Abstract
Intrahepatic cholangiocarcinoma (ICC) is a treatment refractory malignancy with a high mortality and an increasing incidence worldwide. Recent studies have observed that activation of Notch and AKT signalling within mature hepatocytes is able to induce the formation of tumours displaying biliary lineage markers, thereby raising the suggestion that it is hepatocytes, rather than cholangiocytes or hepatic progenitor cells that represent the cell of origin of this tumour. Here we utilise a cholangiocyte-lineage tracing system to target p53 loss to biliary epithelia and observe the appearance of labelled biliary lineage tumours in response to chronic injury. Consequent to this, up-regulation of native functional Notch signalling is observed to occur spontaneously within cholangiocytes and hepatocytes in this model as well as in human ICC. These data prove that in the context of chronic inflammation and p53 loss, frequent occurrences in human disease, biliary epithelia are a target of transformation and an origin of ICC.
Keywords: cholangiocarcinoma, origin, cholangiocyte, Notch, cancer
INTRODUCTION
The unexplained increase in incidence of ICC (1, 2) coupled with its poor response to chemotherapeutics and high mortality necessitate a greater understanding of the biology of this aggressive malignancy, in which the cell of origin remains unclear. The historical assumption that these tumours arise from the oncogenic transformation of mature biliary epithelia has been based on a glandular histological morphology, location within and adjacent to the biliary network and expression of cholangiocyte specific proteins including mucin and biliary cytokeratins 7 and 19 (3). Substantive evidence for this origin however has been lacking. Patients with primary sclerosing cholangitis (PSC) and liver fluke infection, diseases characterised by chronic biliary inflammation and epithelial proliferation, are up to 161 and 27 times more likely to develop biliary tract cancers compared with the general population (4, 5). Bipotential hepatic progenitor cells (HPCs) have also been considered as a cellular source of ICC in light of the existence of combined hepatocellular cholangiocarcinoma (CHC) (6), tumours with features of both cholangiocarcinoma and hepatocellular carcinoma, as well as cholangiolocellular carcinoma (CLC), characterised by ductular reaction and cords resembling the Canals of Hering (7).
Interestingly the incidence of ICC is increased in chronic hepatocellular injury such as HCV and HBV (8) infection indicating a more complex cellular origin of these cancers. Recent work has demonstrated that mature hepatocytes possess potential for transdifferentiation into ICC, a phenomenon dependent on intracellular Notch signalling (9, 10). This concurs with the known role of Notch in the specification of hepatoblasts during ontogeny as well as observations that Notch is able to reprogram postnatal and terminally differentiated hepatocytes into biliary epithelia with capacity to form ductular structures(11-13).
In recent fate-tracing experiments chemically-induced tumours were established in transgenic mice carrying an inducible heritable label for either hepatocyte (Alb-CreERT2) or biliary (CK19-CreERT2) lineages. Unexpectedly in the absence of transgenic Notch overexpression, labelled neoplastic nodules positive for Epithelial Cell Adhesion Molecule (EpCAM) were observed in tumours arising in Alb-CreERT2 but not CK19-CreERT2 animals, which suggested that ICC arose from hepatocytes rather than cholangiocytes in that model (10). Given the unexpected nature of these findings within the clinical context of this disease and their implications for development of future therapy, we set out to assess whether targeted loss of tumour suppressor function within cholangiocytes can precipitate ICC formation using an independent transgenic strategy and hence whether biliary epithelia should still be considered a cell of origin of ICC.
MATERIALS AND METHODS
Mice
CK19CreERTR26ReYFP mice on a mixed genetic background (a kind gift from Guoqiang Gu, Vanderbilt University Medical Center, Nashville Tennessee), and Trp53tm1Brn (Jackson Laboratories) were used in this study.
