Abstract
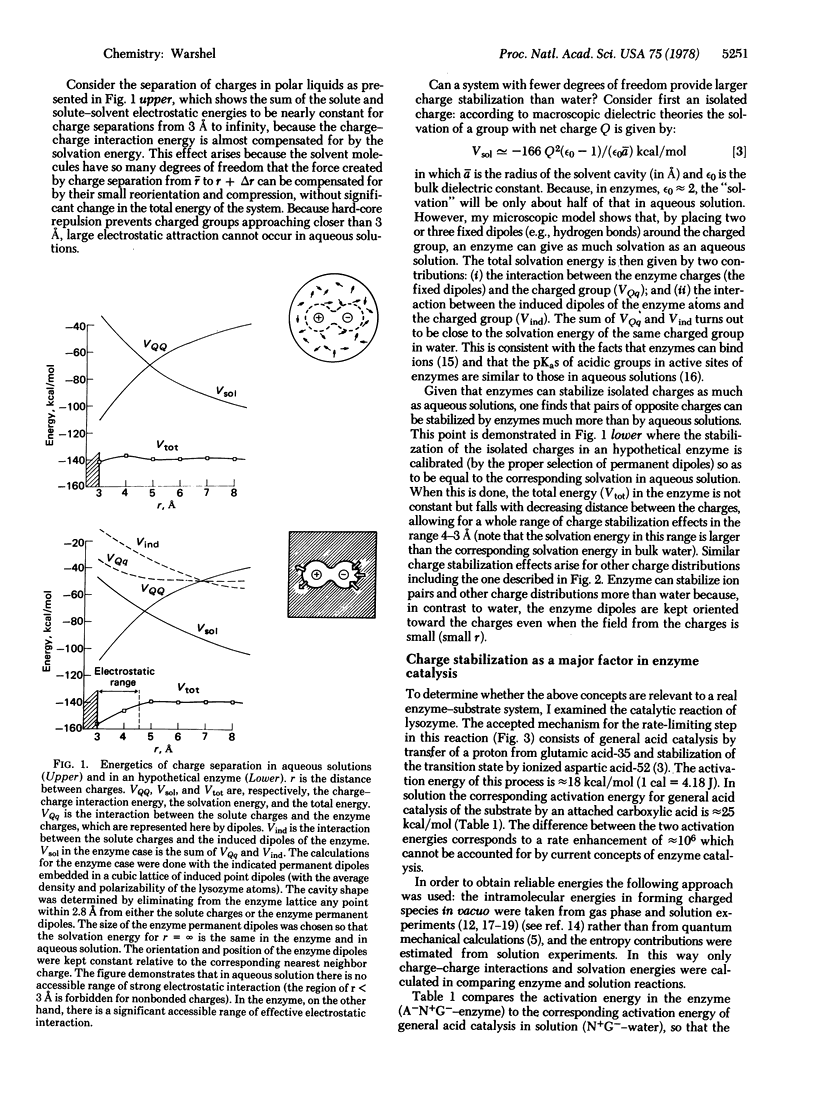
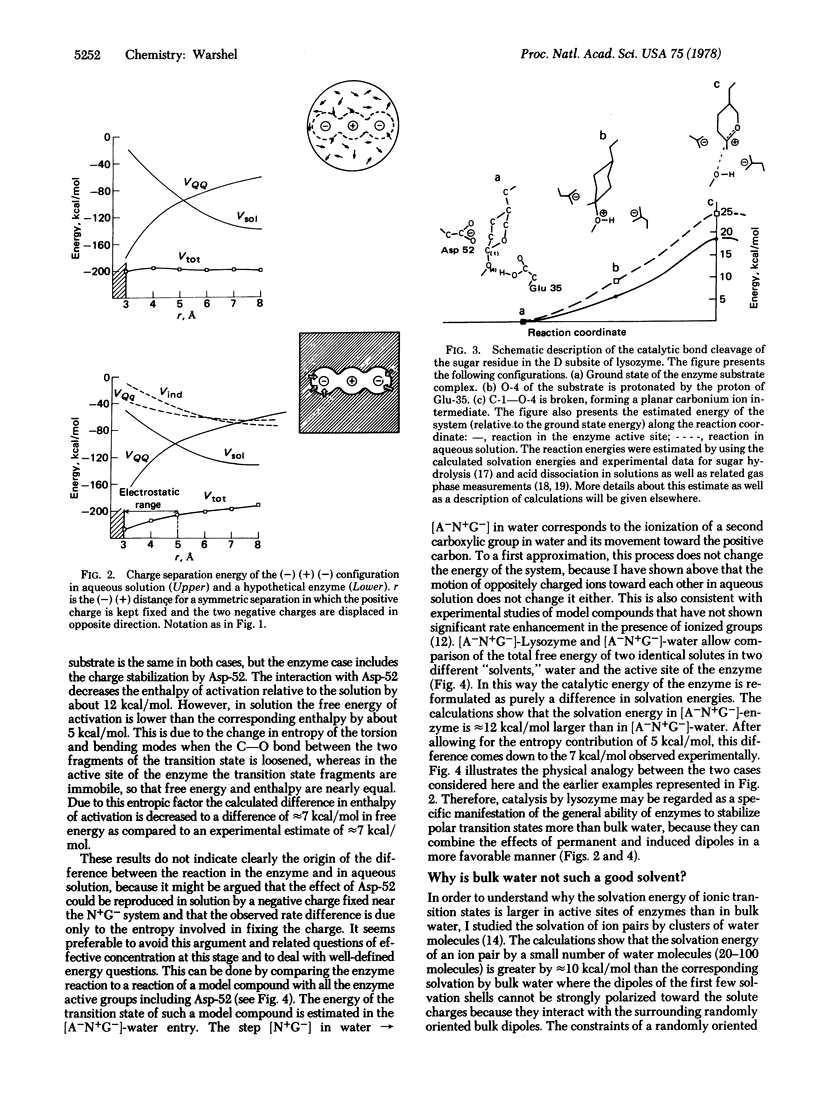
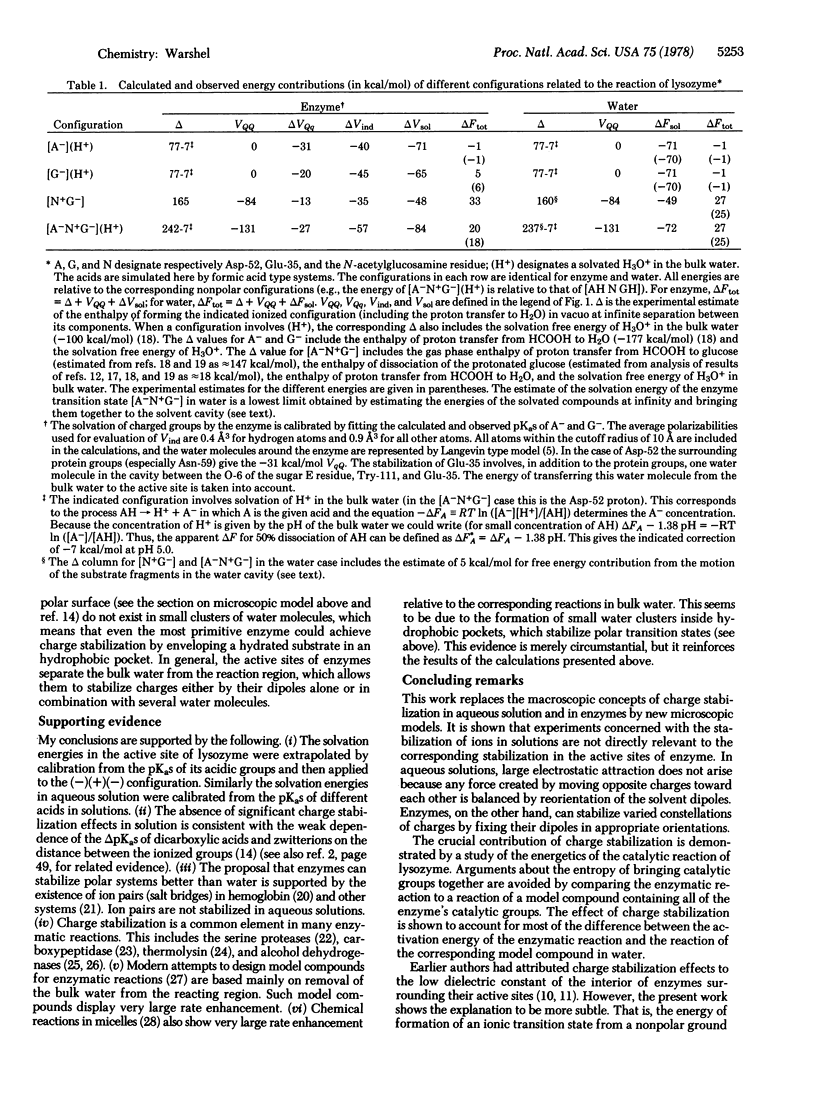
Quantitative studies of the energetics of enzymatic reactions and the corresponding reactions in aqueous solutions indicate that charge stabilization is the most important energy contribution in enzyme catalysis. Low electrostatic stabilization in aqueous solutions is shown to be consistent with surprisingly large electrostatic stabilization effects in active sites of enzymes. This is established quantitatively by comparing the relative stabilization of the transition states of the reaction of lysozyme and the corresponding reaction is aqueous solution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S. K., Kregar I., Turk V., Rupley J. A. Lysozyme-catalyzed reaction of the N-acetylglucosamine hexasaccharide. Dependence of rate on pH. J Biol Chem. 1973 Jul 10;248(13):4786–4792. [PubMed] [Google Scholar]
- Biesecker G., Harris J. I., Thierry J. C., Walker J. E., Wonacott A. J. Sequence and structure of D-glyceraldehyde 3-phosphate dehydrogenase from Bacillus stearothermophilus. Nature. 