Abstract
Numerous dual-systems models of personality have been posited, which propose that behavior is influenced by two complementary systems. A bottom-up system is characterized by emotion-based drive (e.g., urge for rewarding experience), and a top-down system is characterized by the ability to control those urges. Although evidence suggests that these two systems are distinct and may be important in explaining some behaviors, these constructs are also moderately correlated. Notably, there has been little molecular or behavior genetic research on the genetic distinctness of the two systems central to the dual-systems model. The current study used a national twin sample to investigate the degree to which bottom-up and top-down systems, measured here as personality traits of sensation seeking and lack of planning, respectively, covary through genetic and environmental influences. Whereas the overlap between these systems was primarily comprised of unshared environmental influences (e.g., measurement error and unshared systematic variation) in females, a statistically significant proportion of the overlap was accounted for by genetic factors in men. Further, the genetic factors for these systems were moderately to highly correlated in men (rG=.62–.79). These results provide clear support for a dual-systems model in women; however, these systems appear to share some common genetic influences in men.
Keywords: impulsivity, sensation seeking, lack of planning, genetics, risky, twins, dual-systems
Dual-systems models of risky behavior have been posited in which there is an interaction between two distinct and competing systems (Steinberg 2008), a bottom-up, emotion-based system characterized by urges for rewarding stimuli (i.e., appetitive motivation; Gray 1990) and a top-down, cognitive-based system characterized by control over emotion-based urges (e.g., Steinberg 2007). The nature of this interaction is such that individuals high in urges for rewarding stimuli and low in the ability to control those urges are most apt to engage in risky behavior (Galvan et al. 2006). Although these systems are theoretically distinct and importantly related to risky behavior, evidence from personality, developmental, and neuroscience research suggests there is substantial covariation among these systems. In the present study, we investigated the composition of the covariation between these two presumably distinct systems using behavior genetic models, which allow variance in and covariance between constructs to be decomposed into familial factors (i.e., genetic and environment factors that make family members alike) and a unique environmental factor (e.g., measurement error and unshared systematic variation).
Investigations of Dual Process Models
Much of the personality research related to dual-process models of risky behavior has been on impulsivity, which is broadly defined as the tendency to engage in behavior prematurely or without appropriate planning (Evenden 1999). Whiteside and Lynam (2001) conducted exploratory factor analyses on items of impulsivity and extracted four facets—lack of planning, urgency, sensation seeking, and perseverance. Two of these facets are related systems involved in dual-systems models of risky behavior; the bottom-up system conceptually maps onto Gray’s behavioral approach system and at elevated levels reflects trait sensation seeking (Gray 1990), and the top-down system conceptually maps onto executive functioning and at elevated levels of dysfunction reflects trait lack of planning (Bickel et al. 2012). Although evidence from factor analytic models suggests these facets are distinct, these traits are moderately (r=.34–.38; Smith et al. 2007; Steinberg et al. 2008) to strongly correlated (r=.56; Wiers et al. 2010). Notably, urgency is defined as “a tendency to commit rash or regrettable actions as a result of intense negative affect” (Whiteside and Lynam 2001, p. 677), which appears to be an amalgam of emotional and cognitive systems and is moderately correlated with sensation seeking (r=.29) and lack of planning (r=.21) (Smith et al. 2007).
In addition to being at least partially distinct, longitudinal research indicates that these systems follow at least somewhat different developmental trajectories. In a nationally-representative longitudinal study of adolescents (age 12–24), latent growth curve models suggest that quadratic trends best fit both bottom-up and top-down systems, with sensation seeking (three items measuring the enjoyment of taking risks) peaking in early adolescence (age 14) and gradually decreasing thereafter, and impulsivity (three items measuring lack of planning) demonstrating a steep decrease throughout adolescence and a slight increase in young adulthood (Harden and Tucker-Drob 2011). Notably, the correlation between the linear change in these two traits was small and not statistically significant (r=.21, 95% CI −0.01, 0.44), but there was moderate correlation between the quadratic change in these traits (r=.44, 95% CI 0.04, 0.78) . A more recent paper drawn from the same sample using the same measures across a similar timeframe (age 15−26) suggested a strong and statistically significant correlation (r=.67) between changes in sensation seeking and impulsivity (when modeling a non-linear slope variable; Quinn and Harden 2012). These more recent findings suggest that impulsivity and sensation seeking may show substantial developmental overlap, at least during certain time frames or when using specific modeling procedures.
Finally, neuroscience research has provided evidence that distinct neural substrates implement these systems. Notably, the distinction between these systems is partially based in neuroscience; whereas bottom-up systems are simple, fast, automatic, effortless, and implemented by subcortical substrates in the midbrain (e.g., mesolimbic reward systems; Koob and Le Moal 2008), top-down systems are complex, slow, controlled, effortful, and implemented by cortical substrates in the anterior part of the brain (e.g., frontal cortical regions; Goldstein and Volkow 2011). Specifically, bottom-up systems are implemented by neural substrates associated with reward processing, such as the nucleus accumbens and ventral tegmental area (see Depue and Collins 1999 for a review of neural correlates of sensation seeking), and top-down systems are implemented by neural regions associated with executive functioning, such as the ventromedial prefrontal cortex (Diekhof et al. 2011) and dorsolateral prefrontal cortex (Casey et al. 1997). Further, the developmental patterns of neural substrates that implement each system are congruent with change in personality and behavioral measures of each system. For example, the nucleus accumbens exhibits the greatest percent signal change in response to reward during adolescence (when sensation seeking peaks), as opposed to childhood or young adulthood (Galvan et al. 2006). In addition, regions of the prefrontal cortex that implement cognitive control are increasingly recruited throughout the lifespan (see Steinberg 2008 for a review of neurodevelopmental research). The regions implementing bottom-up and top-down systems are connected via corticostriatal pathways, however, and the degree to which one region may influence the other is not clear.
Behavior Genetic Research
Although evidence suggests at least some degree of overlap between trait measures of bottom-up and top-down systems, these findings do not identify sources of their covariation. Construct variance and covariance can be decomposed using classical twin models into three factors—additive genetic, family environment, and unshared environment. Whereas additive genetic and family environmental factors make twins more similar, the unshared environmental factor is comprised of effects that make twins report different scores, primarily measurement error and unshared systematic sources of variation.
