Abstract
Niemann Pick type C disease is an inherited autosomal recessive disorder characterised by the accumulation of unesterified cholesterol and sphingolipids within the endosomal/lysosomal compartments. It has been observed that the administration of hydroxypropyl-β-cyclodextrin (HPBCD) delays onset of clinical symptoms and reduces accumulation of cholesterol and gangliosides within neuronal cells. It was assumed that HPBCD exerts its action by readily entering the CNS and directly interacting with neurones and other brain cells to facilitate removal of stored cholesterol from the late endosomal/lysosomal compartment. Here, we present evidence that refutes this hypothesis. We use two well established techniques for accurately measuring brain uptake of solutes from blood and show that there is no significant crossing of HPBCD into the brain. The two techniques are brain in situ perfusion and intraperitoneal injection followed by multi-time-point regression analysis. Neither study demonstrates significant, time-dependent uptake of HPBCD in either adult or neonatal mice. However, the volume of distribution available to HPBCD (0.113±0.010ml/g) exceeds the accepted values for plasma and vascular volume of the brain. In fact, it is nearly three times larger than that for sucrose (0.039±0.006 ml/g). We propose that this indicates cell surface binding of HPBCD to the endothelium of the cerebral vasculature and may provide a mechanism for the mobilization and clearance of cholesterol from the CNS.
Keywords: Niemann-Pick C Disease, Cyclodextrin, Blood-Brain Barrier
Introduction
Niemann-Pick C disease (NPC) is an autosomal recessive neurodegenerative disease caused by mutations in either the NPC1 or NPC2 genes resulting in the accumulation of unesterified cholesterol and multiple sphingolipid species in the late endocytic system (Pentchev et al 1985; Patterson et al. 2001; Walkley 2004). Specific treatment for this condition is currently limited to substrate reduction therapy using the drug miglustat which slows the course of NPC disease and is approved in Europe for treating CNS manifestations in NPC patients. Recently, it was reported (Griffin et al. 2004) that the neurosteroid allopregnanolone, complexed with hydroxypropyl-β-cyclodextrin (HPBCD), delayed the onset of neurodegeneration and reduced the storage of GM2 and GM3 gangliosides in Npc1−/− mice. However, subsequent studies showed that HPBCD alone was effective in reducing neurodegeneration and cholesterol and ganglioside storage, and prolonging the life-span of Npc1−/− mice (Davidson et al. 2009; Liu et al 2009). As a consequence, attention shifted to cyclodextrins as possible treatments for NPC, as they have been shown in cell-based systems to mediate efflux of cholesterol from within the cell (Yancey et al. 1996; Atger et al. 1997). HPBCD is a complex cyclic carbohydrate composed of seven glycosidic residues assembled into a ring structure (Szejtli 1998). Cyclodextrins form inclusion complexes with many smaller molecules within the hydrophobic cavity formed by the sugar ring (Dodzuik 2006). They readily complex with the sterol cholesterol and have been shown to deplete intracellular cholesterol from macrophages in tissue culture (Kilsdonk et al. 1995). It was assumed that HPBCD was effective in brain because it crossed the blood-brain barrier (BBB) and facilitated clearance of cholesterol from brain cells. In this view, the HPBCD/cholesterol complex would then be cleared from the CNS by bulk flow and drainage of the cerebrospinal fluid to blood from which it could be eliminated by the kidney. However, other smaller carbohydrates that are not transported across the BBB, for example sucrose (350 Da) and inulin (~6000 Da) remain almost exclusively in the cerebral vascular compartment and do not enter brain (Smith et al. 1988). It was therefore imperative to investigate the interaction of HPBCD (MW 1394 Da) with the BBB to clarify its mechanism of action in clearing CNS cholesterol in NPC disease.
