Abstract
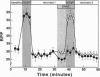
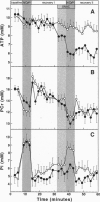
Cardiac myocytes express both constitutive and cytokine-inducible nitric oxide syntheses (NOS). NO and its congeners have been implicated in the regulation of cardiac contractile function. To determine whether NO could affect myocardial energetics, 31P NMR spectroscopy was used to evaluate high-energy phosphate metabolism in isolated rat hearts perfused with the NO donor S-nitrosoacetylcysteine (SNAC). All hearts were exposed to an initial high Ca2+ (3.5 mM) challenge followed by a recovery period, and then, either in the presence or absence of SNAC, to a second high Ca2+ challenge. This protocol allowed us to monitor simultaneously the effect of SNAC infusion on both contractile reserve (i.e., baseline versus high workload contractile function) and high-energy phosphate metabolism. The initial high Ca2+ challenge caused the rate-pressure product to increase by 74 +/- 5% in all hearts. As expected, ATP was maintained as phosphocreatine (PCr) content briefly dropped and then returned to baseline during the subsequent recovery period. Control hearts responded similarLy to the second high Ca2+ challenge, but SNAC-treated hearts did not demonstrate the expected increase in rate-pressure product. In these hearts, ATP declined significantly during the second high Ca2+ challenge, whereas phosphocreatine did not differ from controls, suggesting that phosphoryl transfer by creatine kinase (CK) was inhibited. CK activity, measured biochemically, was decreased by 61 +/- 13% in SNAC-treated hearts compared to controls. Purified CK in solution was also inhibited by SNAC, and reversal could be accomplished with DTT, a sulfhydryl reducing agent. Thus, NO can regulate contractile reserve, possibly by reversible nitrosothiol modification of CK.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak M. I., Ingwall J. S. NMR-invisible ATP in heart: fact or fiction? Am J Physiol. 1992 Jun;262(6 Pt 1):E943–E947. doi: 10.1152/ajpendo.1992.262.6.E943. [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand J. L., Kobzik L., Han X., Kaye D. M., Belhassen L., O'Hara D. S., Kelly R. A., Smith T. W., Michel T. Nitric oxide-dependent parasympathetic signaling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. J Biol Chem. 1995 Jun 16;270(24):14582–14586. doi: 10.1074/jbc.270.24.14582. [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Ungureanu-Longrois D., Simmons W. W., Kobzik L., Lowenstein C. J., Lamas S., Kelly R. A., Smith T. W., Michel T. Induction of NO synthase in rat cardiac microvascular endothelial cells by IL-1 beta and IFN-gamma. Am J Physiol. 1995 Mar;268(3 Pt 2):H1293–H1303. doi: 10.1152/ajpheart.1995.268.3.H1293. [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Ungureanu-Longrois D., Simmons W. W., Pimental D., Malinski T. A., Kapturczak M., Taha Z., Lowenstein C. J., Davidoff A. J., Kelly R. A. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J Biol Chem. 1994 Nov 4;269(44):27580–27588. [PubMed] [Google Scholar]
- Balligand J. L., Ungureanu D., Kelly R. A., Kobzik L., Pimental D., Michel T., Smith T. W. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. J Clin Invest. 1993 May;91(5):2314–2319. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Chen J., Ischiropoulos H., Crow J. P. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- Bittl J. A., Ingwall J. S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985 Mar 25;260(6):3512–3517. [PubMed] [Google Scholar]
- Bolli R. Mechanism of myocardial "stunning". Circulation. 1990 Sep;82(3):723–738. doi: 10.1161/01.cir.82.3.723. [DOI] [PubMed] [Google Scholar]
- Brady A. J., Poole-Wilson P. A., Harding S. E., Warren J. B. Nitric oxide production within cardiac myocytes reduces their contractility in endotoxemia. Am J Physiol. 1992 Dec;263(6 Pt 2):H1963–H1966. doi: 10.1152/ajpheart.1992.263.6.H1963. [DOI] [PubMed] [Google Scholar]
- Brady A. J., Warren J. B., Poole-Wilson P. A., Williams T. J., Harding S. E. Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol. 1993 Jul;265(1 Pt 2):H176–H182. doi: 10.1152/ajpheart.1993.265.1.H176. [DOI] [PubMed] [Google Scholar]
