Abstract
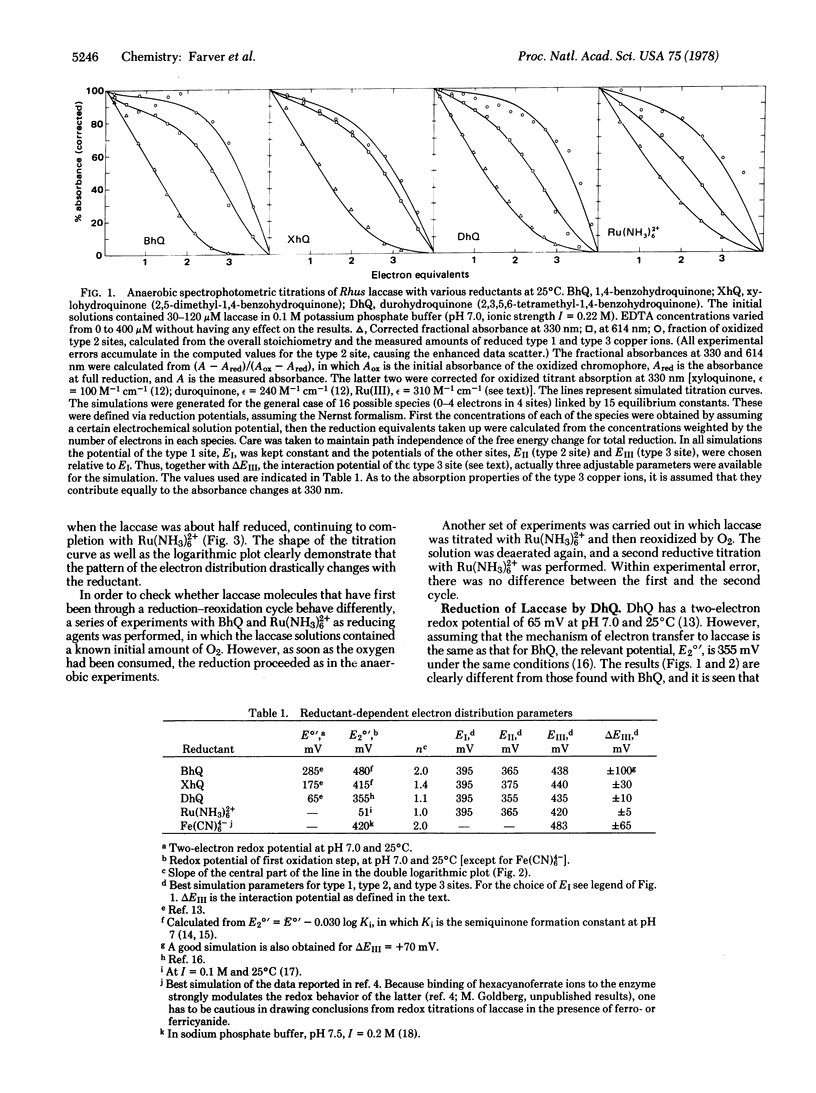
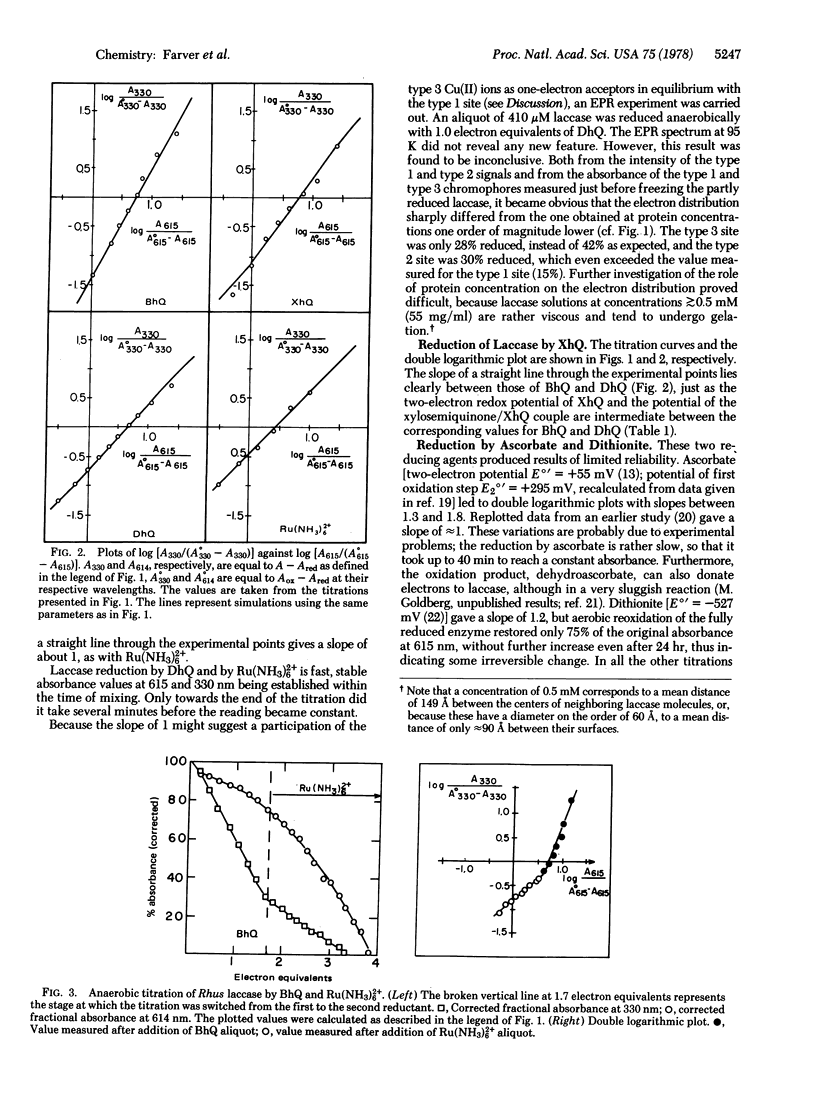
Rhus laccase (monophenol monooxygenase, monophenol,dihydroxyphenylalanine:oxygen oxidoreductase, EC 1.14.18.1) an O2/H2O oxidoreductase containing four copper ions bound to three redox sites (type 1, type 2, and type 3 Cu pair), was titrated anaerobically with several reductants having various chemical and thermodynamic properties. The distribution of electron equivalents among the redox sites was found to be reductant dependent. When the data for titration by various reductants of the type 3 site were plotted against those of the type 1 site according to the Nernst formalism, the slope n varied from 2.0 to 1.0. The redox potential of the reductant's first oxidation step is qualitatively correlated with the value of n and is suggested as the factor that modulates the electron distribution. Such a behavior implies a nonequilibrium situation. A very good simulation of the data was provided by an analysis assuming a formally variable cooperativity between the two type 3 copper ions. This apparent variability is suggested to result from a process whereby sufficiently strong reductants induce a transition of the type 3 site from a cooperative two-electron acceptor to a pair of independent one-electron acceptors. This uncoupled state of the type 3 site is considered metastable. Other possible models were also investigated. Summarizing the available data, we conclude that the two-electron accepting behavior of the 330-nm chromophore is the exception rather than the rule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deinum J., Vänngård T. The stoichiometry of the paramagnetic copper and the oxidation-reduction potentials of type I copper in human ceruloplasmin. Biochim Biophys Acta. 1973 Jun 15;310(2):321–330. doi: 10.1016/0005-2795(73)90112-8. [DOI] [PubMed] [Google Scholar]