Experimental protocol
For induction of Cre activity, 6 week old CK19CreERTeYFPp53f/f mice were administered three intraperitoneal injections of 4mg Tamoxifen (Sigma) reconstituted in olive oil (Sigma) at a concentration of 30mg/ml on alternate days. Mice were harvested 72hours following tamoxifen administration to assess efficiency of Cre recombination. A separate cohort went on to receive either 600mg/ml Thioacetamide (Sigma) in drinking water for 26 weeks to induce tumour formation. To assess non-carcinogenic injury models mice received 1ul/g carbon tetrachloride (Sigma) or olive oil (Sigma) i.p for 16 weeks or 3,5-diethoxycarbonyl-1,4-dihydro-collidine (DDC) (Sigma) diet (0.1% Purina 5015 mouse chow) for 14 days.
Immunohistochemistry
Livers were fixed overnight in 4% aqueous buffered formalin, embedded in paraffin and cut into 5μm sections. Tissue underwent microwave antigen retrieval using Tris-EDTA with 0.1% Tween20 (Sigma) and blocked with H202 and Protein Block (Invitrogen). Sections were incubated overnight with the following primary antibodies: GFP, CK19, Cyp2D6, Sox9 and Notch1 (Abcam), Nanog (eBiosciences) or Oct4 (Santa Cruz). After washing in PBS, the directly conjugated Alexa-488 and Alexa-555/564 conjugated secondary antibodies (Invitrogen) were used according to species with DAPI fluoromount (Southern Biotech). Day 8.5 mouse embryos and the murine epistem cell line C2 were used as positive controls for Oct4 and Nanog immunostaining.
Study approval
All animal experiments were approved by the University of Edinburgh animal ethics committee and conducted with UK Home Office approval. Human specimens were collected prospectively from patients undergoing hepatic resection at the Royal Infirmary of Edinburgh with local ethical approval and informed patient consent.
RESULTS AND DISCUSSION
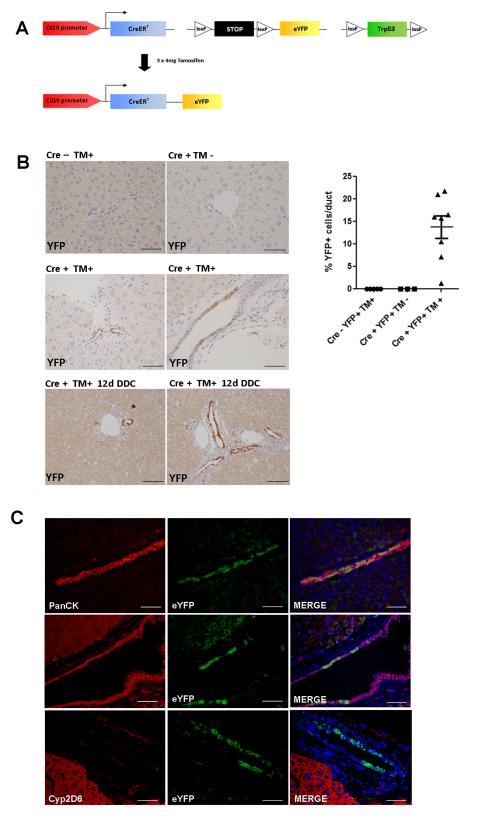
Up to 26% of patients with ICC carry mutations in the Trp53 gene (up to 44.4% in fluke-associated ICC (14)), primarily single base substitutions at CpG sites resulting in loss of tumour suppressor function(15). In our tamoxifen inducible experimental system we have therefore targeted functional loss of p53 in CK19-expressing cells which are synchronously labelled with a Cre inducible eYFP reporter (CK19-CreERT;R26ReYFP;Trp53loxP), where Cre recombination induces excision of exons 2 to 10 of the Trp53 gene, and also the stop locus upstream of eYFP (Figure 1A). In the healthy and injured mouse liver CK19 expression is found on cholangiocytes lining medium and large-sized bile ducts as well as terminal ductules in the Canals of Hering, but not in hepatocytes (Figure 1B). In the absence of Cre or tamoxifen, no eYFP expression was seen in either healthy or injured liver, but following induction with tamoxifen at 6 weeks old, eYFP positivity was observed in 14% of all cholangiocytes (Figure 1C). No cell types other than biliary epithelia were labelled.
