Abstract
Background
Treatment with corticosteroids can reduce the incidence of postextubation stridor (PES) and reintubation in critically ill adult patients, but the mechanisms remain unknown.
Methods
A randomized, controlled clinical trial was conducted in an adult medical and surgical Intensive Care Unit (ICU) of a teaching hospital. Seventy-one patients who had a cuff leak percentage <24% of tidal volume received either a bolus injection of methylprednisolone at 40 mg (treated group, n=38) or normal saline (placebo group, n=33) 4 h prior to a planned extubation. The cuff leak percentage was re-assessed 1 h and 4 h post-injection. Eighty patients who had a cuff leak percentage ≥24% served as a control group. Plasma concentrations of multiple cytokines and C-reactive protein (CRP) were measured at baseline, 4 h and 24 h after the intervention.
Results
The incidences of PES (15.8% vs. 39.4%, P<0.05) and reintubation rate (7.9% vs. 30.3%, P<0.05) were lower in the treated group compared to the placebo group. The plasma concentrations of IL-4 and IL-10 increased while the levels of IL-6 and IL-8 decreased at 24 h in the treated group compared to the placebo group. No difference in CRP levels was observed between the treated and placebo groups.
Conclusion
A single injection of methylprednisolone at the dose used 4 h prior to planned extubation effectively reduced the incidence of PES and the reintubation rate. These beneficial effects were associated with the up-regulation of IL-4 and IL-10 and the down-regulation of IL-6 and IL-8 in the critically ill adult patients.
Keywords: Intubation, intratracheal, Cytokines, Rats, Postextubation stridor
Endotracheal intubation is commonly used for respiratory support in the intensive care unit (ICU). However, intubation/extubation may lead to the development of post-extubation stridor (PES).1, 2 The incidence of PES is up to 22% in patients who are endotracheally intubated for more than 24 h.3 PES is frequently associated with airway inflammation, edema and high mortality rates.4, 5
We have previously reported that treatment with methylprednisolone injections at 40 mg every 6 hours for 24 hours reduced the incidence of PES in critically ill patients.6 Another study showed that the injections of dexamethasone at a dose of 5 mg every 6 hours for 24 hours were effective in reducing the incidence of PES in patients with cuff leak percentage less than 110 mL.7 Moreover, intravenous administration of 20 mg methylprednisolone every 4 hours for 12 hours has been reported to decrease the incidence of PES and reintubation.8 A major concern with the repeated pretreatments 12 or 24 hours prior to a planned extubation is the likelihood of a prolonged ICU stay. However, studies using a single-dose injection of hydrocortisone 100 mg or dexamethasone 8 mg 1 hour prior to extubation did not show clinical benefits in the treatment of PES.3, 9 There is a lack of data regarding the effects of pretreatment with a single injection of methylprednisolone at a relatively reasonable short period of time (i.e., 2–6 h) prior to extubation in adults. We speculated that in routine practice pretreatment with steroid at approximately 4 hours before extubation offers the opportunity to remove the endotracheal tube on the same day, thus reducing the length of stay in ICU and the medical expenditure. We hypothesized that a single intravenous injection of methylprednisolone 4 hours prior to planned extubation reduces the incidence of PES and reintubation due to the anti-inflammatory effects of methylprednisolone in critically ill patients.
Materials and methods
Patients
The study protocol was approved by the institutional Research Ethics Board at Chi Mei Medical Center (IRB No. 9401-003). Informed consent was obtained from the patients or their relatives before the study. Patients were recruited from an adult medical and surgical ICU at the Chi Mei Medical Center between January 2005 and June 2006. All patients were over 18 years of age, intubated for more than 24 h, and met the weaning criteria defined as the respiratory rate <30 breaths/min, negative inspiratory force >25 cm H2O, tidal volume >5 mL/kg of ideal body weight and rapid shallow index <105 breaths/min/L.
The exclusion criteria were any treatment with corticosteroids one week before extubation, nasal or throat disease/surgery, gastrointestinal bleeding, hyperglycemia (blood sugar ≥250 mg%), acute cardiac attack and cardiac surgery, or a history of extubation during the same hospitalization course.
