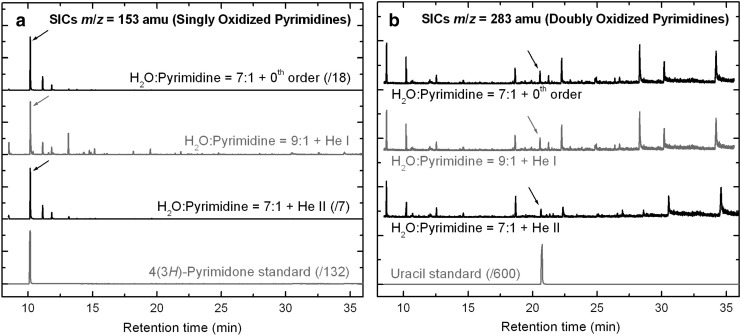
(a) GC-MS single-ion chromatograms (SICs) of the three residues produced from the UV/EUV irradiations of H2O:pyrimidine ice mixtures with the 0th order beam light (top trace, irradiation time: 20 h), the He i line (top middle trace, 32 h), and the He ii line (bottom middle trace, 32 h), for m/z=153 amu, which corresponds to the mass of singly oxidized pyrimidine derivatives. The bottom trace is the SIC of a 4(3H)-pyrimidone standard prepared and injected under the same conditions as the residues. (b) GC-MS SICs of the same three residues for m/z=283 amu, which corresponds to the mass of doubly oxidized pyrimidine derivatives. The bottom trace is the SIC of a uracil standard prepared and injected under the same conditions as the residues. Identifications of 4(3H)-pyrimidone and uracil in the residues are marked with arrows.
Data from the GC-MS single-ion chromatograms (SICs) of the residues could also show the presence of a few other products, including 2,2′-bipyrimidine (158 amu) and urea (231 amu) in all three samples, as well as small quantities of 2-hydroxypyrimidine (153 amu) and 4,6-dihydroxypyrimidine (283 amu) in the samples formed from the irradiations with the 0th order light and the He ii line. All these compounds were previously observed in residues formed from the UV irradiation of H2O:pyrimidine ice mixtures with an H2-discharge lamp (Nuevo et al., 2009). The detection of 2,2′-bipyrimidine, which consists of two molecules of pyrimidine bound via a C–C bond, indicates that UV/EUV can excite and/or ionize pyrimidine to make it reactive and react with another pyrimidine molecule, while that of urea (NH2CONH2) in measurable quantities supports the photo-induced rupture of the pyrimidine ring and the subsequent photo-oxidation of the resulting fragments inferred from the IR spectroscopy data (see Section 3.1).
4. Discussion
4.1. Production of 4(3H)-pyrimidone and uracil
For each HPLC chromatogram of the three samples irradiated with the UV/EUV sources, the peaks assigned to 4(3H)-pyrimidone and uracil were analyzed with the Fityk software in order to separate multiple overlapping peaks. Once these peaks were separated, their areas could be derived; and by comparison with the chromatograms of the corresponding standards, the total quantities of 4(3H)-pyrimidone and uracil in each residue could be estimated.
As absolute quantities can vary from one sample to another, even for residues produced from the same starting ice mixture irradiated with the same UV/EUV light, we used these quantities to estimate the molecular production yields for 4(3H)-pyrimidone and uracil, which are defined here as the numbers of molecules of pyrimidine needed to form one molecule of 4(3H)-pyrimidone or uracil. These molecular yields, summarized in Table 4, were obtained after accounting for the fact that only 10–15% of the pyrimidine deposited on the CaF2 substrate were actually exposed to UV/EUV radiation because of the small synchrotron beam size compared with the deposition area on the substrate. In addition, these molecular production yields were normalized to account for the differences between the irradiation times (20 h for the 0th order light, and 32 h for the He i and He ii lines) and the wavelength ranges of the beams in each experiment (about 275 nm for the 0th order light beam and 0.2 nm for the He i and He ii lines). The irradiation time and wavelength range used for the H2-discharge lamp experiment were 23 h and 70 nm, respectively (Nuevo et al., 2009).
Table 4.