Findings from univariate behavior genetic studies suggest that additive genetic factors account for a significant proportion of variance in trait sensation seeking (34%) and trait lack of planning (29%) (see Bezdjian et al. 2011 for a meta-analysis of behavior genetic studies of impulsivity facets). There have been few studies, however, investigating whether genetic factors account for overlap among bottom-up and top-down systems or the impulsivity facets. This absence is striking given evidence that impulsivity is not a homogeneous construct (Whiteside and Lynam 2001) and a growing awareness of the value of using homogeneous constructs for construct validation and theory testing (Smith et al. 2009).
Only one behavior genetic study (Hur and Bouchard Jr 1997) and one molecular genetic study (Whelan et al. 2012) related to dual-systems models or the impulsivity facets can be found. Hur and Bouchard Jr. (1997) attributed 55% of the genetic variance in control (similar to lack of planning) to sensation seeking, which suggests that there are genetic markers common, and unique, to both lack of planning and sensation seeking. This study, however, used a small sample of twins reared apart (n=106), and there have been no replication attempts. Whelan and his colleagues (2012) found an association between top-down control (activity in the insula and right anterior cingulate during stop trials in the stop-signal task) and a genetic marker for the norepinephrine transporter (rs36024).
Present Study
The present study consisted of three primary aims. First, factor analyses were conducted on personality measures to extract data-driven constructs that map onto the dual-systems model. Second, the genetic and environmental contributions to these personality measures were estimated in an adult twin sample. Finally, the phenotypic, genetic, and environmental correlations between these personality constructs were estimated. Given the moderate overlap between these constructs (e.g., Smith et al. 2007), the ultimate goal of this study was to identify whether this overlap is due to genetic and environmental influences shared by twins or other unshared sources of variation. Stronger support for a dual-systems model would be provided if the covariance between sensation seeking and lack of planning is primarily due to factors not shared by twins, such as unique environment (e.g., measurement error, unshared systematic variation).
Methods
Participants
In 2004–2007, 4,173 members (1,703 men, 2,470 women, 1,482 complete twin pairs) of the national community-based Australian Twin Registry (ATR) Cohort II completed self-report, paper-pencil questionnaires about personality as part of a larger study (80.4% participation rate; Slutske et al. 2009). Participants were 32–43 years of age (M=37.7, SD=2.3). All data collection was approved by the Institutional Review Boards at the University of Missouri—Columbia and the Queensland Institute of Medical Research.
Measures
Personality
To remain consistent with other work in this area, the current study defined bottom-up and top-down systems as two of the facets of impulsivity—sensation seeking and lack of planning, respectively. Sensation seeking is “the seeking of varied, novel, complex, and intense sensations and experiences” (Zuckerman 1994), and lack of planning is the tendency to “act on the spur of the moment and without regard to the consequences” (Whiteside and Lynam 2001).
Participants completed the 40-item Zuckerman Sensation Seeking Scale (ZSS; Zuckerman et al. 1964) and 196-item Multidimensional Personality Questionnaire (MPQ; Tellegen 1982). The ZSS consists of four subscales, Thrill and Adventure Seeking, Experience Seeking, Disinhibition, and Boredom Susceptibility. The MPQ consists of three higher-ordered scales that contain three to four lower-ordered subscales, Positive Emotionality (with subscales of Wellbeing, Social Potency, Social Closeness, and Achievement), Negative Emotionality (Stress Reaction, Alienation, and Aggression), and Constraint (Traditionalism, Control, and Harm Avoidance). In addition, the MPQ includes an Absorption subscale, which does not fall under any of the three higher-ordered scales. Bottom-up and top-down systems were each measured by indices previously used in similar analyses (ZSS total and MPQ Control subscale; Hur and Bouchard Jr 1997) and extracted scales that map onto sensation seeking and lack of planning.
Analytic Procedures
Factor Analyses
Data-driven measures of top-down and bottom-up systems were derived by the following method. First, to be overinclusive (Clark and Watson 1995), 32 and 69 items were selected from the ZSS and MPQ, respectively, that were thought to broadly measure sensation seeking and lack of planning. In addition, items that were distinct but related to both traits (e.g., tendencies to use drugs or experience negative emotionality) were included to demonstrate discriminant validity of the factor solution. Second, participants were randomly split into two halves such that each dataset contained one member of each twin pair. One dataset was used for the entire exploratory factor analyses (EFA) and initial confirmatory factor analysis (CFA), and the other was used for subsequent cross-validation of the CFA solution. To determine the number of factors to retain, a parallel analysis (Horn 1965) was conducted using MATLAB (MathWorks 2005). Eigenvalues from an EFA conducted on the original data were compared to eigenvalues from an EFA conducted on a randomly rearranged set of the data, and observed factors with eigenvalues greater than 95% of the eigenvalues from the randomly generated data were retained. Third, to account for missing data, EFA specifying the number of factors selected from the parallel analyses was then conducted in Mplus (Muthén and Muthén 1989–2011) using full-information maximum likelihood. Fourth, the fit of this solution to the data was assessed by specifying a CFA in Mplus and items with comparatively lower loadings were removed. Fifth, to cross-validate the solution obtained through EFA and CFA in the first half of the data, a CFA was conducted on the remaining half. Finally, CFA was conducted on the entire dataset while accounting for clustering in families. Various indices, including chi-square divided by degrees of freedom, Confirmatory Fit Index (CFI; Bentler 1990), and Root Mean Square Error of Approximation (RMSEA; Steiger 1990), were considered to determine the fit of the factor solution to the data.
Univariate Behavioral Genetic Analyses
Structural equation models (SEMs) were fitted to estimate the degree to which variance in each phenotype was associated with additive genetic (A), common environmental (C), and unshared environmental (E) factors. This was done by using genetically-informed data and imposing variance and covariance constraints on the covariance matrix for each zygosity group, from which latent variables are assumed to represent the biometrical (ACE) factors (e.g., constraining correlations of the A factors to 1.0 for MZ twins and 0.5 for DZ twins). Thus, behavioral genetic models estimated the covariances between MZ-(calculated as A+C) and DZ-twin pairs (calculated as 0.5*A+C) and the percentage of phenotypic variance attributable to the biometrical factors (Neale and Cardon 1992; Prescott 2004). All behavioral genetic analyses were conducted using Mplus, version 6.1.