Cuff leak percentage
The cuff leak percentage was defined as the difference in the actual tidal volume before and after cuff deflation, as previously described.6, 10 Patients with a cuff leak percentage <24% of tidal volume during inflation were considered at high risk of developing PES. Following an injection of methylprednisolone, the cuff leak percentage was re-assessed at 4 h. Extubation was performed in all patients 4 h after treatment with methylprednisolone.
Mechanical ventilation and weaning
All patients were mechanically ventilated in volume-controlled mode (Puritan-Bennett 7200 AE, Carlsbad, CA, or Bird 8400, Palm Springs, CA, USA). The tidal volume was set at 8 mL/kg ideal body weight at a respiratory rate of 20 breaths/min and a zero positive end-expiratory pressure (PEEP) level during cuff leak percentage measurement. Weaning was performed by allowing patients to breathe spontaneously with a T-tube or assisted by pressure support ventilation at 7–10 cm H2O for 30 min to 2 h.11
Protocol
Patients with a cuff leak percentage ≥24% served as a time-matched control group for 48 h. Patients who had a cuff leak percentage <24% were studied in a randomized, double-blinded, and placebo-controlled fashion. The randomization included using computer-generated random numbers. The solutions for the placebo and methylprednisolone were prepared by a respiratory therapist who was not involved in the trial. The appearance, package and volume of the placebo and methylprednisolone solutions were all identical. Neither the physician in charge nor the staff who administered the infusion fluids was aware of the randomization arm to which each patient belonged.
The patients were randomly divided into two groups: 1) the treated group, receiving intravenous infusion of methylprednisolone sodium succinate (Pharmacia Upjohn, Kalamazoo, MI, USA) at 40 mg in 2 mL normal saline and 2) the placebo group, receiving intravenous infusion of 2 mL normal saline. All patients were extubated 4 h after receiving the injection and were continuously monitored for 48 h thereafter.
Stridor symptoms were recognized by the presence of an audible high-pitched wheeze associated with respiratory distress.12–14 Inhalation of epinephrine (2 mL of 1:10000) was administered once PES developed. If the use of three doses of epinephrine inhalation failed, bi-level positive airway pressure (Respironics Inc., Murrysville, PA, USA) was applied. Patients who did not show improvement under epinephrine inhalation and/or ventilator support were reintubated. In the intervention arm, only patients with PES received either bronchoscopy or laryngoscopy to further detect the occurrence of laryngeal edema by the attending physician during reintubation.
To examine inflammatory responses in the presence and absence of methylprednisolone, multiple cytokines (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, GM-CSF and interferon-γ, BioRad Cytokine 10-Plex Panel, Hercules, CA, USA) and C-Reactive protein (CRP, CardioPhase® hsCRP, Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) were measured in plasma at baseline, 4 h and 24 h post-methylprednisolone.
Statistical analysis
The demographic data on the patients were analyzed using the unpaired t-test for continuous variables. Changes in the levels of cytokines and CRP at 4 h and 24 h from baseline were analyzed using ANOVA. The data are reported as the mean ± SD. A P-value <0.05 is considered statistically significant.
Results
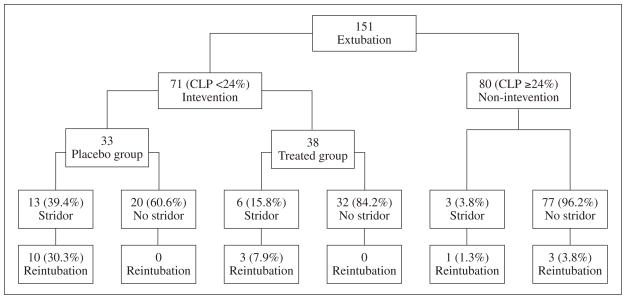
A total of 151 patients were enrolled in the study protocol, including 80 patients with a cuff leak percentage ≥24% who served as a control group. Seventy-one patients with a cuff leak percentage <24% were randomized to a placebo (n=33) or a treated group (n=38). The patients’ characteristics were similar between the placebo and the treated groups (Table I). The incidence of PES was 3.8% in the control group and 39.4% among the patients who had a cuff leak percentage <24% (Figure 1). We examined upper airway flow obstruction in 13 patients who required reintubation under laryngoscopy and found that 8 of the patients had laryngeal edema.