- Brown G. C., Cooper C. E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994 Dec 19;356(2-3):295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- Buechter D. D., Medzihradszky K. F., Burlingame A. L., Kenyon G. L. The active site of creatine kinase. Affinity labeling of cysteine 282 with N-(2,3-epoxypropyl)-N-amidinoglycine. J Biol Chem. 1992 Feb 5;267(4):2173–2178. [PubMed] [Google Scholar]
- Castro L., Rodriguez M., Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994 Nov 25;269(47):29409–29415. [PubMed] [Google Scholar]
- Dimmeler S., Lottspeich F., Brüne B. Nitric oxide causes ADP-ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1992 Aug 25;267(24):16771–16774. [PubMed] [Google Scholar]
- Doupnik C. A., Lim N. F., Kofuji P., Davidson N., Lester H. A. Intrinsic gating properties of a cloned G protein-activated inward rectifier K+ channel. J Gen Physiol. 1995 Jul;106(1):1–23. doi: 10.1085/jgp.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield R. A., Swain J. L. Disruption of myofibrillar energy use: dual mechanisms that may contribute to postischemic dysfunction in stunned myocardium. Circ Res. 1987 Feb;60(2):283–289. doi: 10.1161/01.res.60.2.283. [DOI] [PubMed] [Google Scholar]
- Grocott-Mason R., Anning P., Evans H., Lewis M. J., Shah A. M. Modulation of left ventricular relaxation in isolated ejecting heart by endogenous nitric oxide. Am J Physiol. 1994 Nov;267(5 Pt 2):H1804–H1813. doi: 10.1152/ajpheart.1994.267.5.H1804. [DOI] [PubMed] [Google Scholar]
- Grocott-Mason R., Fort S., Lewis M. J., Shah A. M. Myocardial relaxant effect of exogenous nitric oxide in isolated ejecting hearts. Am J Physiol. 1994 May;266(5 Pt 2):H1699–H1705. doi: 10.1152/ajpheart.1994.266.5.H1699. [DOI] [PubMed] [Google Scholar]
- Hamman B. L., Bittl J. A., Jacobus W. E., Allen P. D., Spencer R. S., Tian R., Ingwall J. S. Inhibition of the creatine kinase reaction decreases the contractile reserve of isolated rat hearts. Am J Physiol. 1995 Sep;269(3 Pt 2):H1030–H1036. doi: 10.1152/ajpheart.1995.269.3.H1030. [DOI] [PubMed] [Google Scholar]
- Han X., Shimoni Y., Giles W. R. An obligatory role for nitric oxide in autonomic control of mammalian heart rate. J Physiol. 1994 Apr 15;476(2):309–314. doi: 10.1113/jphysiol.1994.sp020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. M., Keaney J. F., Jr, Balligand J. L., Loscalzo J., Smith T. W., Colucci W. S. Role of nitric oxide in parasympathetic modulation of beta-adrenergic myocardial contractility in normal dogs. J Clin Invest. 1995 Jan;95(1):360–366. doi: 10.1172/JCI117664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. M., Loh E., Creager M. A., Colucci W. S. Nitric oxide inhibits the positive inotropic response to beta-adrenergic stimulation in humans with left ventricular dysfunction. Circulation. 1995 Oct 15;92(8):2198–2203. doi: 10.1161/01.cir.92.8.2198. [DOI] [PubMed] [Google Scholar]
- Hausladen A., Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem. 1994 Nov 25;269(47):29405–29408. [PubMed] [Google Scholar]
- Hou L. X., Vollmer S. The activity of S-thiomethyl modified creatine kinase is due to the regeneration of free thiol at the active site. Biochim Biophys Acta. 1994 Mar 16;1205(1):83–88. doi: 10.1016/0167-4838(94)90095-7. [DOI] [PubMed] [Google Scholar]
- King C. E., Melinyshyn M. J., Mewburn J. D., Curtis S. E., Winn M. J., Cain S. M., Chapler C. K. Canine hindlimb blood flow and O2 uptake after inhibition of EDRF/NO synthesis. J Appl Physiol (1985) 1994 Mar;76(3):1166–1171. doi: 10.1152/jappl.1994.76.3.1166. [DOI] [PubMed] [Google Scholar]
- Kobzik L., Reid M. B., Bredt D. S., Stamler J. S. Nitric oxide in skeletal muscle. Nature. 1994 Dec 8;372(6506):546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Kobzik L., Stringer B., Balligand J. L., Reid M. B., Stamler J. S. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun. 1995 Jun 15;211(2):375–381. doi: 10.1006/bbrc.1995.1824. [DOI] [PubMed] [Google Scholar]
- Krause S. M. Effect of global myocardial stunning on Ca2(+)-sensitive myofibrillar ATPase activity and creatine kinase kinetics. Am J Physiol. 1990 Sep;259(3 Pt 2):H813–H819. doi: 10.1152/ajpheart.1990.259.3.H813. [DOI] [PubMed] [Google Scholar]