Molecular Production Yields (Number of Molecules of Pyrimidine Deposited Needed to Produce One Molecule of a Given Product) and Relative Proportions (Ratios) Estimated for 4(3H)-Pyrimidone and Uracil in the Residues Produced from the UV/EUV Irradiation of H2O:Pyrimidine Ices with the 0th Order Beam Light, the He i Line, and the He ii Line
| Sample | 4(3H)-Pyrimidone | Uracil | Ratio |
|---|---|---|---|
| 0th order light | 1/450 | 1/1900 | 4.4 |
| He i line | 1/1.4×104 | 1/3.6×106 | 254 |
| He ii line | 1/3400 | 1/1.2×105 | 36.5 |
| H2 lamp | 1/670 | 1/9300 | 13.9 |
Values are compared with what was obtained for a residue produced from an H2O:pyrimidine=20:1 ice mixture photo-irradiated with an H2-discharge lamp (Nuevo et al., 2009).
As suggested by previous experimental and theoretical studies, 4(3H)-pyrimidone is mostly formed from the photo-oxidation of pyrimidine, while uracil is formed from the photo-oxidation of two possible singly oxidized derivatives of pyrimidine, among which 4(3H)-pyrimidone is the most abundant and stable isomer and, therefore, the most probable precursor of uracil in irradiation experiments (Nuevo et al., 2009; Bera et al., 2010). Consequently, the production yield of 4(3H)-pyrimidone is directly related to the photooxidation efficiency of pyrimidine, while that of uracil is strongly correlated with the photo-oxidation efficiency of 4(3H)-pyrimidone.
Nonetheless, production yields should not be seen merely as the efficiency of forming a compound from its precursor but rather as the net result of the competition between a set of photo-induced reactions leading to the formation of more complex molecules and an independent set of photo-induced reactions leading to the destruction of the starting compounds and newly formed photo-products. In addition, it must be noted that the photo-induced reactions that take place in the experiments described in the present study may be the consequence of the absorption of a UV/EUV photon not only by pyrimidine and its derivatives but also by H2O, even though the following discussion will only focus on the production of pyrimidine-based compounds.
Molecular yields show that the 0th order light is more efficient for producing both 4(3H)-pyrimidone and uracil than any other light source employed (Table 4). The second most efficient light source for producing those two photo-products is the H2-discharge lamp, while the molecular yields obtained for 4(3H)-pyrimidone and uracil with the monochromatic sources (He i and He ii lines) are significantly smaller than those obtained for the broad energy sources (0th order light and H2-discharge lamp). The He i line was found to be the least efficient light source among all those studied here, with production yields for 4(3H)-pyrimidone and uracil about 8 and 65 times smaller than those derived for the 0th order light experiment, respectively, and about 4 and 29 times smaller than those derived for the He ii line experiment, respectively (Table 4). Such a result was expected from the very small number of peaks seen in the HPLC chromatogram of this residue (Fig. 4) and from the longer photo-destruction half-life derived for pyrimidine in this experiment (Table 2). It also shows that the absorption of higher-energy photons emitted by a monochromatic light source (He ii, 40.8 eV) results in higher production yields than when a monochromatic source with lower-energy photons is employed (He i, 21.2 eV).
The other interesting parameter to be compared between the samples is the relative abundances—or molecular yield—of 4(3H)-pyrimidone and uracil. These ratios, given in the last column of Table 4, were obtained by dividing the abundance—or molecular yield—of 4(3H)-pyrimidone by that of uracil in each residue. The 4(3H)-pyrimidone to uracil ratio in the sample irradiated with the 0th order light (4.4) is about 3 times smaller than that measured for the sample irradiated with the H2-discharge lamp (13.9) (Nuevo et al., 2009), which indicates that the production of uracil from its precursors is more efficient when the 0th order beam is employed. This difference is most probably due to the fact that the energy range of the 0th order light beam is broader (about 4 times) than that of the H2-discharge lamp. Indeed, the broader the UV/EUV range, the higher the probability for a photon with the right energy to be absorbed by the newly formed photo-produced pyrimidine derivatives, including 4(3H)-pyrimidone and its isomers—in their neutral, cationic, and/or radical forms—and thus the higher the probability for those species to be converted into more complex products, including uracil and its isomers.