Bivariate Behavioral Genetic Analyses
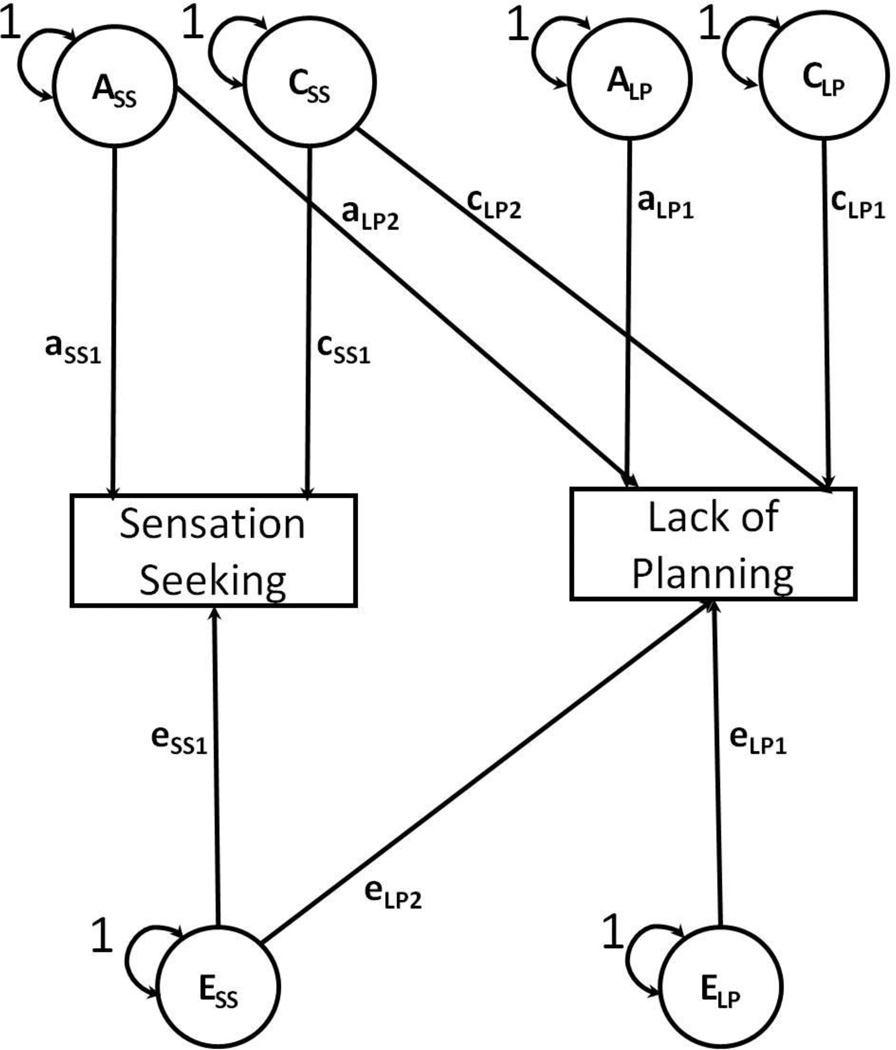
Structural equation models were fitted to estimate the covariances between personality traits attributed to the biometrical factors (see Figure 1). The bivariate model was based on the Cholesky decomposition approach, from which three triangular matrices containing parameter estimates for the biometrical factors are derived (Loehlin 1996; Neale and Cardon 1992). In bivariate models, each matrix contains three elements, two on the diagonal accounting for the variance in each phenotype and one on the off-diagonal accounting for the covariance between both phenotypes. Using tracing rules, variance in a phenotype is computed as the sum of every path estimate, squared, from the biometrical factors (e.g., Ass, Css, Ess) to a phenotype (e.g., sensation seeking), and the proportion of variance due to a factor is its component divided by the sum of all factors (e.g., ass2 / [ass2 + css2 + ess2]). Similarly, covariance between two phenotypes is computed as the product of every path going from one phenotype (e.g., sensation seeking), through a biometrical factor (e.g., Ass, Css, Ess), and to the other phenotype (e.g., lack of planning), and the proportion of covariance is its component divided by the sum of all paths (e.g., [aSS*aLP] / [aSS*aLP + cSS*cLP + eSS*eLP]). Thus, the variances and covariance are decomposed into the biometrical factors.
Figure 1. Bivariate behavior genetic model decomposing the covariation between sensation seeking and lack of planning into genetic and environmental factors.
The Cholesky decomposition approach to bivariate behavioral genetic modeling. Models were used to obtain estimates of the proportion of covariance between sensation seeking (a bottom-up trait) and lack of planning (a top-down trait) associated with genetic and environmental factors. The variances for the latent variables, additive genetic factors (A), common environmental factors (C), and unique environmental factors (E), were fixed to 1. Subscripts refer to the specific latent variables (A,C,E) and their respective parameter estimates (a,c,e) for sensation seeking (SS) and lack of planning (LP).
Results
Factor Analyses
Exploratory Factor Analysis
Parallel analysis on the 101 items suggested that a 10-factor solution should be retained. Three factors were not included in subsequent analyses because they contained less than three items and appeared to reflect method effects (e.g., items related to sail boating), and five factors were not included because they measured constructs not related to the current paper (harm avoidance, narcissism, achievement, substance use, and identifying with countercultures). As a result, two factors were included in subsequent analyses that mapped onto sensation seeking (comprised of eight items from the Thrill and Adventure Seeking subscale of the ZSS, three items from the Harm Avoidance subscale of the MPQ, and one item from the Disinhibition subscale of the ZSS) and lack of planning (comprised of 16 items from the Control and two items from the Traditionalism subscales of the MPQ Control scale) (see Table A in the Appendix).