Methods
Wild type BALB/c (Npc1+/+) mice and Npc1−/− were generated by heterozygous mating of BALB/c/NPCnih mice. The radiolabelled HPBCD had an average degree of substitution of propyl groups 5.1 and was obtained from the same source as the radiolabelled HPCD used in study of Camargo (2001), the unlabelled HPCD used in this study was obtained from the same source, Sigma-Aldrich (H107) as for the study of Davidson et al. (2009) and had a quoted degree of substitution of propyl groups of 5.6 to 4.2. 2-hydroxypropyl-[14C]-propyl-β-cyclodextrin ([14C]-HPBCD), was purchased from Cyclodextrin Technologies Development Inc, Florida 32843, USA at a specific activity 6.95mCi/g. Radioactivity eluted as a single peak on a Sephadex G25 column. Brain uptake was determined in Npc1+/+ and Npc1−/− mice using two methods: in situ brain perfusion and multi-time-point regression analysis following intraperitoneal administration. For in situ brain perfusion a cannula was inserted into the aorta via the left ventricle, the descending aorta tied off, the jugular veins opened, and the upper part of the animal perfused at a rate of 10ml/min with a buffered saline solution containing tracer [14C]-HPBCD at a radioactive concentration of 0.3μCi/ml. We have established in a separate series of experiments, using diazepam as a flow marker, that this perfusion rate gives physiological rates of flow of perfusate in all of the brain regions examined (unpublished results). After two or four minutes of perfusion the experiment was terminated by decapitation and the brain removed from the skull. Brain regions were then manually dissected out and their radioactivity determined by scintillation counting. Under these conditions a volume of distribution (Vd) is given by the expression Vd = Cbr/Cp, where Cbr is the HPBCD radioactive concentration in brain, Cp the radioactive concentration of HPBCD in the perfusate. Units are ml/g. For multi-time-point regression analysis animals were injected intraperitoneally with 10μCi of [14C]-cyclodextrin and the experiment terminated by decapitation after varied times of up to one hour. The Vd in brain at different experimental times were then plotted against experimental time (min). A rising slope of the resultant line indicates brain uptake with time and the slope of the line gives a unidirectional influx constant (Kin) and the intercept represents an instantaneous volume of distribution (Vi) or approximates to the plasma volume if the tracer is restricted to the vascular compartment and does not bind to the blood-brain barrier (Smith et al. 1988; Begley 1999; Smith et al. 2003). For the high dose experiments the tracer [14C]-cyclodextrin was combined with “cold” HPBCD, purchased from Sigma –Aldrich (H107), at a dose of 4000mg/kg. This is equivalent to the subcutaneous dose given to mice in previous studies (Camargo et al. 2001; Davidson et al. 2009). All experiments were conducted under general anaesthesia using a mixture of medetomidine HCl 1mg/kg and ketamine HCl 75 mg/kg under the provisions of the Animals Scientific Procedures Act 1986 and Home Office Project Licence 70/6381.
Results
Brain perfusion studies with HPCD
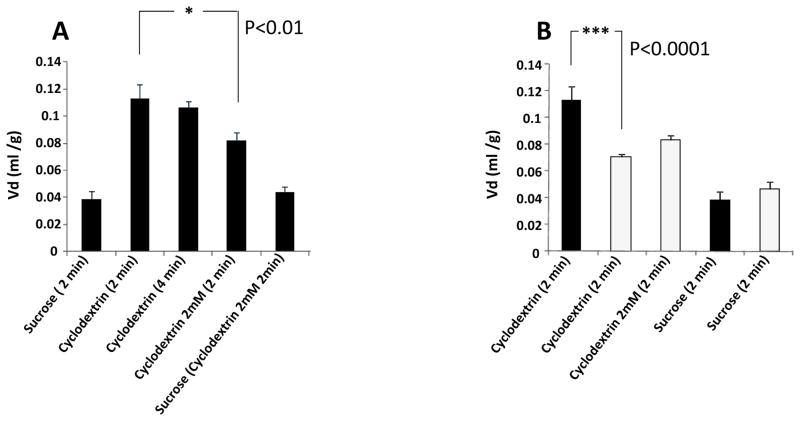
These studies were conducted for either a two or four minute duration. The volume of distribution (Vd) for sucrose at two minutes was 0.039ml/g of brain tissue in both Npc1+/+ and Npc1−/− mice indicating that this tracer occupies a total brain vascular volume of approximately 4.0% at 6–8 weeks of age in both the wild type and the genetically modified mice (Fig. 1A). In Npc1+/+ mice the volume of distribution for HPBCD at two minutes was 0.113ml/g of tissue and did not increase significantly after four minutes of perfusion. The addition of 2mM HPBCD to the perfusate containing radiolabelled tracer sucrose had no effect on the Vd of sucrose in Npc1+/+ mice but when added to the perfusate containing tracer levels of cyclodextrin, the Vd for HPBCD was significantly reduced (p<0.01) to 0.082ml/g. The volume of distribution for tracer cyclodextrin in Npc1−/− mice was significantly lower (p<0.001) than in the Npc1+/+ mice, but conversely the addition of a 2mM competing concentration of HPCD had no significant effect on the Vd in these mice (Fig. 1B).