1977 Mar 24;266(5600):328–333. doi: 10.1038/266328a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Dunn B. M., Bruice T. C. Physical organic models for the mechanism of lysozyme action. Adv Enzymol Relat Areas Mol Biol. 1973;37:1–60. doi: 10.1002/9780470122822.ch1. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Conformational equilibria and the salt bridge in chymotrypsin. Cold Spring Harb Symp Quant Biol. 1972;36:71–73. doi: 10.1101/sqb.1972.036.01.012. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Mechanism of tertiary structural change in hemoglobin. Proc Natl Acad Sci U S A. 1977 Mar;74(3):801–805. doi: 10.1073/pnas.74.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester W. R., Matthews B. W. Crystallographic study of the binding of dipeptide inhibitors to thermolysin: implications for the mechanism of catalysis. Biochemistry. 1977 May 31;16(11):2506–2516. doi: 10.1021/bi00630a030. [DOI] [PubMed] [Google Scholar]
- Moras D., Olsen K. W., Sabesan M. N., Buehner M., Ford G. C., Rossmann M. G. Studies of asymmetry in the three-dimensional structure of lobster D-glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1975 Dec 10;250(23):9137–9162. doi: 10.2210/pdb1gpd/pdb. [DOI] [PubMed] [Google Scholar]
- Page M. I., Jencks W. P. Entropic contributions to rate accelerations in enzymic and intramolecular reactions and the chelate effect. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1678–1683. doi: 10.1073/pnas.68.8.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Kraut J., Alden R. A., Birktoft J. J. Subtilisin; a stereochemical mechanism involving transition-state stabilization. Biochemistry. 1972 Nov 7;11(23):4293–4303. doi: 10.1021/bi00773a016. [DOI] [PubMed] [Google Scholar]
- Schindler M., Assaf Y., Sharon N., Chipman D. M. Mechanism of lysozyme catalysis: role of ground-state strain in subsite D in hen egg-white and human lysozymes. Biochemistry. 1977 Feb 8;16(3):423–431. doi: 10.1021/bi00622a013. [DOI] [PubMed] [Google Scholar]
- Vernon C. A. The mechanisms of hydrolysis of glycosides and their revelance to enzyme-catalysed reactions. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):389–401. doi: 10.1098/rspb.1967.0036. [DOI] [PubMed] [Google Scholar]
- Warshel A. Energy-structure correlation in metalloporphyrins and the control of oxygen binding by hemoglobin. Proc Natl Acad Sci U S A. 1977 May;74(5):1789–1793. doi: 10.1073/pnas.74.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]