Confirmatory Factor Analysis
A CFA was conducted in the first half of the sample using items from the two factors extracted from the EFA. This initial CFA indicated a relatively poor fit to the data [χ2=1927.69, 526 df, p<.001, RMSEA=.049 (90% CI=.047, .052), CFI=.86]. Three items (one from the sensation seeking factor, two from the lack of planning factor) with standardized loadings lower than 0.3 were removed, resulting in a model that provided adequate fit to the data [χ2=1303.00, 404 df, p<.001, RMSEA=.045 (90% CI=.042, .048), CFI=.90]. This solution was then cross-validated in the second half of the sample. Model fit was again adequate [χ2=1274.64, 404 df, p<.001, RMSEA=.045 (90% CI=.042, .047), CFI=.91], suggesting that a robust factor solution was achieved, particularly considering the number of items per factor. Finally, CFA was conducted on the entire dataset (i.e., involving all twin pairs), accounting for clustering. This final model provided adequate fit to the data [χ2=4169.28, 404 df, p<.001, RMSEA=.046 (90% CI=.045, .047), CFI=.90]. Standardized loadings are provided for each item in the Appendix. The correlation between the two factors was a statistically significant −.40 and comparable to correlations between similar measures in other studies. The original and extracted scales all demonstrated excellent internal consistency in the current sample (α=.77–.84).
Descriptive Statistics
Sample means and standard deviations for males and females were estimated for sum scores of items comprising the scales and extracted factors. For bottom-up systems, males had higher scores than females on the total ZSS (M=18.68 vs. 14.01; χ2=277.14, 1 df, p<.001) and the extracted sensation seeking factor (M=7.73 vs. 4.76; χ2=450.07, 1 df, p<.001). There was not a sex difference in variability, however, on either the total ZSS (SD=6.24 vs. 6.32; χ2=0.21, 1 df, p=.65) or the extracted sensation seeking factor (SD=3.17 vs. 3.27; χ2=1.31, 1 df, p=25). For top-down systems, males had lower scores than females on the MPQ Control scale (M=−12.63 vs. −14.16; χ2=83.59, 1 df, p<.001) and higher scores on the lack of planning factor (M=−12.31 vs. −13.34; χ2=54.03, 1 df, p<.001)1. In addition, there was more variability among males in scores on the MPQ Control scale (SD=4.27 vs. 4.01; χ2=5.66, 1 df, p=.02) and the extracted lack of planning factor (SD=3.60 vs. 3.36; χ2=6.93, 1 df, p=.01). Although there were sex differences in the means and variances of these measures, there were no sex differences in the correlations between the total ZSS and MPQ Control scales (males=.43, females=.47; χ2=0.31, 1 df, p=.58) or the scales of the extracted sensation seeking and lack of planning factors (males=.25, females=.27; χ2=0.12, 1 df, p=.73). This difference in correlations between the actual scales and extracted factors is likely to due to the ZSS being comprised of a heterogeneous set of items, some of which may be related to top-down systems (e.g., the Disinhibition subscale), whereas the sensation seeking factor is comprised of a homogenous set of items that are more distinct from these top-down systems.
Twin correlations are presented in Table 1. All within-trait correlations were consistently larger in MZ (r=.31–.57) than DZ (r=.13–.33) twin pairs for both males and females suggesting genetic influences on variation in bottom-up and top-down systems. Interestingly, cross-trait correlations between bottom-up and top-down systems were relatively similar in female MZ (mean r=.24) and DZ (mean r=.18) pairs, but noticeably larger in male MZ (mean r=.30) than in DZ (mean r=.13) male pairs. This pattern of correlations suggested that genetic factors accounted for some of the overlap between sensation seeking and lack of planning in males but not females. Therefore, behavioral genetic modeling was conducted separately for data from male and female twin pairs.
Table 1.
Twin correlations for measures of sensation seeking and lack of planning.
| Twin 2 phenotype | Twin 2 phenotype | |||||||
|---|---|---|---|---|---|---|---|---|
| Monozygotic Women (659 pairs) | Monozygotic Men (461 pairs) | |||||||
| Twin 1 Phenotype | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| 1) Lack of Planning Factora | .31 | .40 | ||||||
| 2) MPQ Control Scale | .31 | .34 | .42 | .45 | ||||
| 3) Sensation Seeking Factora | .10 | .13 | .49 | .22 | .23 | .51 | ||
| 4) ZSS Total | .22 | .23 | .43 | .57 | .26 | .27 | .42 | .48 |
| Twin 2 phenotype | Twin 2 phenotype | |||||||
| Dizygotic Women (529 pairs) | Dizygotic Men (403 pairs) | |||||||
| Twin 1 Phenotype | ||||||||
| 1) Lack of Planning Factora | .21 | .17 | ||||||
| 2) MPQ Control Scale | .21 | .21 | .14 | .13 | ||||
| 3) Sensation Seeking Factora | .13 | .12 | .27 | .09 | .11 | .33 | ||
| 4) ZSS Total | .20 | .18 | .24 | .27 | .10 | .08 | .28 | .32 |
Note: MPQ=Multidimensional Personality Questionnaire (Tellegen, 1982); SSF=Sensation Seeking measure extracted from factor analyses in the current study; ZSS=Total Zuckerman Sensation Seeking Scale (Zuckerman et al., 1964). The lack of planning factor and MPQ Control scale were correlated .96. The SSF and ZSS were correlated .78. Also included in the analyses were data from 786 single twins: 190 MZ women, 166 MZ men, 197 DZ women, and 233 DZ men.
Univariate Behavior Genetic Analyses
Estimates of the variance associated with genetic and environmental factors in sensation seeking and lack of planning are presented in Table 2. Unshared environmental factors (i.e., environmental factors affecting twins differently) explained the largest proportion of variance for most personality measures, accounting for at least half of the variance in all measures except the total ZSS scale in females (E=.42, 95% confidence interval [CI]=.36, .49) and the sensation seeking factor for males (E=.49, 95% CI=.41, .57). In addition, a substantial proportion of variance in most phenotypes was due to genetic factors, ranging from .21 (the lack of planning factor) to .55 (the total ZSS) in females and from .34 (the total ZSS) to .43 (the MPQ Control scale) in males. Shared environmental factors (i.e., environmental factors affecting siblings similarly) showed negligible influences on all phenotypes.
Table 2.