Figure 1. Data from in situ brain perfusion studies.
A. From left to right; volumes of distribution (Vd) for sucrose, 2 min (0.039±0.006 ml/g); Vd for cyclodextrin 2 minutes perfusion (0.113±0.010 ml/g); Vd for cyclodextrin 4 minutes perfusion (0.106±0.005ml/g) Vd for cyclodextrin 2 minutes in the presence of 2mM cyclodextrin (0.082±0.006 ml/g); Vd of sucrose 2 minutes in the presence of 2mM cyclodextrin (0.044±0.009ml/g). All data Npc1+/+. (n=4–6 for each column ± SEM, significance 1-way anova)
B. From left to right; volumes of distribution (Vd) for cyclodextrin 2 minutes Npc1+/+ (0.113±0.010ml/g); Vd for cyclodextrin 2 minutes Npc1−/− (0.071±0.002ml/g); Vd for cyclodextrin 2 minutes in the presence of 2mM cyclodextrin Npc1−/− (0.083±0.003ml/g); Vd for sucrose, 2 min Npc1+/+ (0.039±0.006ml/g); Vd for sucrose, 2 min Npc1−/− (0.047±0.005ml/g). (n=4–6 for each column ± SEM, significance 1-way anova)
Intraperitoneal administration of HPBCD
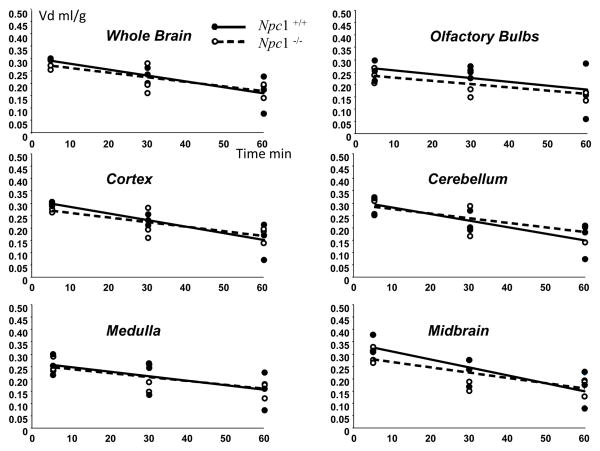
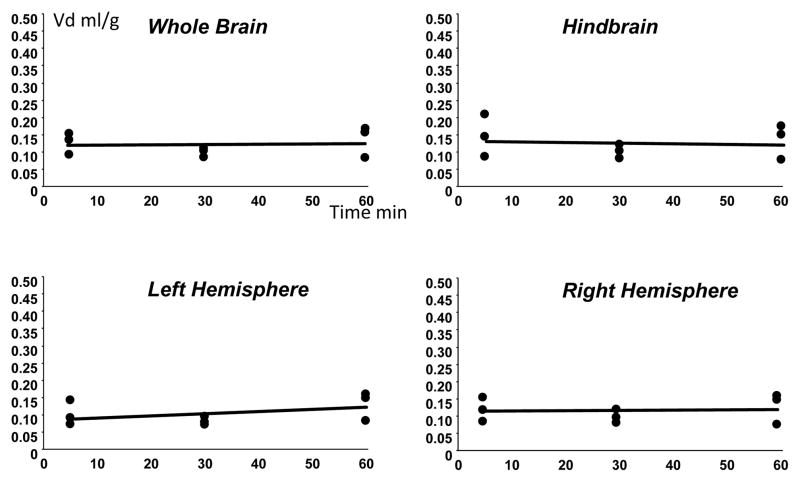
This second approach was adopted to allow longer term CNS uptake to be determined for time periods of up to one hour. Intraperitoneal administration of tracer levels of HPBCD resulted in rapid rise in plasma levels of cyclodextrin which were sustained over the one hour experimental period (Fig. 2). Plasma levels of high dose cyclodextrin rose more slowly after intraperitoneal injection than tracer quantities, probably as a result of slower absorption of the more viscous mixture. Multiple time point plots of the Vd of cyclodextrin in 6–8 week old Npc1+/+ and Npc1−/− mice resulted in a mean of 15 to 20% in all brain regions with no increase in Vd with time, indicating no penetration of the cyclodextrin into brain during the time course of the experiment (Fig. 3). There was no significant difference between the Npc1+/+ and Npc1−/− mice. With the high dose studies again there was no discernible entry of the cyclodextrin into brain with time (Fig. 4). In the high dose studies the multi-time point plots resulted in a slightly negative slope as a result of the slower entry into plasma of the injected material from the peritoneum due to the increased viscosity of the injectate. However, at the one hour time point the volumes of distribution for cyclodextrin at both tracer levels and at high dose were the same. Identical experiments performed with 7 day Npc1+/+ mice resulted in exactly the same result as in Npc1+/+ and Npc1−/− 6–8week old mice with a 15–20% Vd with no evidence of brain accumulation with time (Fig. 5). These data indicate that the BBB to HPBCD is equally robust in both newborn and adult mice and that HPBCD does not significantly cross the BBB at either age.
Figure 2.
Plasma radioactivity (cpm/50μl) with time after intraperitoneal injection of tracer quantities of [14C]-cyclodextrin or at high dose (same radioactive quantity mixed with 4000mg cyclodextrin/kg, in Npc1+/+ and Npc1−/− mice. (n=3 for each time point)