Table I.
Patient’s characteristics.
| Study group (N.=38) | Placebo group (N.=33) | P value | |
|---|---|---|---|
| Gender (M/F)a | 8/30 | 8/25 | 0.748 |
| Age (years)c | 59.7±16.6 | 61.4±16.9 | 0.453 |
| BMI (kg/m2)b | 23.3±4.1 | 22.8±4.3 | 0.641 |
| APACHE IIb | 17.4±7.9 | 18.0±6.3 | 0.725 |
| TISSb | 27.9±8.6 | 26.3±7.5 | 0.427 |
| GCSc | 9.3±3.8 | 8.9±3.8 | 0.580 |
| Duration of intubation (h)b | 114.9±55.3 | 124.7±72.0 | 0.534 |
| Endotracheal tube size a | 0.875 | ||
| Endo size 6.5/7.0 (mm) | 12 | 11 | |
| Endo size 7.5/8.0 (mm) | 26 | 22 | |
| Patient’s Sourcea | 0.349 | ||
| Medical ICU | 17 | 11 | |
| Surgical ICU | 21 | 22 |
Data shown as Mean±SD, BMI=Body Mass Index, APACHE II=Acute Physiology and Chronic Health Evaluation II, TISS=Thearapeutic Intervention Score System, GCS=Glasgow Coma Scale.
Chi-Square;
Unpaired t-test,
Mann-Whitney test.
Figure 1.
Distribution of patient groups. CLP=cuff leak percentage.
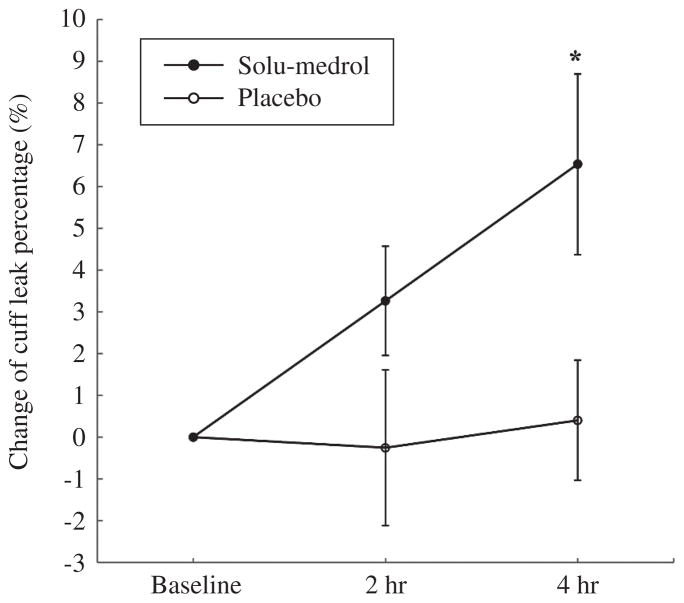
The administration of methylprednisolone 4 h prior to extubation resulted in a significant reduction in the incidence of PES and in the reintubation rate in the treated group compared to the placebo group (15.8% vs. 39.4%, P=0.025, and 7.9% vs. 30.3%, P=0.015, respectively) (Figure 1). The cuff leak percentage increased significantly 4 h after injection in the treated group compared with the placebo group (Figure 2). The length of stay in the ICU was significantly shortened in the treated group compared to the placebo group (8.6±5.2 vs. 13±6.8 days, P=0.004). ICU expenditure was lower in the treated group compared to the placebo group (4 687±2 740 vs. 6 750±3 711 USD, P=0.01).
Figure 2.
Changes in cuff leak percentage (%) compared to baseline at 2 h and 4 h after the injection of methylprednisolone in the treated group and normal saline in the placebo group. *P<0.05 vs. placebo group.