In contrast, photons emitted by monochromatic light sources such as the He i and He ii lines will have a significantly smaller probability to be absorbed by the potential precursors of uracil and its isomers, resulting in smaller conversion rates and final production yields. However, as mentioned earlier, increasing the probability of absorbing photons does not necessarily result in an increase of the production of more complex molecules, since photons of given energies can also selectively destroy specific chemical bonds and lead to the photo-destruction or fragmentation of both the starting and the newly formed species. Nonetheless, the results summarized in Table 4 clearly indicate that the combinations of those photo-induced production and destruction processes in the experiments described in the present study always result in the production of more complex molecules, whose yields are correlated with the energy range of the incident light source.
4.2. Astrobiological implications
Studying the photo-formation and photo-stability of molecules such as pyrimidine and derivatives including nucleobases is important from an astrobiological point of view because such compounds have been detected in meteorites, together with a number of other purine-based nucleobases such as adenine, guanine, xanthine, and hypoxanthine (Hayatsu, 1964, 1975; Folsome et al., 1971, 1973; van der Velden and Schwartz, 1977; Stoks and Schwartz, 1979, 1981; Callahan et al., 2011). In particular, the reported detections of 4(3H)-pyrimidone (sometimes named 4-hydroxy-pyrimidine) and uracil in the carbonaceous chondrites Murchison, Murray, and Orgueil (Folsome et al., 1971, 1973; Lawless et al., 1972; Stoks and Schwartz, 1979) suggest that these compounds can survive ionizing radiation from the astrophysical environment where they formed to the formation of the Solar System and their delivery to telluric planets via comets and asteroids.
The results obtained from our experiments are in agreement with such a scenario, as they demonstrated that the irradiation of simple H2O:pyrimidine ice mixtures with high-energy UV/EUV sources always leads to the formation of complex molecules including the nucleobase uracil. Although the three light sources employed in our experiments have different effects on the photo-destruction of pyrimidine, the formation of photo-products, and the photo-stability of the newly formed products to the incident photo-irradiation (Section 3), the photo-destruction half-lives derived for pyrimidine indicate that, when embedded in an H2O ice matrix, pyrimidine may survive for long periods of time in astrophysical environments.
In these environments, ice compositions are far more complex, as they usually consist of mixtures of H2O (dominant component) as well as CH3OH, CO, CO2, CH4, and NH3 (Gibb et al., 2000, 2004; Dartois, 2005). Therefore, if pyrimidine and its products of photo-irradiation, including 4(3H)-pyrimidone and uracil, are shielded from harsh UV/EUV radiation by such complex ices, nucleobases as well as a host of other molecules of prebiotic interest including amino acids and sugars (Kvenvolden et al., 1970; Cronin and Pizzarello, 1999; Cooper et al., 2001; Sephton, 2002) may have formed abiotically, survived throughout the formation of the Solar System, and seeded the early Earth during the heavy meteoritic bombardment (Chyba and Sagan, 1992). This primordial prebiotic soup on Earth (Oró, 1961) may have subsequently triggered the first biological reactions that led to the emergence of life on our planet about 3.8 billion years ago.
5. Conclusions
This experimental study shows that the irradiation of pyrimidine in pure H2O ice at low temperature with several UV/EUV light sources (0th order beam light, He i line, and He ii line) always leads to the formation of the nucleobase uracil and its precursor 4(3H)-pyrimidone. These two compounds were detected in the residues obtained after warming the samples to room temperature. However, the energy and the energy range of the incident photon sources had significantly different effects on both the photodestruction of pyrimidine and its conversion into photoproducts, as well as on the photo-stability of these newly formed photo-products such as uracil and its precursor 4(3H)-pyrimidone. In particular, our results show that the energy range of the light source employed is correlated with the production yields of 4(3H)-pyrimidone and uracil.