- Krause S. M., Jacobus W. E. Specific enhancement of the cardiac myofibrillar ATPase by bound creatine kinase. J Biol Chem. 1992 Feb 5;267(4):2480–2486. [PubMed] [Google Scholar]
- Kôrge P., Campbell K. B. Iron effects on myocardial enzymes depend on redox state. J Mol Cell Cardiol. 1994 Feb;26(2):151–162. doi: 10.1006/jmcc.1994.1018. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989 Apr;413(6):685–687. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Méry P. F., Lohmann S. M., Walter U., Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Paulus W. J., Vantrimpont P. J., Shah A. M. Acute effects of nitric oxide on left ventricular relaxation and diastolic distensibility in humans. Assessment by bicoronary sodium nitroprusside infusion. Circulation. 1994 May;89(5):2070–2078. doi: 10.1161/01.cir.89.5.2070. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Vodovotz Y., Roche N. S., Sporn M. B., Nathan C. F. Role of nitric oxide in antagonistic effects of transforming growth factor-beta and interleukin-1 beta on the beating rate of cultured cardiac myocytes. Mol Endocrinol. 1992 Nov;6(11):1921–1930. doi: 10.1210/mend.6.11.1282674. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Schulz R., Nava E., Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992 Mar;105(3):575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun. 1994 Oct 14;204(1):169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- Shah A. M., Spurgeon H. A., Sollott S. J., Talo A., Lakatta E. G. 8-bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ Res. 1994 May;74(5):970–978. doi: 10.1161/01.res.74.5.970. [DOI] [PubMed] [Google Scholar]
- Shen W., Xu X., Ochoa M., Zhao G., Wolin M. S., Hintze T. H. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res. 1994 Dec;75(6):1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- Stamler J. S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994 Sep 23;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Chai Y. C., Jung C. H. Protein S-thiolation and dethiolation. Methods Enzymol. 1994;233:385–395. doi: 10.1016/s0076-6879(94)33045-x. [DOI] [PubMed] [Google Scholar]
- Ungureanu-Longrois D., Balligand J. L., Kelly R. A., Smith T. W. Myocardial contractile dysfunction in the systemic inflammatory response syndrome: role of a cytokine-inducible nitric oxide synthase in cardiac myocytes. J Mol Cell Cardiol. 1995 Jan;27(1):155–167. doi: 10.1016/s0022-2828(08)80015-6. [DOI] [PubMed] [Google Scholar]
- Ungureanu-Longrois D., Balligand J. L., Okada I., Simmons W. W., Kobzik L., Lowenstein C. J., Kunkel S. L., Michel T., Kelly R. A., Smith T. W. Contractile responsiveness of ventricular myocytes to isoproterenol is regulated by induction of nitric oxide synthase activity in cardiac microvascular endothelial cells in heterotypic primary culture. Circ Res. 1995 Sep;77(3):486–493. doi: 10.1161/01.res.77.3.486. [DOI] [PubMed] [Google Scholar]
- Ungureanu-Longrois D., Balligand J. L., Simmons W. W., Okada I., Kobzik L., Lowenstein C. J., Kunkel S. L., Michel T., Kelly R. A., Smith T. W. Induction of nitric oxide synthase activity by cytokines in ventricular myocytes is necessary but not sufficient to decrease contractile responsiveness to beta-adrenergic agonists. Circ Res. 1995 Sep;77(3):494–502. doi: 10.1161/01.res.77.3.494. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H. M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992 Jan 1;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. X., Preiss B., Tsou C. L. Kinetics of inactivation of creatine kinase during modification of its thiol groups. Biochemistry. 1988 Jul 12;27(14):5095–5100. doi: 10.1021/bi00414a022. [DOI] [PubMed] [Google Scholar]
- Yang X., Chowdhury N., Cai B., Brett J., Marboe C., Sciacca R. R., Michler R. E., Cannon P. J. Induction of myocardial nitric oxide synthase by cardiac allograft rejection. J Clin Invest. 1994 Aug;94(2):714–721. doi: 10.1172/JCI117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. M., Tsou C. L. The presence of reactive SH groups in the enzymatically active dicyano derivative of creatine kinase. Biochim Biophys Acta. 1987 Jan 30;911(2):136–143. doi: 10.1016/0167-4838(87)90002-1. [DOI] [PubMed] [Google Scholar]