Figure 1. Transgenic system of tamoxifen inducible, Cre mediated cell tracking with Trp53 deletion in CK19CreERTeYFPR26p53f/f mice.
(A) Transgenic construct of fluorescent labelling and tumour suppressor deletion in CK19 positive cells in response to tamoxifen in 6 week old mice. (B) In the presence of Cre and tamoxifen, eYFP activity is seen within small ductules as well as large bile ducts. The eYFP+ population expands following 14 days of dietary 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) (Scalebars: 50μm). Quantitative analysis of Cre efficiency 72 hours post injection in Cre negative mice exposed to tamoxifen (n=5), Cre positive mice without tamoxifen (n=3) and Cre positive mice exposed to tamoxifen (n=8). (C) Following tamoxifen injection eYFP positivity is seen only in CK19 expressing cells. These are cholangiocytes that also express the biliary markers Sox9. No co-localisation is seen with the mature hepatocyte marker Cyp2D6 (Scalebars: 50μm).
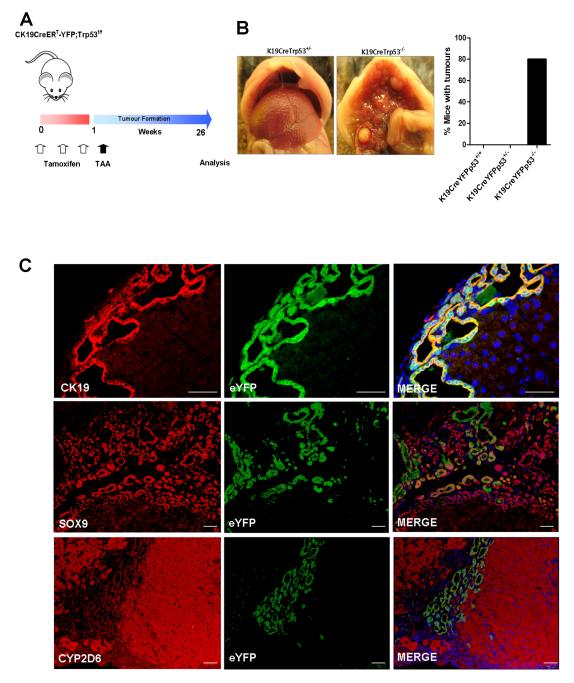
One week following Cre induction, mice were initiated on thioacetamide (TAA) to induce tumour formation (16) (Figure 2A). After 26 weeks multifocal tumours were observed in the livers of CK19CreERTeYFPp53−/− (80%) but not CK19CreERTeYFPp53+/− (0%) or CK19CreERTeYFPp53+/+ (0%) animals (Figure 2B). eYFP positivity was observed in all histologically identified neoplastic nodules, and this co-localised with expression of the ductular markers CK19 and Sox9. No cells were dually positive for eYFP and the mature hepatocyte marker Cyp2D6 (Figure 2C).
Figure 2. ICC is derived from CK19 positive cholangiocytes.
(A) Experimental strategy of tamoxifen induction in CK19CreERTeYFPR26p53f/f mice followed by oral administration of 600mg/ml thioacetamide for 26 weeks. (B) Multifocal tumours developed only in CK19CreERTeYFPp53−/− (homozygous for p53 deletion) (n=5) and not CK19CreERTeYFPR26p53+/− (n=14) or CK19CreERTeYFPR26p53+/+ (n=5) animals, and only following TAA administration. (C) Co-immunofluorescent staining of eYFP with the biliary lineage markers CK19 and Sox9. All eYFP+ cells were seen to be CK19 positive. eYFP positivity did not overlap with the mature hepatocyte marker Cyp2D6. Nuclei are stained with DAPI (Scalebars: 50μm).