In all cases, our study indicates that pyrimidine and its derivatives, including nucleobases, can survive the high-energy UV/EUV irradiation field present during and after the formation of the Solar System if embedded in—and shielded by—an H2O-rich ice matrix, as is the case in cold, primitive astrophysical objects such as comets. The detection of pyrimidine-based compounds in meteorites strongly supports our results and a scenario in which organic molecules of prebiotic interest such as nucleobases were formed in astrophysical environments before they were delivered to the early Earth, eventually leading to the emergence of life.
Footnotes
Available at http://fityk.nieto.pl.
Acknowledgments
This work was supported by the NSC grants #99-2112-M-008-011-MY3 and #101-2811-M-008-023 (T.-S.Y.), the National Synchrotron Radiation Research Center, and the NSF Planetary Astronomy Program under grant AST-1108898 (C.-Y.R.W.). M.N. acknowledges S.A. Sandford for the use of the HPLC and GC-MS devices at the Astrophysics & Astrochemistry Laboratory of NASA Ames Research Center. The authors are grateful for the helpful reviews of two anonymous reviewers, from which this manuscript benefited significantly.
Author Disclosure Statement
No competing financial interests exist.
Abbreviations
EUV, extreme ultraviolet; GC-MS, gas chromatography coupled with mass spectrometry; HPLC, high-performance liquid chromatography; ISM, interstellar medium; ML, monolayers; MTBSTFA, N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide; PAHs, polycyclic aromatic hydrocarbons; PANHs, polycyclic aromatic nitrogen heterocycles; SICs, single-ion chromatograms; TICs, total-ion chromatograms; UHV, ultrahigh vacuum.
References
- Allamandola L.J., Tielens A.G.G.M., and Barker J.R. (1989) Interstellar polycyclic aromatic hydrocarbons—the infrared emission bands, the excitation/emission mechanism, and the astrophysical implications. Astrophys J Suppl Ser 71:733–775 [DOI] [PubMed] [Google Scholar]
- Barr J.D., De Fanis A., Dyke J.M., Gamblin S.D., Hooper N., Morris A., Stranges S., West J.B., and Wright T.G. (1999) Study of the OH and OD radicals with photoelectron spectroscopy using synchrotron radiation. J Chem Phys 110:345–354 [Google Scholar]
- Bera P.P., Nuevo M., Milam S.N., Sandford S.A., and Lee T.J. (2010) Mechanism for the abiotic synthesis of uracil via UV-induced oxidation of pyrimidine in pure H2O ices under astrophysical conditions. J Chem Phys 133:104303.(7pp) [DOI] [PubMed] [Google Scholar]
- Bernstein M.P., Sandford S.A., and Allamandola L.J. (2000) H, C, N, and O isotopic substitution studies of the 2165 cm−1 (4.62 μm) “XCN” feature produced by UV photolysis of mixed molecular ices. Astrophys J 542:894–897 [Google Scholar]
- Bernstein M.P., Sandford S.A., and Allamandola L.J. (2005) The mid-infrared absorption spectra of neutral polycyclic aromatic hydrocarbons in conditions relevant to dense interstellar clouds. Astrophys J Suppl Ser 161:53–64 [Google Scholar]
- Brünken S., McCarthy M.C., Thaddeus P., Godfrey P.D., and Brown R.D. (2006) Improved line frequencies for the nucleic acid base uracil for a radioastronomical search. Astron Astrophys 459:317–320 [Google Scholar]