Figure 3.
Volumes of distribution (Vd).of cyclodextrin after intraperitoneal injection of tracer levels with time in whole brain, cortex, medulla, olfactory bulb, cerebellum and midbrain in Npc1+/+ and Npc1−/− mice. (n=3 for each time point)
Figure 4.
Volumes of distribution (Vd) of cyclodextrin after intraperitoneal injection of high dose with time in whole brain, cortex, medulla, olfactory bulb, cerebellum and midbrain in Npc1+/+ and Npc1−/− mice. (n=3 for each time point)
Figure 5.
Volumes of distribution (Vd)of cyclodextrin after intraperitoneal injection of tracer levels with time in whole brain, hind brain and left and right hemispheres in Npc1+/+ at 7 days of age. (n=3 for each time point)
Discussion
The experimental techniques used in this study to examine possible brain penetration of HPBCD are well-established methods for determining the brain uptake of both slow and rapidly penetrating tracers (Begley 1999; Smith 2003). The results obtained indicate no significant penetration of the HPBCD into any of the examined brain regions with time. With the in situ perfusion studies the Vd of sucrose after two minutes is 0.039ml/g (3.9%) which equates well with the expected intravascular space, determined with albumin under similar conditions (Urayama et al 2004). In Npc1+/+ mice the Vd for HPBCD was larger than sucrose at 0.113ml/g (11.3%) even though it is a much larger molecule. When the perfusion time was extended to four minutes there was no increase in the Vd for the HPBCD. When 2mM excess unlabelled cyclodextrin was added to the perfusate in Npc1+/+ mice there was a significant (p<0.01) displacement of the radiolabelled HPBCD with a reduction of some 25% in the measured Vd. The addition of 2mM HPBCD to a perfusate containing tracer sucrose had no effect on the Vd of sucrose indicating no competition for binding to the vasculature. The fact that sucrose exhibits a Vd similar to the expected vascular volume indicated little or no binding for this molecule. The much larger Vd for HPBCD suggests a significant binding of HPBCD to the brain vasculature even though there is no further transport across the BBB into brain tissue in wild-type mice. The displacement of radiolabelled HPBCD by an excess of unlabelled cyclodextrin indicates reversible binding to the luminal surface of the brain vasculature, possibly to the proteoglycan/glycosphingolipid/glycosaminoglycan-rich glycocalyx which lines the microvessels of the brain (Van den Berg et al. 2006). This glycocalyx is approximately 0.5μm in thickness. A significant non-specific adsorption of the HPBCD to this layer could result in the observed initial Vd, significantly larger than the intravascular space, as exemplified by the sucrose Vd, that does not increase with time. The ready displaceability of the adsorbed tracer HPBCD with excess HPBCD is consistent with very little internalization of the molecule into the endothelium of the microvasculature.