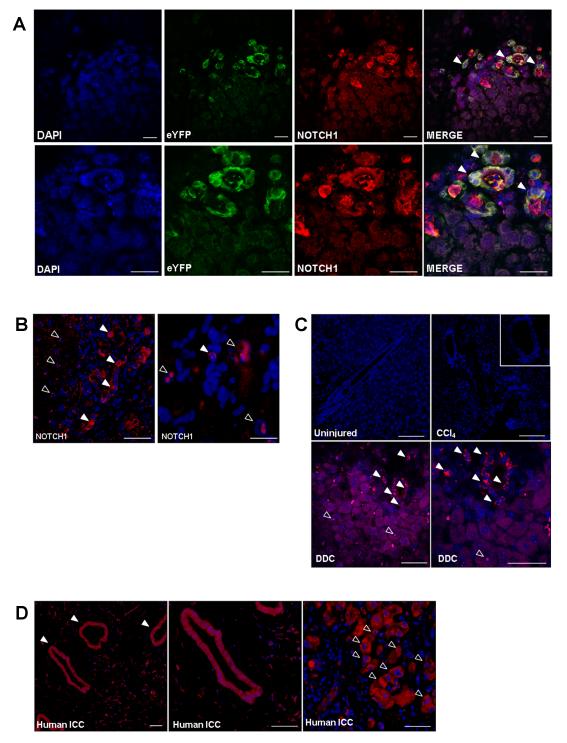
In light of the emerging role for Notch in driving cholangiocarcinogenesis, we then looked to identify the cellular expression of the Notch 1 receptor within this model. Membranous and nuclear positivity of activated Notch1 was observed widely in the epithelium of the malignant ducts and frequently co-localised with eYFP staining (Figure 3A). Interestingly, positivity was also seen to occur within nuclei of hepatocytes, particularly those located adjacent to the cancerous stroma (Figure 3B). We went on to assess whether Notch1 was also expressed in non-malignant models of liver injury and observed strong ductular positivity in the context of the 3,5-diethoxycarbonyl-1,4-dihydro-collidine (DDC) biliary injury dietary model, but none during chronic hepatocyte regeneration with carbon tetrachloride or in the uninjured mouse liver (Figure 3C). Furthermore this pattern of Notch activity is recapitulated in human resected ICC specimens, where the strongest positivity is observed within malignant ducts, as well as in hepatocytes adjacent to the invasive front of the tumours (Figure 3D).
Figure 3. Native Notch signalling is activated in ICC.
(A) Immunofluorescent staining of activated Notch1 in the membranes of malignant ductules of TAA-induced ICC in CK19CreERTeYFPp53−/− mice frequently co-localises with eYFP positivity (filled arrowheads) (Scalebars: 50μm). (B) Immunostaining of activated Notch1 within nuclei of peri-tumoral hepatocytes (open arrowheads) (Scalebars: 50μm and 125μm (Second photomicrograph taken under oil). (C) Activated Notch1 immunostaining in uninjured mouse liver; CCl4 induced fibrosis (16weeks) and DDC diet (Scalebars: 50μm) (D) Activated Notch1 immunostaining in human ICC specimens. Staining in malignant biliary epithelia (filled arrowheads) and peri-tumoral hepatocytes (open arrowheads) (Scalebars: 50μm).