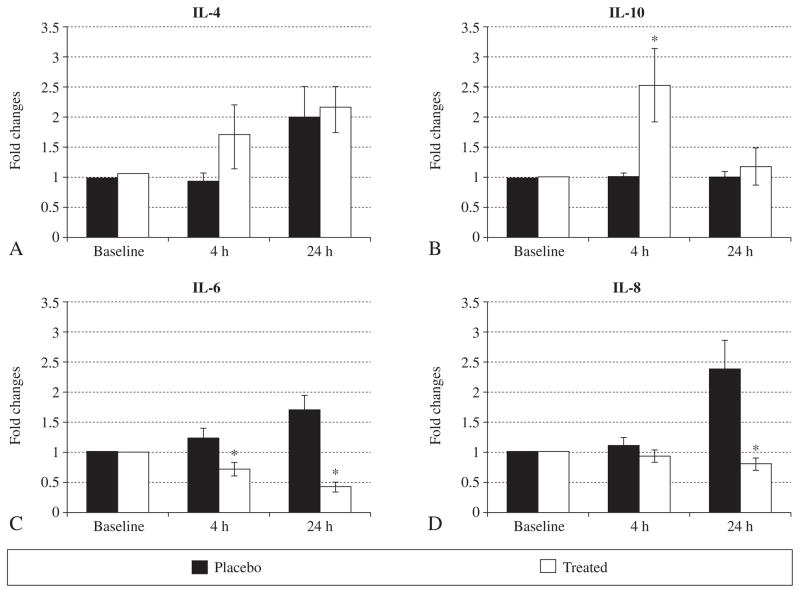
To explore the possible mechanisms by which a single dose of methylprednisolone reduced the incidence of PES, a panel of pro- and anti-inflammatory cytokines were measured in plasma. Out of the ten cytokines assayed, only the levels of IL-4 and IL-10 increased at 4 h in the study group compared to the placebo group (Figure 3A, B); IL-6 levels decreased at 4 h and 24 h, while IL-8 decreased at 24 h in the study group (Figure 3C, D). There was no significant difference in CRP levels between the two groups (data not shown).
Figure 3.
Fold changes in the levels of cytokines (IL-4, IL-6, IL-8 and IL-10) at 4 h and 24 h after the injection of methylprednisolone in the placebo and treated groups. *P<0.05 vs. placebo in identical conditions, respectively.
There were no obvious complications, such as gastrointestinal bleeding, high blood sugar, or increased risk of infection.
Discussion
Intratracheal intubation and mechanical ventilation is routinely used for life support in critically ill patients. Despite the use of improved techniques for the use of high-volume and lower-pressure cuffs in endotracheal tubes, PES is still a major complication in practice.15, 16 Using a cut-off point of ≥24% of cuff leak percentage, we showed that this population of patients had an incidence of 3.8% with respect to PES development, compared to 39.4% if cuff leak percentage was <24%. These results are consistent with our previous study,6 suggesting that cuff leak percentage is a relatively reliable predictor to identify a population at high risk of developing PES in the ICU. The overall incidence of postextubation is 15% in the present study, which is somewhat lower than the overall incidence of 22% reported by a previous study.3
Several previous studies have shown that the administration of corticosteroids can reduce the incidence of PES in a heterogeneous population of patients at different dosages and frequencies of injection.6–8 Francois et al. showed that a 12-h pretreatment with methylprednisolone at 20 mg every 4 h lowered the incidence of PES from 22% to 10% in patients who were not selected based on cuff leak percentage.8 Lee et al. reported a decrease in the incidence of PES from 28% to 10% after the administration of dexamethasone of 5 mg every 6 h for 24 h before extubation in patients with a cuff leak percentage <110 mL.7 We demonstrate that a single dose of methylprednisolone 4 hours before planned extubation reduced the incidence of PES from 39% to 16%.