In contrast, in the Npc1−/− mice the initial Vd is significantly smaller (p<0.0001) than in the Npc1+/+ mice and was not significantly displaced with excess 2mM HPBCD. This observation is interesting as the glycocalyx is known to thin in acute hyperglycaemia and other inflammatory conditions affecting the microvasculature (Nieuwdorp et al. 2008). A change in glycocalyx thickness or composition in NPC may explain the reduced binding of HPBCD to the BBB and perhaps changes in the affinity of the binding, altering the displaceabilty of the labelled material.
These results and conclusions are fully supported by the intraperitoneal injection studies over periods of up to one hour. The results of these longer-term studies are the same for Npc1+/+ and Npc1−/− mice at 6 to 8 weeks of age and in P7 neonatal mice. The multi-time point regression studies suggest larger initial volumes of distribution for HPBCD than the short time period in situ perfusion studies but this difference is probably not significant and results from systemic differences in the two protocols used. The volumes of distribution for HPBCD obtained are again well in excess of, in this case, the expected plasma volume as the mice are auto-perfusing, and are again consistent with binding of HPBCD to the walls of the vasculature. The plots of the volumes of distribution with time do not show a sustained upward slope which would indicate penetration of the labelled material into brain (Begley 1999; Smith et al. 2003).
An earlier study (Camargo et al. 2001) also suggested that there was little brain penetration of HPBCD from blood. However the results of that study are difficult to interpret as the authors measured the total brain content of HPBCD and sucrose, one hour after intravenous injection, and did not relate this brain concentration to the brain plasma volume or plasma HPBCD concentration. That study (Camargo 2001) also suggested a doubling of the brain content for both sucrose and HPBCD in Npc1−/− mice compared to Npc1+/+ mice. However, this may be due to different plasma concentrations of tracer achieved in the two groups of mice after one hour and thus simply reflect radiolabel trapped in the cerebral vasculature.
Taken together, both methods used in this current study to examine the interaction of HPBCD with the BBB indicate a significant and rapid binding of HPBCD to the blood vessel walls but no further penetration into the CNS in both 6 to 8 week old Npc1+/+ and Npc1−/− mice and in 7 day old mice. It has been suggested (Liu et al. 2009) that treatment early in life with HPBCD was the most effective as the BBB might be more permeable in the neonatal mammal. However, this suggestion is not supported by this study as the BBB to HPBCD is just as robust in the 7 day old mice as in the adult mice. Numerous other studies with a variety of intravascular tracers (reviewed by Saunders et al. 1999) have also refuted the idea that the BBB is leaky in neonates. The greater efficacy of early initiation of treatment with HPBCD in NPC is probably due to the fact that any neurological damage is less advanced in the neonate, neuronal plasticity is maximal, and the arrest or slowing of further damage to the CNS thus becomes more apparent.