Hepatic lineage tracing experiments have proven problematic; indeed the CK19CreERTR26RYFP mouse has hitherto not been widely adopted for cell-specific gene deletion experiments due to poor efficiency. p53 deletion at the point of tamoxifen administration does not result in increased labelling efficiency, but is likely to cause a preferential expansion of the eYFP positive compartment in response to TAA-induced injury, making it more probable that a transforming event will occur in this population of cells compared with labelled cells in a similar fate tracing system without p53 deletion. We believe this to be a robust and representative model of biliary carcinogenesis given the frequent combination of p53 loss and chronic biliary inflammation observed in human disease. eYFP positivity was observed in all animals in which tumours arose as well as in each and every focus of malignancy. We observed co-localisation between eYFP and the M3 Acetylcholine receptor, a marker of mature cholangiocytes, occasional co-localisation with CD44 and no co-localisation with the stem cell markers Nanog and Oct 4 [17] (Supplementary Figure 1). A likely cell of origin is therefore the mature cholangiocyte although we cannot eliminate the possibility of stem cells, progenitors or intermediates as targets of transformation. Interestingly given the lineage tracing system used here, these would be CK19 positive cells. Given the CK19CreERTR26RYFP mouse has not hitherto exhibited lineage labelling of hepatocytes, we can conclude that the eYFP positive tumour cells here arise from cholangiocytes rather than hepatocytes. It is unclear why labelled tumours were not observed after 30 weeks of TAA administration in the CK19CreERT2R26RYFP system published by Sekiya et al., however our data clearly and definitively attest that biliary epithelia can be a cell of origin of ICC in an independent CK19-based transgenic system.
Primary liver cancers are a phenotypically and molecularly heterogeneous group of malignancies without a stereotypical mutational signature. It has been suggested that such heterogeneity reflects in part the diversity of the underlying cells of origin (17), although this remains unproven. What is evident however is the plasticity of hepatic lineages. Following oncogenic transduction, mature hepatocytes, hepatic progenitor cells and hepatoblasts all have potential for reprogramming into tumour initiating cells with acquisition of CD133+ expression, side population fractions as well as tumour-forming and metastatic capacity(18). Cellular differentiation appears to trigger distinct transcriptional programs in response to the same oncogenic stimulus; however all transduced cells independent of origin, are able to form tumours of multiple lineages.
Our data support the published evidence for Notch as driver of biliary oncogenesis (19) by demonstrating active signalling within the ductular epithelium in ICC in both human and mouse. The observation of strong Notch1 intracellular domain expression within hepatocyte nuclei adjacent to the desmoplastic stroma substantiates previous experiments that have shown Notch1 activation within these cells acts as a transdifferentiating factor (9, 10). Moreover this model of reprogramming is further strengthened by the capacity of constitutively activated Notch2 in albumin-expressing cells to induce ICC formation and accelerate DEN-induced HCC which is less differentiated than wild type controls (20). This Notch high state, able to prime the peritumoral parenchyma for transdifferentiation, has significant therapeutic implications for the many patients who develop ICC on a background of chronic hepatocellular injury (8). We believe these findings unify and clarify previous reports and explain how chronic biliary damage can lead to cholangiocarcinoma arising from biliary epithelium.
In conclusion we have definitively shown that even in the absence of transgenic Notch activation ICC can arise from the biliary epithelia. Future therapeutic strategies should target the Notch pathway as a driver of tumorigenesis in this aggressive malignancy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Scottish Centre for Regenerative Medicine, University of Edinburgh UK. Funding was provided by the Wellcome Trust, the Medical Research Council UK and Cancer Research UK. The authors would like to thank Guoqiang Gu, Vanderbilt University Medical Center, Nashville Tennessee USA for the provision of mice.
Financial Support: RV Guest is supported by a Wellcome Trust Clinical Research Training Fellowship; L Boulter is supported by a Cancer Research UK project grant and an MRC research grant; TJ Kendall is supported by a Wellcome Trust Intermediate Clinical Fellowship; SE Minnis-Lyons is supported by an MRC Scottish Clinical Pathology Fellowship; R Walker is supported by a Cancer Research UK project grant; SJ Wigmore is supported by the Scottish Higher Education Funding Council ; OJ Sansom is supported by Cancer Research UK and the European Research Council ; SJ Forbes is supported by the MRC and a Cancer Research UK project grant.