- Callahan M.P., Smith K.E., Cleaves H.J., II, Ruzicka J., Stern J.C., Glavin D.P., House C.H., and Dworkin J.P. (2011) Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc Natl Acad Sci USA 108:13995–13998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal S., Mendes E., Fernandes J.O., Oliveira M.B.P.P., and Ferreira M.A. (2004) Analysis of heterocyclic aromatic amines in foods by gas chromatography–mass spectrometry as their tert.-butyldimethylsilyl derivatives. J Chromatogr A 1040:105–114 [DOI] [PubMed] [Google Scholar]
- Charnley S.B., Kuan Y.-J., Huang H.-C., Botta O., Butner H.M., Cox N., Despois D., Ehrenfreund P., Kisiel Z., Lee Y.-Y., Markwick A.J., Peeters Z., and Rodgers S.D. (2005) Astronomical searches for nitrogen heterocycles. Adv Space Res 36:137–145 [Google Scholar]
- Chen Y.-J., Chuang K.-Y., Muñoz Caro G.M., Nuevo M., Chu C.-C., Yih T.-S., Ip W.-H., and Wu C.-Y.R. (2014) Vacuum ultraviolet emission spectrum measurement of a microwave-discharge hydrogen-flow lamp in several configurations: application to photodesorption of CO ice. Astrophys J 781:15.(13pp) [Google Scholar]
- Chyba C.F. and Sagan C. (1992) Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 335:125–132 [DOI] [PubMed] [Google Scholar]
- Cooper G., Kimmich N., Belisle W., Sarinana J., Brabham K., and Garrel L. (2001) Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 414:879–883 [DOI] [PubMed] [Google Scholar]
- Cronin J.R. and Pizzarello S. (1999) Amino acid enantiomer excesses in meteorites: origin and significance. Adv Space Res 23:293–299 [Google Scholar]
- Danger G., Bossa J.-B., de Marcellus P., Borget F., Duvernay F., Theulé P., Chiavassa T., and d'Hendecourt L. (2011) Experimental investigation of nitrile formation from VUV photochemistry of interstellar ices analogs: acetonitrile and amino acetonitrile. Astron Astrophys 525:A30.(6pp) [Google Scholar]
- Dartois E. (2005) The ice survey opportunity of ISO. Space Sci Rev 119:293–310 [Google Scholar]
- Delwiche J., Gochel-Dupuis M., Collin J.E., and Heinesch J. (1993) High resolution HeI photoelectron spectrum of acrylonitrile. J Electron Spectros Relat Phenomena 66:65–74 [Google Scholar]
- Demyk K., Dartois E., d'Hendecourt L., Jourdain de Muizon M., Heras A.M., and Breitfellner M. (1998) Laboratory identification of the 4.62 μm solid state absorption band in the ISO-SWS spectrum of RAFGL 7009S. Astron Astrophys 339:553–560 [Google Scholar]
- Folsome C.E., Lawless J., Romiez M., and Ponnamperuma C. (1971) Heterocyclic compounds indigenous to the Murchison meteorite. Nature 232:108–109 [DOI] [PubMed] [Google Scholar]
- Folsome C.E., Lawless J., Romiez M., and Ponnamperuma C. (1973) Heterocyclic compounds recovered from carbonaceous chondrites. Geochim Cosmochim Acta 37:455–465 [Google Scholar]
- Frenklach M. and Feigelson E.D. (1989) Formation of polycyclic aromatic hydrocarbons in circumstellar envelopes. Astrophys J 341:372–384 [Google Scholar]
- Gerakines P.A., Schutte W.A., and Ehrenfreund P. (1996) Ultraviolet processing of interstellar ice analogs. I. Pure ices. Astron Astrophys 312:289–305 [Google Scholar]
- Gerakines P.A., Moore M.H., and Hudson R.L. (2004) Ultraviolet photolysis and proton irradiation of astrophysical ice analogs containing hydrogen cyanide. Icarus 170:202–213 [Google Scholar]
- Gibb E.L., Whittet D.C.B., Schutte W.A., Boogert A.C.A., Chiar J.E., Ehrenfreund P., Gerakines P.A., Keane J.V., Tielens A.G.G.M., van Dishoeck E.F., and Kerkhof O. (2000) An inventory of interstellar ices toward the embedded protostar W33A. Astrophys J 536:347–356 [Google Scholar]
- Gibb E.L., Whittet D.C.B., Boogert A.C.A., and Tielens A.G.G.M. (2004) Interstellar ice: the Infrared Space Observatory legacy. Astrophys J Suppl Ser 151:35–73 [Google Scholar]