The present study indicates no significant penetration of HPBCD into the CNS and raises the crucial question of how does this agent actually reduce the observed storage in neurons of Npc1−/− mice. Studies with HPBCD with cells in vitro have suggested that cyclodextrin may be able to extract cholesterol directly from the cell membrane (Yancey et al. 1996). A recent study has suggested that HPBCD can induce exocytosis of endosomal vesicles containing cholesterol in cells derived from the liver of Npc1−/− mice in a calcium dependent manner (Chen et al. 2010). A further study (Rosenbaum et al. 2010) employing NPC fibroblasts suggests that both methyl-β-cyclodextrin and HPBCD are taken up by the cells and sequestered into the lysosomal compartment. However, these studies were conducted over 24 hours and the cells were fibroblasts not cerebral endothelial cells. A further study by Plazzo et al. (2012) has also indicated a progressive uptake of fluorescent methyl-β-cyclodextrin in HeLa cells and hamster kidney cells over a two hour period. It is possible that some HPBCD is internalised by the cerebral endothelial cells in vivo in our study as only about 25% of the HPBCD can be displaced by 2mM HPBCD. However, in the brain perfusion studies there was no significant difference in the Vd between two and four minutes of perfusion. Also, in the experiments conducted over one hour there was no continued uptake of HPBCD with time. The total volume of the endothelial cells in brain capillaries is less than 1% of the total brain volume. Thus, achieving a Vd of 15–20% for HPBCD in brain in vivo (greatly in excess of that for sucrose) would require a considerable concentration of HPBCD within endothelial cells relative to that present in plasma. It would seem unlikely that endothelial cells could take up sufficient HPBCD into their endosomal/lysosomal compartment to achieve this ratio. A similarly large intracellular concentration would have to be reached, by two minutes, within the endothelium compared to perfusate concentrations in the short-term in situ perfusion studies in order to achieve the observed result. The most likely explanation for the large Vd is that there is a rapid binding of HPBCD to the cell surface by a non-specific interaction with the glycocalyx. Earlier studies (Monneart et al. 2004a; Monneart et al. 2004b) with monolayers of bovine cerebral endothelial cells in vitro suggested some movement of both methyl-β-cyclodextrin and HPBCD across the cell monolayers when up to 1mM concentrations of the appropriate cyclodextrin were applied. With concentrations of cyclodextrin in the medium of 2mM the permeability of the monolayer to sucrose was increased by a factor of 2 to 5 times suggesting a disruption of tight junctions. One of these in vitro studies (Monneart et al. 2004b) using a monolayer of bovine cerebral endothelial cells indicated that the permeability of the monolayer to hydroxpropyl-γ-cyclodextrin was the same as inulin up to a concentration of 10mM cyclodextrin, after which permeability to both the cyclodextrin and inulin increased. Permeability to the smaller molecular weight inulin began to increase at a lower cyclodextrin concentration than with cyclodextrin itself, suggesting molecular weight selectivity. The authors suggest that this phenomenon is probably related to cholesterol removal from the cell membrane by the cyclodextrin with subsequent disruption of tight junctions (Monneart et al. 2004b). No changes in BBB permeability were noted in the present in vivo study to either HPBCD or sucrose in doses up to the 2mM dose ranges used in in situ perfusion. It is thus concluded that under these conditions the integrity of the tight junctions is not affected at these concentrations of HPBCD.
In the present high dose studies conducted with a dose of 4000mg/kg HPBCD intraperitoneally “spiked” with radioactive HPBCD, the same dose as administered subcutaneously in previous studies (Griffin et al. 2004; Davidson et al. 2009), we calculate from the plasma radioactivity that the plasma HPBCD concentration achieved in mice in the high dose study was approximately 0.36mM. If HPBCD binds to the glycocalyx of the brain vasculature, it would result in a relatively high concentration of HPBCD immediately adjacent to the endothelial cell membrane. Given the thickness of the normal glycocalyx (0.5μm) and the observation that the apparent vascular volumes for HPBCD are three to four times larger than expected, then by adsorption the local endothelial cell surface concentrations of HPBCD could be maintained at high levels. If the HPBCD bound to the endothelium can exchange with circulating HPBCD as the binding is displaceable, this might form sink conditions thus removing cholesterol from the endothelium. However, it has been recently suggested (Taylor et al. 2012) that HPBCD may not simply mobilise cholesterol from cells by a sink action from the cell membrane and thus need not necessarily act principally as a carrier of cholesterol from tissue to the kidney for excretion. It is possible that the reduction of cholesterol initiated by HPBCD within the capillary endothelium may then further catalyse shifts in cholesterol content between other brain cells and facilitate overall clearance of the sterol out of the brain. Taylor et al. (2012) have demonstrated that HPBCD can trigger the movement of cholesterol from the late-endosome/lysosome to the cytosol thus allowing further normal sterol processing and metabolism. In the brain, mobilization of cholesterol within the endothelium may then initiate cholesterol release from the late-endosomal lysosomal compartments of other brain cells via cell- contact or signalling (Chen et al 2010; Taylor et al. 2012). Glial cells are well positioned to achieve this as they form direct cellular pathways between the capillary endothelial cells, neurones and other brain cells (Begley 2012) and can, for example, transmit calcium waves over large areas.