Footnotes
Conflict of interest statement: The authors disclose no potential conflicts of interest
REFERENCES
- 1.Taylor-Robinson SDTM, Arora S, Keegan TJ, Hargreaves S, Beck A, Khan SA, Elliott P, Thomas HC. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48:816–20. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witjes CDMK-KH, Visser O, de Vries E, Ijzermans JNM, de Man RA, Coebergh JWW, Verhoed C. Intrahepatic cholangiocarcinoma in a low endemic area: rising incidence and improved survival. HPB. 2012;14:777–81. doi: 10.1111/j.1477-2574.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishak KGAP, Sobin LH. Histological typing of tumours of the liver. WHO International Classification of Tumour. Springer Verlag; Berlin: 1994. [Google Scholar]
- 4.Bergquist AEA, Olsson R, Kornfeldt D, Loof L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzen H, Almer S, Granath F, Broome U. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. Journal of Hepatology. 2002;36:321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 5.Sripa BKS, Sithithaworn P, Mairiang E, laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley P. Liver fluke induces cholangiocarcinoma. Plos Medicine. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman ZDIK, Langloss JM, Sesterhenn IA, Rabin L. Combined Hepatocellular-Cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124–35. doi: 10.1002/1097-0142(19850101)55:1<124::aid-cncr2820550120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Komuta MSB, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fuiji H, Desmet VI, Kojiro M, Roskams T. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–56. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 8.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan BMY, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. Journal of Clinical Investigation. 2012;122:2911–5. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekiya SSA. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. Journal of Clinical Investigation. 2012;122:3914–8. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zong YPA, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger1 BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–39. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanger KZY, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes and Development. 2013;27:719–24. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulter LGO, Bird TG, Radulescu S, Aucott RL, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage derived Wnt signalling opposes Notch signalling in a NUMB mediated manner to specify HOC fate in chronic liver disease in liver and mouse. Nature Medicine. 2012 doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong CKSC, Pairojkul, Wongkham S, Cutcutache I, Yu W, McPherson JR, Allen GE, Ng CCY, Wong BH, Myint SS, Rajasegaran V, Heng HL, Gan A, Zang ZJ, Wu Y, Wu J, Lee MH, Huang D, Ong P, Chan-on W, Cao Y, Qian C-N, Lim KH, Ooi A, Dykema K, Furge K, Kukongviriyapan V, Sripa B, Wongkham C, Yongvanit P, Futreal PA, Bhudhisawasdi V, Rozen S, Tan P, Teh BT. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nature Genetics. 2012;44:690–3. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 15.Khan SATH, Toledano MB, Cox IJ, Taylor-Robinson SD. p53 mutations in human cholangiocarcinoma: a review. Liver International. 2005;25:704–16. doi: 10.1111/j.1478-3231.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 16.Yeh C-NMA, Lee K-F, Jan Y-Y, Chen M-F. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631–6. doi: 10.1093/carcin/bgh037. [DOI] [PubMed] [Google Scholar]
- 17.Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–88. doi: 10.1002/hep.25595. [DOI] [PubMed] [Google Scholar]
- 18.Holczbauer AFV, Andersen JB, Marquardt JU, Kleiner D, Raggi C, Kitade M, Seo D, Akita H, Durkin M, Thorgiersson SS. Modeling Pathogenesis of Primary Liver Cancer in Lineage-Specific Mouse Cell types. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.03.013. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zender S, Nickeleit I, Wuestefeld T, Sorensen I, Dauch D, Bozko P, et al. A Critical Role for Notch Signaling in the Formation of Cholangiocellular Carcinomas. Cancer Cell. 2013 doi: 10.1016/j.ccr.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Dill MT, Tornillo L, Fritzius T, Terracciano L, Semela D, Bettler B, et al. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2013;57:1607–19. doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.