The proportion of variance in lack of planning and sensation seeking attributed to genetic and environmental factors.
| Females |
Males |
|||||
|---|---|---|---|---|---|---|
| Phenotypes | Additive Genetic |
Shared Environment |
Unshared Environment |
Additive Genetic |
Shared Environment |
Unshared Environment |
| Lack of Planning Factor a | .21 (−.06, .48) | .11 (−.12, .34) | .68 (.60, .77) | .38 (.29, .48) | .00 (.00, .00) | .62 (.52, .71) |
| MPQ Control Scale | .25 (−.02, .51) | .09 (−.14, .31) | .67 (.59, .75) | .43 (.34, .52) | .00 (.00, .00) | .57 (.48, .66) |
| Sensation Seeking Factor a | .46 (.22, .69) | .03 (−.18, .24) | .51 (.45, .58) | .36 (.05, .66) | .15 (−.13, .43) | .49 (.41, .57) |
| ZSS Total | .55 (.30, .80) | .03 (−.20, .26) | .42 (.36, .49) | .34 (−.04, .72) | .15 (−.20, .50) | .51 (.42, .60) |
Notes: MPQ=Multidimensional Personality Questionnaire (Tellegen, 1982); ZSS=Zuckerman Sensation Seeking Scale (Zuckerman, 1964)
The Lack of planning factor and sensation seeking factor were both derived from factor analyses conducted in the current study.
Bivariate Behavior Genetic Analyses
Estimates of the covariance of the sensation seeking and lack of planning measures associated with genetic and environmental factors are presented in Table 3. Bivariate analyses produced results reflecting the univariate analyses; unshared environmental factors accounted for the largest proportion of covariance between all personality measures. The covariance attributable to additive genetic factors for females’ sensation seeking and lack of planning was not significantly different from zero for the extracted factors (.00, 95% CI=−.07, .07) or the actual ZSS and MPQ Control scales (.17, 95% CI=−.03, .37). In males, however, additive genetic factors accounted for a statistically significant proportion of covariance between the extracted factors (.24, 95% CI=.18, .31) and ZSS and MPQ Control scales (.27, 95% CI=.19, .34). Shared environmental factors did not account for a statistically significant proportion of any of the covariances between bottom-up and top-down systems in either females or males (.00–.12). The largest proportion of covariance between bottom-up and top-down systems for both females (.74– .88) and males (.73–.76) was accounted for by unshared environmental factors, which includes error variance (both random and systematic factors that do not correspond to the construct of interest). In summary, whereas overlap between sensation seeking and lack of planning is partially due to common genetic factors in males, it is almost entirely accounted for by unshared environmental factors in females.
Table 3.
Phenotypic correlations between measures of sensation seeking and lack of planning, and the proportion of correlations attributed to genetic and environmental factors.
| Proportion of Correlation Due to: |
||||
|---|---|---|---|---|
| Phenotypic Correlation |
Additive Genetics |
Shared Environment |
Unshared Environment |
|
| Sensation Seeking & Lack of Planning Factorsa | ||||
| Females | .2 7 | .00 (−.0 7, .07) | .12 (−.0 5, .20) | .88 (.8 2, .93) |
| Males | .24 | .24 (.18, .31) | .00 (−.02, .02) | .76 (.69, .82) |
| ZSS Total & MPQ Control Scales | ||||
| Females | .4 7 | .17 (−.0 3, .37) | .09 (−.0 8, .27) | .74 (.6 8, .80) |
| Males | .42 | .27 (.19, .34) | .00 (−.02, .02) | .73 (.67, .80) |
Notes: MPQ=Multidimensional Personality Questionnaire (Tellegen, 1982); ZSS=Zuckerman Sensation Seeking Scale (Zuckerman, 1964)
The Lack of planning factor and sensation seeking factor were both derived from factor analyses conducted in the current study.
The correlations between the genetic factors influencing sensation seeking with the genetic factors influencing lack of planning (i.e., the genetic correlations; rG), as well as analogous correlations for the unshared environmental (rE) factor, are presented in Table 4. In females, the genetic correlation reached statistical significance for the ZSS total and MPQ Control scale (rG=.44, 95% CI=.13, .75) but not for the extracted factors (rG=.01, 95% CI=−.27, .29); unshared environmental correlations (rE=.28–.44) reached statistical significance for both measurement types. In males, both genetic correlations were large and statistically significant (rG=.62–.79), but the unshared environmental correlation reached statistical significance only for the ZSS total and MPQ Control scale (rE=.30). Because there were no shared environmental influences associated with variance in the lack of planning or sensation seeking measures, the shared environmental correlation was either zero or inestimable.
Table 4.
The genetic and environmental correlations between measures of bottom-up and top-down systems.
| Correlation Between Factors: |
||
|---|---|---|
| rG | rE | |
| Sensation Seeking & Lack of Planning Factors a | ||
| Females | 0.01 (−0.2 7, 0.29) | 0.28 (0.2 0, 0.35) |
| Males | 0.62 (0.29, 0.94) | 0.04 (−0.07, 0.15) |
| ZSS Total & MPQ Control Scales | ||
| Females | 0.44 (0.1 3, 0.75) | 0.44 (0.3 7, 0.52) |
| Males | 0.79 (0.27, 1.00b) | 0.30 (0.18, 0.41) |
Notes: MPQ=Multidimensional Personality Questionnaire (Tellegen, 1982); ZSS=Zuckerman Sensation Seeking Scale (Zuckerman, 1964). Estimates are from full ACE models. The shared environmental correlations (rC) were either 0 or not estimable, primarily due to shared environmental influences accounting for a non-significant proportion of variance for all measures of bottom-up and top-down systems.
The Lack of planning factor and sensation seeking factor were both derived from factor analyses conducted in the current study.
Estimated bounded at 1.0.
Sex Differences
The observed sex differences in the bivariate behavior genetic models were empirically evaluated by comparing models with freely estimated parameter estimates for males and females to models with constrained parameter estimates. For the extracted factors there were sex differences in the proportion of the correlation (reported in Table 3) attributable to unshared environment (χ2=6.18, 1 df, p=.01), and sex differences in the proportion of the correlation attributable to genetic influences approached statistical significance (χ2=3.28, 1 df, p=.07). Similarly, the unshared environmental correlations (reported in Table 4) were significantly different in males and females (χ2=12.01, 1 df, p=.001), and the difference in genetic correlations approached statistical significance (χ2=3.81, 1 df, p=.05). The only significant sex difference for the total ZSS and MPQ Control scales was for the unshared environmental correlation (χ2=4.44, 1 df, p=.04). These results provide evidence that the sources of the overlap between sensation seeking and lack of planning were different in men and women.