We did not measure the plasma concentration of methylprednisolone. It has been reported that methylprednisolone sodium succinate reaches a peak plasma concentration at approximately 0.8 h after intravenous administration, with a halflife of about 2.4 h.17 Interestingly, this effect of a single injection was similar to that found by our previous study using the same dose of methylprednisolone injected 19 h before extubation.6 This observation suggests that the onset of methylprednisolone action is short and that the dose used is effective and persistent. This rapid effect is also reflected by an increase in the cuff leak percentage from 3.3% at 2 h to 6.5% at 4 h. Our previous study showed that the effects of methylprednisolone in increasing cuff leak percentage were well sustained 7 h after a single-dose injection.6
The present study showed that the length of the ICU stay and expenditure were significantly reduced in the treated group. The incidence of reintubation was reduced from 30% to 8% in the present study, which is surprisingly similar to our previous study showing that the rate of reintubation decreased from 26% to 7% after a single injection of methylprednisolone 19 h prior to extubation.6 Our study is consistent with a study by Francois, et al., which reported a 50% reduction in the reintubation rate following treatment with methylprednisolone.8 The same group of investigators further demonstrated that the administration of methylprednisolone significantly reduced the incidence of reintubation secondary to laryngeal edema.8
The novelty of the study is that we examined the possible mechanisms by which the administration of methylprednisolone protects against the development of PES. We observe that the levels of IL-4 and IL-10 increase significantly at 4 h, while the levels of IL-6 and IL-8 decrease at 24 h in the methylprednisolone treated group compared to the placebo group. IL-4 was originally considered as a key regulator in humoral and adaptive immunity, including the stimulation of activated B-cell and T-cell proliferation and is an important cytokine in allergic inflammation.18 Investigators have also demonstrated that the administration of recombinant IL-4 decreased lung levels of myeloperoxidase and reduced neutrophil infiltration and the production of pulmonary vascular intercellular adhesion molecule-1 (ICAM-1) in IgG immune complex induced lung injury.19 IL-10 is an anti-inflammatory cytokine and is capable of inhibiting the production of pro-inflammatory cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Previous studies have suggested that IL-4 and IL-10 have significant protective effects on lung inflammatory injury by various mechanisms.19–20 We indeed observe enhanced levels of IL-4 and IL-10 at 4 hours after the administration of methylprednisolone, followed by decreased levels of IL-6 and IL-8 at 24 hours. IL-6 has been used as an inflammatory marker to guide the administration of polyclonal ovine anti-TNF-α fragment antigen binding fragments in critically ill patients.21 IL-8 is a chemoattractant for neutrophils that plays an important role in the development of inflammatory responses.22 The decrease in IL-6 and IL-8 suggests that the inflammatory responses were attenuated following the administration of methylprednisolone, which may contribute to the protection against PES in high-risk patients. We believe that the decreased inflammatory responses after systemic administration of methylprednisolone would not only contribute to local protection but also reflect an improved peripheral inflammatory process.
CRP is a stable molecule and has been considered as an inflammatory marker; levels peak at 48 hours in acute inflammation.23 No significant difference in CRP levels was found between two groups of patients over the 4-h study. We speculate that the lack of difference in the CRP level between the treated and placebo groups was due to the short duration of the study; CRP metabolism did not change rapidly enough.
We conclude that a single injection of 40 mg methylprednisolone starting 4 hours before a planned extubation can effectively reduce the incidence of PES and reintubation rate associated with the up-regulation of IL-4 and IL-10 and the down-regulation of IL-6 and IL-8 in critically ill adult patients.
Acknowledgments
The authors thank Ai-Chin Cheng and Mei-Yi Sung for their assistance with patient screening and cuff leak percentage measurements.
Funding.—Supported by Chi Mei Medical Center (grant # CMFHR-9408 and Canadian Institutes of Health Research (MOP77818)).