- Everling F. B., Weis W., Staudinger H. Bestimmung des Standardredoxpotentials(pH 7,0) von L-(+)-Ascorbat-Semidehydro-L(+)-ascorbinsäure durch nichtenzymatische Reaktion von L(+)-Ascorbat-Semidehydro-L(+)-ascorbinsäure mit Cytochrom b5 (Fe+2)-Cytochrom b5 (Fe+3) Hoppe Seylers Z Physiol Chem. 1969 Jul;350(7):886–888. [PubMed] [Google Scholar]
- Farver O., Goldberg M., Lancet D., Pecht I. Oxidative titrations of Rhus vernicifera laccase and its specific interaction with hydrogen peroxide. Biochem Biophys Res Commun. 1976 Nov 22;73(2):494–500. doi: 10.1016/0006-291x(76)90734-8. [DOI] [PubMed] [Google Scholar]
- Makino N., Ogura Y. Oxidation-reduction titrations of copper ions in Rhus-laccase. Properties of two types of copper ions in the molecule. J Biochem. 1971 Jan;69(1):91–100. doi: 10.1093/oxfordjournals.jbchem.a129463. [DOI] [PubMed] [Google Scholar]
- NAKAMURA T. Purification and physico-chemical properties of laccase. Biochim Biophys Acta. 1958 Oct;30(1):44–52. doi: 10.1016/0006-3002(58)90239-7. [DOI] [PubMed] [Google Scholar]
- O'Reilly J. E. Oxidation-reduction potential of the ferro-ferricyanide system in buffer solutions. Biochim Biophys Acta. 1973 Apr 5;292(3):509–515. doi: 10.1016/0005-2728(73)90001-7. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. R. Oxidation-reduction potentials of the electron acceptors in laccases and stellacyanin. Biochim Biophys Acta. 1972 Aug 17;275(2):245–259. doi: 10.1016/0005-2728(72)90045-x. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. R., Vänngård T. I. The electron-accepting sites in Rhus vernicifera laccase as studied by anaerobic oxidation-reduction titrations. Eur J Biochem. 1971 Feb;18(4):463–468. doi: 10.1111/j.1432-1033.1971.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Dooley D. M., Wang R. H., Gray H. B., Credonio M., Mogno F., Romani G. L. Letter: Susceptibility studies of laccase and oxyhemocyanin using an ultrasensitive magnetometer. Antiferromagnetic behavior of the type 3 copper in Rhus laccase. J Am Chem Soc. 1976 Feb 18;98(4):1029–1031. doi: 10.1021/ja00420a035. [DOI] [PubMed] [Google Scholar]