To address these sex differences, analyses were conducted to investigate measurement invariance across men and women. An initial baseline model freely estimated thresholds and factor loadings of all items for men and women, and a nested model constrained these parameters to be equal across groups (see Table B in the Online Supplementary Material). Chi-square difference tests indicated that constraining item loadings and thresholds across groups resulted in significantly diminished fit for all measures of top-down and bottom-up traits (Δχ2=43.94, df=16, p<.001 for lack of planning factor; Δχ2=144.66, df=18, p<.001 for MPQ control scale; Δχ2=316.29, df=10, p<.001 for sensation seeking factor; Δχ2=397.06, df=38, p<.001 for ZSS total scale). A comparison of other fit indices, however, suggested that there is no substantial change in fit for these measures (lack of planning factor: ΔCFI=.01, ΔRMSEA=.003; MPQ control scale: ΔCFI<.01, ΔRMSEA=.002; sensation seeking factor: ΔCFI=−.02, ΔRMSEA=− .008; ZSS total scale: ΔCFI<.01, ΔRMSEA=.001). These results provide mixed evidence in regards to whether these measures function differently in men and women, and the sex differences found in the current study should be interpreted with caution.
Discussion
The current study provides at least partial support for a dual-systems model of risky behavior, with overlap between bottom-up and top-down systems being almost entirely due to non-familial factors in females. That is, these findings support the distinction between two systems that have been incorporated into models of self-regulation (Hofmann et al. 2008), delayed gratification (Metcalfe and Mischel 1999), and substance abuse (Hutchison 2010; Wiers et al. 2010). Results were less consistent with a dual-systems model in males, however, for which genetic factors accounted for a statistically significant proportion of correlation between sensation seeking and lack of planning (24%–27%) and genetic correlations between these constructs were large (r=0.62–0.79). Notably, a perfect genetic correlation (i.e., rG=1.0) between sensation seeking and lack of planning could not be ruled out in males.
The genetic overlap between these constructs in males is consistent with previous work by Hur and Bouchard (1997), who found 55% of the genetic variance in top-down systems (the MPQ Control scale) overlapped with bottom-up systems (the total ZSS). The lack of genetic overlap in females, however, is inconsistent with previous results. This might be attributable to the larger sample size in the current study, relative to the previous study, providing adequate statistical power to separately analyze the data obtained from men and women. The current study was also distinctive in that it included data-driven as well as established measures of the specific constructs of interest. Although both approaches produced similar results, it can be helpful to derive measures from factor analyses when testing theory or validating constructs, as this system can lead to more homogeneous constructs (e.g., lack of planning and sensation seeking rather than general impulsivity; Smith et al. 2009). Finally, Hur and Bouchard took an analytic approach that combined all four ZSS subscales, including Boredom Susceptibility and drug preference (from the Disinhibition subscale), which did not lend itself to testing broad assumptions of a dual-systems model. In this regard, this is the first behavior genetic study directly investigating the dual-systems model.
Molecular genetic research has implicated several genetic markers in bottom-up and top-down systems. Bottom-up systems have been associated with genes involved in dopaminergic activity, including dopamine transporters (e.g., DAT1/SLC6A3; Enter et al. 2012) and receptors (e.g., DRD2 and DRD4; Eisenberg et al. 2007). Top-down systems have been linked to genes involved in serotonergic activity, as markers for the serotonin transporter have been associated with impulsiveness (5-HTTLPR; Lesch et al. 1996) and attention deficit hyperactivity disorder (5-HTR1B; Faraone and Mick 2010). In addition, top-down systems, like bottom-up systems, are associated with dopamine genes (Congdon et al. 2008). It should be noted that significant associations of genetic markers with personality traits are often followed by numerous null findings (Munafò and Flint 2011), and evidence linking specific genetic markers with specific personality should be interpreted cautiously.
Although measurement error may comprise part of the overlap due to unshared environmental factors, all measures demonstrated excellent internal consistency and these factors likely include actual environmental factors. For example, both sensation seeking and lack of planning have been linked to social influences outside the family. Association with delinquent peers during adolescence correlates with risky behavior (Yanovitzky 2005) and extraversion (of which sensation seeking is a facet; Baker and Daniels 1990). Similarly, spouses may encourage or discourage the development or expression of these personality traits. Notably, an individual’s personality traits correlate with those of friends and spouses (e.g., Eaves et al. 1999), which may be indicative of individuals selecting peers with personality profiles similar to their own (i.e., gene-environment correlations; Scarr and McCartney 1983). Unshared environmental factors accounting for overlap between measures of bottom-up and top-down systems may, therefore, also include complex interplays between genetic predisposition and unshared environmental factors, such as social influences.
Limitations & Future Directions
Although the current study is the first behavior genetic investigation of a dual-systems model of risky behavior, it is important to note that measures of between-person, not within-person, variation in bottom-up and top-down systems were used. Measures of within-person variation incorporated into dual-systems models of risky behavior include behavioral tasks (Thush et al. 2008; Houben and Wiers 2009) and neuroimaging (Steinberg 2008; Galvan et al. 2006; Casey et al. 2005), neither of which correlate strongly with self-report measures (r=−.06− .10; Cyders and Coskunpinar 2012; Steinberg et al. 2008).
Additional criticisms have been made of dual-systems models of risky behavior, which may point to important directions of future research. Specifically, interactions between these systems may be bidirectional and more complex than acknowledged by current models (Crone and Dahl 2012), and the developmental trajectories of these systems may not follow those typically posited in dual-systems models of risky behavior (see Pfeifer and Allen 2012 for review).
Significance
Clarifying how bottom-up and top-down systems are related to each other in adults is important for at least four reasons. First, if these systems are truly distinct and differentially predict various outcomes, any model including only one system would be incomplete. If these systems account for the same variation in an outcome, however, models including both systems may be unnecessarily complex. Second, clarifying the relationship between these systems may lead to uniformity in the ways in which these systems are measured and discussed. For example, some studies have intentionally combined measures of bottom-up and top-down systems, whereas others have intended to measure one system but actually measured the other (see Magid et al. 2007). Third, much of the work on dual-systems models focuses on outcomes in adolescence and, less frequently, emerging adulthood; the current study extends this work to middle adulthood (age 32–43) , when top-down systems should be fully matured. The nature and context of risky behavior in adulthood may differ from adolescence, and further work is needed to investigate whether the current findings generalize to adolescent samples, as well as whether the dual-systems models of risk behavior in adolescence generalize to adulthood. Finally, clarifying the role of the bottom-up and top-down systems in general risky behavior, well as whether the dual-systems models of risk behavior in adolescence generalize to adulthood.