- Hashiguchi K., Hamada Y., Tsuboi M., Koga Y., and Kondo S. (1984) Pyrolysis of amines: infrared spectrum of ethylideneimine. J Mol Spectrosc 105:81–92 [Google Scholar]
- Hayatsu R. (1964) Orgueil meteorite: organic nitrogen contents. Science 146:1291–1293 [DOI] [PubMed] [Google Scholar]
- Hayatsu R., Anders E., Studier M.H., and Moore L.P. (1975) Purines and triazines in the Murchison meteorite. Geochim Cosmochim Acta 39:471–488 [Google Scholar]
- Hudgins D.M., Sandford S.A., Allamandola L.J., and Tielens A.G.G.M. (1993) Mid- and far-infrared spectroscopy of ices: optical constants and integrated absorbances. Astrophys J Suppl Ser 86:713–870 [DOI] [PubMed] [Google Scholar]
- Hudson R.L. and Moore M.H. (2004) Reactions of nitriles in ices relevant to Titan, comets, and the interstellar medium: formation of cyanate ion, ketenimines, and isonitriles. Icarus 172:466–478 [Google Scholar]
- Itikawa Y., Ichimura K., Onda K., Sakimoto K., Takayanagi K., Hatano Y., Hayashi M., Nishimura H., and Tsurubuchi S. (1989) Cross sections for collisions of electrons and photons with oxygen molecules. Journal of Physical and Chemical Reference Data 18:23–42 [Google Scholar]
- Judge P.G. and Pietarila A. (2004) On the formation of extreme-ultraviolet helium lines in the Sun: analysis of SOHO data. Astrophys J 606:1258–1275 [Google Scholar]
- Kobayashi K. and Tsuji T. (1997) Abiotic synthesis of uracil from carbon monoxide, nitrogen and water by proton irradiation. Chem Lett 1997:903–904 [Google Scholar]
- Kuan Y.-J., Yan C.-H., Charnley S.B., Kisiel Z., Ehrenfreund P., and Huang H.-C. (2003) A search for interstellar pyrimidine. Mon Not R Astron Soc 345:650–656 [Google Scholar]
- Kuan Y.-J., Charnley S.B., Huang H.-C., Kisiel Z., Ehrenfreund P., Tseng W.-L., and Yan C.-H. (2004) Searches for interstellar molecules of potential prebiotic importance. Adv Space Res 33:31–39 [Google Scholar]
- Kvenvolden K., Lawless J., Pering K., Peterson E., Flores J., and Ponnamperuma C. (1970) Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 228:923–926 [DOI] [PubMed] [Google Scholar]
- Lawless J.G., Folsome C.E., and Kvenvolden K.A. (1972) Organic matter in meteorites. Sci Am 226:38–46 [Google Scholar]
- MacKenzie S.L., Tenaschuk D., and Fortier G. (1987) Analysis of amino acids by gas-liquid chromatography as tert-butyldimethylsilyl derivatives: preparation of derivatives in a single reaction. J Chromatogr 387:241–253 [DOI] [PubMed] [Google Scholar]
- Martins Z., Botta O., Fogel M.L., Sephton M.A., Glavin D.P., Watson J.S., Dworkin J.P., Schwartz A.W., and Ehrenfreund P. (2008) Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet Sci Lett 270:130–136 [Google Scholar]
- Materese C.K., Nuevo M., Bera P.P., Lee T.J., and Sandford S.A. (2013) Thymine and other prebiotic molecules produced from the ultraviolet photo-irradiation of pyrimidine in simple astrophysical ice analogs. Astrobiology 13:948–962 [DOI] [PubMed] [Google Scholar]
- McCluskey M. and Frei H. (1993) Transfer of methylene from ketene to nitric oxide by photoexcitation of reactant pairs in solid argon below the CH2:C:O dissociation limit. J Phys Chem 97:5204–5207 [Google Scholar]
- Meier R.R. (1991) Ultraviolet spectroscopy and remote sensing of the upper atmosphere. Space Sci Rev 58:1–185 [Google Scholar]