HPBCD is certainly most active in slowing disease progression and in clearing cholesterol from the brain if it is injected intrathecally (Ward et al. 2010) or intraventricularly (Aqul et al. 2011) and thus direct contact with brain cells is clearly most effective. However, it is still debateable whether HPBCD needs to be internalised into a cell to catalyse the export of cholesterol from the late-endosomal/lysosomal compartment or whether further intracellular or extracellar messengers are involved (Chen et al. 2010; Taylor et al. 2012). A mechanism depleting the brain capillary endothelial late endosomal/lysosomal compartment of cholesterol may also allow the brain to more effectively clear further cholesterol to the periphery by the synthesis and export of 24S-hydroxycholesterol from the CNS by the oatp2 transporter (Ohtsuki et al. 2007). However, as HPBCD is also known to bind many other molecules (Yancey et al. 1996) we cannot rule out or rule in that mobilisation of cholesterol is the primary or sole mechanism of action of HPBCD in NPC disease. The actions of cyclodextrin on the brain and the blood-brain barrier in vivo and in vitro and possible changes in the blood-brain barrier in Niemann-Pick C disease clearly merit further investigation.
Take Home Message.
2-hydroxypropyl-β-cyclodextrin displays no significant time-dependent crossing of the blood-brain barrier in wild type Npc1+/+, wild type neonate Npc1+/+ (P7) or Niemann-Pick C Npc−/− disease mice.
Acknowledgments
This work was supported by a research grant from The Hadley Hope Fund and The Addi and Cassi Fund. The generation and genotyping of the Npc1−/− mice was funded by a grant to FMP from SOAR-NPC. Contributions made by SW and CD were made possible through funding by NIH (NS053677). The authors would also like to thank Dr. William Sly and members of his lab at the Edward A. Doisy Department of Biochemistry and Molecular Medicine, St. Louis School of Medicine, St. Louis, MO, USA who have contributed useful critical comments.
Footnotes
Author Contributions: DJB and CCP designed the research and analysed the data, CCP performed the experiments, CDD, SUW and FMP contributed to the experimental design and discussion and FMP contributed Npc1−/− mice, DJB and CCP wrote the paper.
References
- Aqul AA, Liu B, Ramirez CM, et al. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J Neuroscience. 2011;31:9404–9413. doi: 10.1523/JNEUROSCI.1317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atger VM, de la Llera Moya M, Stoudt GW, Rodrigueza WV, Phillips MC, Rothblat GH. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J Clin Invest. 1997;99:773–780. doi: 10.1172/JCI119223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley DJ. Brain Superhighways. Sci Transl Med. 2012;4:147fs29. doi: 10.1126/scitranslmed.3004611. [DOI] [PubMed] [Google Scholar]
- Begley DJ. Methods for Determining CNS Drug Transport in Animals. In: Paulson O, Moos-Knudsen G, Moos T, editors. Brain Barrier Systems. Alfred Benzon Symposium 45. Munksgaard; Copenhagen: 1999. pp. 91–109. [Google Scholar]
- Camargo F, Erickson RP, Garver WS, Hossain GS, Carbone PN, Heidenreich RA, Blanchard J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sciences. 2001;70:131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- Chen FW, Gordon RE, Ioannou YA. Cyclodextrin induces calcium-dependent lysosomal exocytosis. PLoS One. 2010;5:e15054. doi: 10.1371/journal.pone.0015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson CD, Ali NF, Micseyi MC, et al. Chronic cyclodextrin treatment of murine Nieman-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodzuik H. Molecules with holes- cyclodextrins. In: Dodzuik H, editor. Cyclodextrins and their complexes. Wiley-VCH; Weinheim, Germany: 2006. pp. 1–30. [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteriodogenesis and responds to allopregnanalone. Nature Medicine. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- Kilsdonk EPC, Yancey PG, Stoudt GW, Bangertert FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc Natl Acad Sci USA. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneart V, Tilloy S, Bricout H, Fenart L, Cecchelli R, Monfiler E. Behaviour of α-, β- and γ-cyclodextrins and their derivatives on an in vitro model of blood brain barrier. J Pharm Exp Ther. 2004a;310:745–751. doi: 10.1124/jpet.104.067512. [DOI] [PubMed] [Google Scholar]