Conclusion
The current study is the first genetically-informed investigation of a dual-systems model; there was some support for there being two distinct traits underlying risky behavior. Whereas the overlap between sensation seeking and lack of planning was almost entirely attributed to unshared environmental factors (i.e., measurement error and systematic sources of variation) in females, a statistically significant proportion of the covariance between these constructs was attributed to genetic factors in males. These findings provide support for a dual-systems model, particularly in females for whom the covariance between bottom-up and top-down systems was primarily explained by factors not shared by twins. The specific sources of these environmental and genetic factors remain unknown; additional research will be needed to identify the composition of these latent factors.
Supplementary Material
To address these sex differences, analyses were conducted to investigate measurement invariance across men and women. An initial baseline model freely estimated thresholds and factor loadings of all items for men and women, and a nested model constrained these parameters to be equal across groups (see Table B in the Online Supplementary Material). Chi-square difference tests indicated that constraining item loadings and thresholds across groups resulted in significantly diminished fit for all measures of top-down and bottom-up traits (Δχ2=43.94, df=16, p<.001 for lack of planning factor; Δχ2=144.66, df=18, p<.001 for MPQ control scale; Δχ2=316.29, df=10, p<.001 for sensation seeking factor; Δχ2=397.06, df=38, p<.001 for ZSS total scale). A comparison of other fit indices, however, suggested that there is no substantial change in fit for these measures (lack of planning factor: ΔCFI=.01, ΔRMSEA=.003; MPQ control scale: ΔCFI<.01, ΔRMSEA=.002; sensation seeking factor: ΔCFI=−.02, ΔRMSEA=− .008; ZSS total scale: ΔCFI<.01, ΔRMSEA=.001). These results provide mixed evidence in regards to whether these measures function differently in men and women, and the sex differences found in the current study should be interpreted with caution.
Acknowledgments
This work was supported by National Institutes of Health Grants MH066206 (WSS), T32AA13526 (AKL, JME), F31AA019596 (AKL), and F31AA022294 (JME). Thanks to Dixie Statham, Bronwyn Morris, and Megan Fergusson for coordinating the data collection for the twins, and to David Smyth, Olivia Zheng, and Harry Beeby for data management of the Australian Twin Registry. We thank the Australian Twin Registry twins for their continued participation.
Appendix
Table A.
Factor loadings and subscales for each item entered into the final confirmatory factor analysis of sensation seeking and lack of planning
| Subscale |
Item |
Loading |
|---|---|---|
| Sensation Seeking | ||
| TAS | A: I often wish I could be a mountain climber. B: I can’t understand people who risk their necks climbing mountains. |
0.67 |
| TAS | A: A sensible person avoids activities that are dangerous. B: I sometimes like to do things that are a little frightening. |
0.77 |
| TAS | A: I would like to take up the sport of water skiing. B: I would not like to take up water skiing. |
0.59 |
| TAS | A: I would like to try surfboard riding. B: I would not like to try surfboard riding. |
0.70 |
| TAS | A: I would not like to learn to fly an airplane. B: I would like to learn to fly an airplane. |
0.60 |
| TAS | A: I prefer the surface of the water to the depths. B: I would like to go scuba diving. |
0.68 |
| TAS | A: I would like to try parachute jumping. B: I would never want to try jumping out of plane, with or without a parachute. |
0.74 |
| TAS | A: Skiing down a high mountain slope is a good way to end up on crutches. B: I think I would enjoy the sensations of skiing very fast down a high mountain slope. |
0.69 |
| HA | I might enjoy riding in an open lift to the top of a tall building under construction. |
0.64 |
| HA | Of these two things I would dislike more: (A) Riding a long stretch of rapids in a canoe, or (B) Waiting for someone who’s late. |
0.69 |
| HA | I would not like to try bungy-jumping. | 0.63 |
| DIS | A: I am not interested in experience for its own sake. B: I like to have new and exciting experiences and sensations even if they are a little frightening, unconventional, or illegal. |
0.74 |
| Lack of Planning | ||
| CN | I keep close track of where my money goes. | 0.48 |
| CN | I often stop one thing before completing it and start on another. | 0.31 |
| CN | When forced with a decision I usually take time to consider and weigh all possibilities. |
0.55 |
| CN | I often act without thinking. | 0.48 |
| CN | I often prefer to “play things by ear” rather than to plan ahead. | 0.59 |
| CN | I don’t like to start a project until I know exactly how to do it. | 0.58 |
| CN | I am more likely to be fast and careless than to be slow and plodding. | 0.50 |
| CN | I tend to value and follow a rational, “sensible” way of doing things. | 0.75 |
| CN | I am often not as cautious as I should be. | 0.59 |
| CN | I plan and organize my work in detail. | 0.56 |
| CN | I often start projects with little idea of what the end result will be. | 0.51 |
| CN | People say that I am well-organized (that I do things in a systematic manner). | 0.51 |
| CN | I am a cautious person. | 0.68 |
| CN | I am very level headed and always like to keep my feet on the ground. |
0.75 |
| CN | Whenever I go out to have fun I like to have a pretty good idea of what I’m going to do. |
0.49 |
| CN | Before I get into a new situation I like to find out what to expect from it. |
0.68 |
| TRA | Whenever I decide anything I try to remember the basic rules of right and wrong. |
0.31 |
| TRA | I very much dislike it when someone breaks the rules of good conduct. |
0.36 |
NOTE: For items with A and B options, participants were asked to choose the activity that they would most prefer. The choice that indicates sensation seeking is italicized. For other items, reverse-scored items are italicized.
TAS: Thrill and Adventure Seeking Subscale from the Zuckerman Sensation Seeking Scale (Zuckerman et al., 1964)
HA: Harm Avoidance Subscale from the Multidimensional Personality Questionnaire (Tellegen, 1982)
CN: Control Subscale from the Multidimensional Personality Questionnaire (Tellegen, 1982)
TRA: Traditionalism Subscale from the Multidimensional Personality Questionnaire (Tellegen, 1982)
Footnotes
To facilitate model fitting, measures of top-down systems were reverse-scored so that bottom-up and top-down systems were positively correlated. Being high in the lack of planning factor and low in the MPQ Control scale were therefore equivalent.