- Mendoza E., Almeida G.C., Andrade D.P.P., Luna H., Wolff W., Rocco M.L.M., and Boechat-Roberty H.M. (2013) X-ray photodesorption and proton destruction in protoplanetary discs: pyrimidine. Mon Not R Astron Soc 433:3440–3452 [Google Scholar]
- Miyakawa S., Yamanashi H., Kobayashi K., Cleaves H.J., and Miller S.L. (2002) Prebiotic synthesis from CO atmosphere: implications for the origins of life. Proc Natl Acad Sci USA 99:14628–14631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.H. and Hudson R.L. (2003) Infrared study of ion-irradiated N2-dominated ices relevant to Triton and Pluto: formation of HCN and HNC. Icarus 161:486–500 [Google Scholar]
- Nuevo M., Milam S.N., Sandford S.A., Elsila J.E., and Dworkin J.P. (2009) Formation of uracil from the ultraviolet photo-irradiation of pyrimidine in pure H2O ices. Astrobiology 9:683–695 [DOI] [PubMed] [Google Scholar]
- Nuevo M., Milam S.N., and Sandford S.A. (2012) Nucleobases and prebiotic molecules in organic residues produced from the ultraviolet photo-irradiation of pyrimidine in NH3 and H2O+NH3 ices. Astrobiology 12:295–314 [DOI] [PubMed] [Google Scholar]
- Öberg K.I., Fuchs G.W., Awad Z., Fraser H.J., Schlemmer S., van Dishoeck E.F., and Linnartz H. (2007) Photodesorption of CO ice. Astrophys J 662:L23–L26 [Google Scholar]
- Öberg K.I., van Dishoeck E.F., and Linnartz H. (2009) Photodesorption of ices I: CO, N2, and CO2. Astron Astrophys 496:281–293 [Google Scholar]
- Oró J. (1961) Comets and the formation of biochemical compounds on the primitive Earth. Nature 190:389–390 [DOI] [PubMed] [Google Scholar]
- Peeters Z., Botta O., Charnley S.B., Kisiel Z., Kuan Y.-J., and Ehrenfreund P. (2005) Formation and photostability of N-heterocycles in space. I. The effect of nitrogen on the photostability of small aromatic molecules. Astron Astrophys 433:583–590 [Google Scholar]
- Peter H., Gudiksen B.V., and Nordlund Å. (2006) Forward modeling of the corona of the Sun and solar-like stars: from a three-dimensional magnetohydrodynamic model to synthetic extreme-ultraviolet spectra. Astrophys J 638:1086–1100 [Google Scholar]
- Pilling S., Andrade D.P.P., do Nascimento E.M., Marinho R.R.T., Boechat-Roberty H.M., de Coutinho L.H., de Souza G.G.B., de Castilho R.B., Cavasso-Filho R.L., Lago A.F., and de Brito A.N. (2011) Photostability of gas- and solid-phase biomolecules within dense molecular clouds due to soft X-rays. Mon Not R Astron Soc 411:2214–2222 [Google Scholar]
- Puget J.L. and Léger A. (1989) A new component of the interstellar matter—small grains and large aromatic molecules. Annu Rev Astron Astrophys 27:161–198 [Google Scholar]
- Rees M.H. (1989) Physics and Chemistry of the Upper Atmosphere, Cambridge University Press, Cambridge, UK, pp 8–11 [Google Scholar]
- Ricca A., Bauschlicher C.W., and Bakes E.L.O. (2001) A computational study of the mechanisms for the incorporation of a nitrogen atom into polycyclic aromatic hydrocarbons in the Titan haze. Icarus 154:516–521 [Google Scholar]
- Roelfsema P.R., Cox P., Tielens A.G.G.M., Allamandola L.J., Baluteau J.P., Barlow M.J., Beintema D., Boxhoorn D.R., Cassinelli J.P., Caux E., Churchwell E., Clegg P.E., de Graauw T., Heras A.M., Huygen R., van der Hucht K.A., Hudgins D.M., Kessler M.F., Lim T., and Sandford S.A. (1996) SWS observations of IR emission features towards compact HII regions. Astron Astrophys 315:L289–L292 [Google Scholar]
- Ruscic B., Wagner A.F., Harding L.B., Asher R.L., Feller D., Dixon D.A., Peterson K.A., Song Y., Qian X., Ng C.-Y., Liu J., Chen W., and Schwenke D.W. (2002) On the enthalpy of formation of hydroxyl radical and gas-phase bond dissociation energies of water and hydroxyl. J Phys Chem A 106:2727–2747 [Google Scholar]