References
- 1.Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med. 1981;70:65–76. doi: 10.1016/0002-9343(81)90413-7. [DOI] [PubMed] [Google Scholar]
- 2.Kastanos N, Estopa Miro R, Marin Perez A, Xaubet Mir A, Agusti-Vidal A. Laryngotracheal injury due to endotracheal intubation: incidence, evolution, and predisposing factors. A prospective long-term study. Crit Care Med. 1983;11:362–7. doi: 10.1097/00003246-198305000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Ho LI, Harn HJ, Lien TC, Hu PY, Wang JH. Postextubation laryngeal edema in adults. Risk factor evaluation and prevention by hydrocortisone. Intensive Care Med. 1996;22:933–6. doi: 10.1007/BF02044118. [DOI] [PubMed] [Google Scholar]
- 4.Epstein SK, Ciubotaru RL. Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am J Respir Crit Care Med. 1998;158:489–93. doi: 10.1164/ajrccm.158.2.9711045. [DOI] [PubMed] [Google Scholar]
- 5.Demling RH, Read T, Lind LJ, Flanagan HL. Incidence and morbidity of extubation failure in surgical intensive care patients. Crit Care Med. 1988;16:573–7. doi: 10.1097/00003246-198806000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Cheng KC, Hou CC, Huang HC, Lin SC, Zhang H. Intravenous injection of methylprednisolone reduces the incidence of postextubation stridor in intensive care unit patients. Crit Care Med. 2006;34:1345–50. doi: 10.1097/01.CCM.0000214678.92134.BD. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Peng MJ, Wu CL. Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study. Crit Care. 2007;11:R72. doi: 10.1186/cc5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francois B, Bellissant E, Gissot V, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet. 2007;369:1083–9. doi: 10.1016/S0140-6736(07)60526-1. [DOI] [PubMed] [Google Scholar]
- 9.Darmon JY, Rauss A, Dreyfuss D, et al. Evaluation of risk factors for laryngeal edema after tracheal extubation in adults and its prevention by dexamethasone. A placebo-controlled, double-blind, multicenter study. Anesthesiology. 1992;77:245–51. doi: 10.1097/00000542-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu RS, Pasquale MD, Miller K, Wasser TE. Measurement of endotracheal tube cuff leak to predict postextubation stridor and need for reintubation. J Am Coll Surg. 2000;190:682–7. doi: 10.1016/s1072-7515(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 11.Esteban A, Alia I, Gordo F, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1997;156:459–65. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- 12.Miller RL, Cole RP. Association between reduced cuff leak volume and postextubation stridor. Chest. 1996;110:1035–40. doi: 10.1378/chest.110.4.1035. [DOI] [PubMed] [Google Scholar]
- 13.Daley BJ, Garcia-Perez F, Ross SE. Reintubation as an outcome predictor in trauma patients. Chest. 1996;110:1577–80. doi: 10.1378/chest.110.6.1577. [DOI] [PubMed] [Google Scholar]
- 14.Chevron V, Menard JF, Richard JC, Girault C, Leroy J, Bonmarchand G. Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med. 1998;26:1049–53. doi: 10.1097/00003246-199806000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Agro FECR, Mattei A. New devices and techniques for airway management. Minerva Anestesiol. 2009;75:141–9. [PubMed] [Google Scholar]
- 16.Mauri TpS, Bigatello LM. Prolonged mechanical ventilation after critical illness. Minerva Anestesiol. 2008;74:297–301. [PubMed] [Google Scholar]
- 17.Czock DKF, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Alexis N, Griffith K, Almond M, Peden DB. IL-4 induces IL-6 and signs of allergic-type inflammation in the nasal airways of nonallergic individuals. Clin Immunol. 2002;104:217–20. doi: 10.1006/clim.2002.5236. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan MS, Jones ML, Vaporciyan AA, Howard MC, Ward PA. Protective effects of IL-4 and IL-10 against immune complex-induced lung injury. J Immunol. 1993;151:5666–74. [PubMed] [Google Scholar]
- 20.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int. 2005;67:1216–33. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 21.Rice TW, Wheeler AP, Morris PE, et al. Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection (CytoFab) in severe sepsis. Crit Care Med. 2006;34:2271–81. doi: 10.1097/01.CCM.0000230385.82679.34. [DOI] [PubMed] [Google Scholar]
- 22.Herzum I, Renz H. Inflammatory markers in SIRS, sepsis and septic shock. Curr Med Chem. 2008;15:581–7. doi: 10.2174/092986708783769704. [DOI] [PubMed] [Google Scholar]
- 23.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]