References
- Baker LA, Daniels D. Nonshared environmental influences and personality differences in adult twins. Journal of Personality and Social Psychology. 1990;58(1):103–110. doi: 10.1037//0022-3514.58.1.103. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bezdjian S, Baker LA, Tuvblad C. Genetic and environmental influences on impulsivity: A meta-analysis of twin, family and adoption studies. Clinical Psychology Review. 2011;31(7):1209–1223. doi: 10.1016/j.cpr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes?. A conceptual reconstruction with special reference to addiction. Psychopharmacology. 2012;221(3):361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Constructing validity: Basic issues in objective scale development. Psychological Assessment. 1995;7(3):309–319. doi: 10.1037/pas0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(1):27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. The relationship between self-report and lab task conceptualizations of impulsivity. Journal of Research in Personality. 2012;46(1):121–124. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22(3):491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Nerenberg L, Falkai P, Dechent P, Baudewig J, Gruber O. Impulsive personality and the ability to resist immediate reward: An fMRI study examining interindividual differences in the neural mechanisms underlying self-control. Human Brain Mapping. 2011 doi: 10.1002/hbm.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Heath AC, Martin NG, Neale MC, Meyer JM, Silberg JL, Corey LA, Truett K, Walters E. Biological and cultural inheritance of stature and attitudes. In: Cloninger CR, editor. Personality and Psychopathology. American Psychi. 1999. pp. 269–308. [Google Scholar]
- Eisenberg DT, MacKillop J, Modi M, Beauchemin J, Dang D, Lisman SA, Lum JK, Wilson DS. Examining impulsivity as an endophenotype using a behavioral approach: A DRD2 TaqI A and DRD4 48-bp VNTR association study. Behavior and Brain Function. 2007;3(2) doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enter D, Colzato LS, Roelofs K. Dopamine transporter polymorphisms affect social approach–avoidance tendencies. Genes, Brain and Behavior. 2012 doi: 10.1111/j.1601-183X.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146(4):348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatric Clinics of North America. 2010;33(1):159–180. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition & Emotion. 1990;4(3):269–288. [Google Scholar]
- Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: Further evidence for a dual systems model. Developmental Psychology. 2011;47(3):739–746. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Gschwendner T, Friese M, Wiers RW, Schmitt M. Working memory capacity and self-regulatory behavior: Toward an individual differences perspective on behavior determination by automatic versus controlled processes. Journal of Personality and Social Psychology. 2008;95(4):962–977. doi: 10.1037/a0012705. [DOI] [PubMed] [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965:30179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW. Response inhibition moderates the relationship between implicit associations and drinking behavior. Alcoholism: Clinical and Experimental Research. 2009;33(4):626–633. doi: 10.1111/j.1530-0277.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- Hur YM, Bouchard TJ., Jr. The genetic correlation between impulsivity and sensation seeking traits. Behavior Genetics. 1997;27(5):455–463. doi: 10.1023/a:1025674417078. [DOI] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology. 2010:6577–6589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008:5929–5953. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. The Cholesky approach: A cautionary note. Behavior Genetics. 1996;26(1):65–69. [Google Scholar]
- Magid V, MacLean MG, Colder CR. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addictive Behaviors. 2007;32(10):2046–2061. doi: 10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MathWorks I. MATLAB: the language of technical computing. Desktop tools and development environment, version 7. MathWorks. 2005 [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological review. 1999;106(1):3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Flint J. Dissecting the genetic architecture of human personality. Trends in Cognitive Sciences. 2011;15(9):395–400. doi: 10.1016/j.tics.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 1989–2011 [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Netherlands: Springer; 1992. [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA. Using the Mplus computer program to estimate models for continuous and categorical data from twins. Behavior Genetics. 2004;34(1):17–40. doi: 10.1023/B:BEGE.0000009474.97649.2f. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Harden KP. Differential changes in impulsivity and sensation seeking and the escalation of substance use from adolescence to early adulthood. Development and Psychopathology. 2012 doi: 10.1017/S0954579412000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype → environment effects. Child development. 1983;54(2):424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG. The Australian Twin Study of Gambling (OZ-GAM): Rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Research and Human Genetics. 2009;12(1):63–78. doi: 10.1375/twin.12.1.63. [DOI] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14(2):155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- Smith GT, McCarthy DM, Zapolski TCB. On the value of homogeneous constructs for construct validation, theory testing, and the description of psychopathology. Psychological Assessment. 2009;21(3):272–284. doi: 10.1037/a0016699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research. 1990;25(2):173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence new perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16(2):55–59. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Brief manual for the multidimensional personality questionnaire. Minneapolis: Unpublished manuscript, University of Minnesota; 1982. [Google Scholar]
- Thush C, Wiers RW, Ames SL, Grenard JL, Sussman S, Stacy AW. Interactions between implicit and explicit cognition and working memory capacity in the prediction of alcohol use in at-risk adolescents. Drug and Alcohol Dependence. 2008;94(1–3):116–124. doi: 10.1016/j.drugalcdep.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Büchel C, Byrne M, Cummins TDR, Fauth B, Flor H, Gallinat J, Heinz A, Ittermann B, Mann K, Martinot JL, Lalor EC, Lathrop M, Loth E, Nees F, Paus T, Rietschel M, Smolka MN, Spanagel R, Stephens DN, Struve M, Thyreau B, Vollstaedt-Klein S, Robbin TW, Schumann G, Garavan H IMAGEN Consortium. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience. 2012;15(6):920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- Wiers RW, Ames SL, Hofmann W, Krank M, Stacy AW. Impulsivity, impulsive and reflective processes and the development of alcohol use and misuse in adolescents and young adults. Frontiers in Psychology. 2010;1 doi: 10.3389/fpsyg.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovitzky I. Sensation seeking and adolescent drug use: The mediating role of association with deviant peers and pro-drug discussions. Health Communication. 2005;17(1):67–89. doi: 10.1207/s15327027hc1701_5. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge University Press; 1994. [Google Scholar]
- Zuckerman M, Kolin EA, Price L, Zoob I. Development of a sensation-seeking scale. Journal of Consulting Psychology; Journal of Consulting Psychology. 1964;28(6):477–182. doi: 10.1037/h0040995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.