- Sandford S.A. and Allamandola L.J. (1990) The physical and infrared spectral properties of CO2 in astrophysical ice analogs. Astrophys J 355:357–372 [DOI] [PubMed] [Google Scholar]
- Sandford S.A., Allamandola L.J., Tielens A.G.G.M., and Valero G. (1988) Laboratory studies of the infrared spectral properties of CO in astrophysical ices. Astrophys J 329:498–510 [DOI] [PubMed] [Google Scholar]
- Sandford S.A., Bernstein M.P., and Allamandola L.J. (2004) The mid-infrared laboratory spectra of naphthalene (C10H8) in solid H2O. Astrophys J 607:346–360 [Google Scholar]
- Schummer C., Delhomme O., Appenzeller B.M.R., Wenning R., and Millet M. (2009) Comparison of MTSBTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 77:1473–1482 [DOI] [PubMed] [Google Scholar]
- Schutte W.A. and Greenberg J.M. (1997) Further evidence for the OCN− assignment to the XCN band in astrophysical ice analogs. Astron Astrophys 317:L43–L46 [Google Scholar]
- Sephton M.A. (2002) Organic compounds in carbonaceous meteorites. Nat Prod Rep 19:292–311 [DOI] [PubMed] [Google Scholar]
- Shimanouchi T. (1972) Tables of Molecular Vibrational Frequencies Consolidated, Vol. I, National Bureau of Standards, U.S. Government Printing Office, Washington, DC [Google Scholar]
- Simon M.N. and Simon M. (1973) Search for interstellar acrylonitrile, pyrimidine, and pyridine. Astrophys J 184:757–762 [Google Scholar]
- Stephens J.A. and McKoy V. (1987) Photoionization of the valence orbitals of OH. J Chem Phys 88:1737–1742 [Google Scholar]
- Stoks P.G. and Schwartz A.W. (1979) Uracil in carbonaceous meteorites. Nature 282:709–710 [Google Scholar]
- Stoks P.G. and Schwartz A.W. (1981) Nitrogen-heterocyclic compounds in meteorites: significance and mechanisms of formation. Geochim Cosmochim Acta 45:563–569 [Google Scholar]
- Stolkin I., Ha T.-K., andGünthard Hs.H. (1977) N-methylmethyleneimine and ethylideneimine: gas- and matrix-infrared spectra, ab initio calculations and thermodynamic properties. Chem Phys 21:327–347 [Google Scholar]
-
Thompson W.E. and Jacox M.E. (2001) The infrared spectra of the
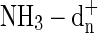 cations trapped in solid neon. J Chem Phys 114:4846–4854 [Google Scholar]
cations trapped in solid neon. J Chem Phys 114:4846–4854 [Google Scholar] - van Broekhuizen F.A., Keane J.V., and Schutte W.A. (2004) A quantitative analysis of OCN− formation in interstellar ice analogs. Astron Astrophys 415:425–436 [Google Scholar]
- van der Velden W. and Schwartz A.W. (1977) Search for purines and pyrimidines in the Murchison meteorite. Geochim Cosmochim Acta 41:961–968 [Google Scholar]
- Wu C.-Y.R., Judge D.L., Cheng B.-M., Shih W.-H., Yih T.-S., and Ip W.-H. (2002) Extreme ultraviolet photon-induced chemical reactions in the C2H2–H2O mixed ices at 10 K. Icarus 156:456–473 [Google Scholar]
- Wu Y.-J., Wu C.-Y.R., Chou S.-L., Lin M.-Y., Lu H.-C., Lo J.-I., and Cheng B.-M. (2012) Spectra and photolysis of pure nitrogen and methane dispersed in solid nitrogen with vacuum-ultraviolet light. Astrophys J 746:175.(11pp) [Google Scholar]
- Yamanashi H., Takeda S., Murasawa K.-I., Miyakawa S., Kaneko T., and Kobayashi K. (2001) Identification and determination of nucleic acid bases abiotically formed in simulated planetary atmospheres. Anal Sci (Suppl) 17:i1639–i1642 [Google Scholar]