- Monnaert V, Betbeder D, Fenart L, et al. Effects of γ-and hydroxypropyl-γ-cyclodextrins on the transport of doxorubicin across an in vitro model of the blood-brain barrier. J Pharm Exp Ther. 2004b;311:1115–1120. doi: 10.1124/jpet.104.071845. [DOI] [PubMed] [Google Scholar]
- Nieuwdorp M, Meuwese MC, Mooij HL, et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J Appl Physiol. 2008;104:845–852. doi: 10.1152/japplphysiol.00440.2007. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Ito S, Matsuda A, Hori S, Abe T, Terasaki T. Brain-to-blood elimination of 24S-hydroxycholesterol from rat brain is mediated by organic anion transporting polypeptide 2 (oatp2) at the blood-brain barrier. J Neurochem. 2007;103:1430–1438. doi: 10.1111/j.1471-4159.2007.04901.x. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Vanier MT, Suzuki K, et al. Niemann-Pick disease type C: a lipid trafficking disorder. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. 8. Vol. 3. New York: McGraw-Hill Medical Publishing Division; 2001. pp. 3611–3633. [Google Scholar]
- Pentchev PG, Comley ME, Kruth HS, Vanier MT, Wegner DA, Patel S, Brady RO. A defect in cholesterol esterification in Niemann-Pick disease (type C) patients. PNAS USA. 1985;82:8247–8251. doi: 10.1073/pnas.82.23.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazzo AP, Höfer CT, Jicsinszky L, et al. Uptake of a fluorescent methyl-β-cyclodextrin via clathrin-dependent endocytosis. Chem and Phys of Lipids. 2012;165:505–511. doi: 10.1016/j.chemphyslip.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum AI, Zhang G, Warren JD, Mayfield FR. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc Natl Acad Sci USA. 2010;107:5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders N, Habgood MP, Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clin Exp Pharmacol Physiol. 1999;26:85–91. doi: 10.1046/j.1440-1681.1999.02987.x. [DOI] [PubMed] [Google Scholar]
- Smith QR, Ziylan YZ, Robinson PJ, Rapoport SI. Kinetics and distribution volumes for tracers of different sizes in the brain plasma space. Brain Res. 1988;462:1–9. doi: 10.1016/0006-8993(88)90577-x. [DOI] [PubMed] [Google Scholar]
- Smith QR. A review of blood-brain barrier transport techniques. The Blood-Brain Barrier Biology and Research Protocols. In: Nag S, editor. Methods in Molecular Medicine. Vol. 89. Humana Press; Totowa, NJ, USA: 2003. pp. 193–208. [DOI] [PubMed] [Google Scholar]
- Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743–1753. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Liu B, Mari Y, Liu B, Repa JJ. Cyclodextrin mediates rapid changes in lipid balance in Npc−/− mice without carrying cholesterol through the bloodstream. J Lipid Res. 2012;53:2331–2342. doi: 10.1194/jlr.M028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg BM, Nieuwdorp M, Stroes ESG, Vink H. Glycocalyx and endothelial (dys) function: From mice to men. Pharmacological Reports. 2006;58(suppl):75–80. [PubMed] [Google Scholar]
- Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in neurons. Biochem Biophys Acta. 2004;1685:16026–16034. doi: 10.1016/j.bbalip.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ward S, O’Donnell P, Fernanadez S, Vite CH. 2-hydroxypropyl-β-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Ped Res. 2010;68:52–56. doi: 10.1203/PDR.0b013e3181df4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PG, Rodrigueza WV, Kilsdonk EPC, Stoudt GW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1996;271:16026–16034. doi: 10.1074/jbc.271.27.16026. [DOI] [PubMed] [Google Scholar]