Abstract
BACKGROUND
Arteriovenous graft stenosis leading to thrombosis is a major cause of complications in patients undergoing hemodialysis. Procedural interventions may restore patency but are costly. Although there is no proven pharmacologic therapy, dipyridamole may be promising because of its known vascular antiproliferative activity.
METHODS
We conducted a randomized, double-blind, placebo-controlled trial of extended-release dipyridamole, at a dose of 200 mg, and aspirin, at a dose of 25 mg, given twice daily after the placement of a new arteriovenous graft until the primary outcome, loss of primary unassisted patency (i.e., patency without thrombosis or requirement for intervention), was reached. Secondary outcomes were cumulative graft failure and death. Primary and secondary outcomes were analyzed with the use of a Cox proportional-hazards regression with adjustment for prespecified covariates.
RESULTS
At 13 centers in the United States, 649 patients were randomly assigned to receive dipyridamole plus aspirin (321 patients) or placebo (328 patients) over a period of 4.5 years, with 6 additional months of follow-up. The incidence of primary unassisted patency at 1 year was 23% (95% confidence interval [CI], 18 to 28) in the placebo group and 28% (95% CI, 23 to 34) in the dipyridamole–aspirin group, an absolute difference of 5 percentage points. Treatment with dipyridamole plus aspirin significantly prolonged the duration of primary unassisted patency (hazard ratio, 0.82; 95% CI, 0.68 to 0.98; P = 0.03) and inhibited stenosis. The incidences of cumulative graft failure, death, the composite of graft failure or death, and serious adverse events (including bleeding) did not differ significantly between study groups.
CONCLUSIONS
Treatment with dipyridamole plus aspirin had a significant but modest effect in reducing the risk of stenosis and improving the duration of primary unassisted patency of newly created grafts. (ClinicalTrials.gov number, NCT00067119.)
A functioning vascular access is necessary for hemodialysis. However, vascular-access failure is common and is a major source of complications.1 Vascular access for hemodialysis can be provided by means of an autogenous fistula, arteriovenous graft, or central venous catheter. The arteriovenous graft, typically created by using a synthetic tube connecting an artery and a vein, was until recently the most common type of access used in the United States.2 Grafts are generally more readily cannulated than fistulas and can be used for hemodialysis sooner after surgery. With grafts, however, stenosis frequently develops at the venous anastomosis, leading to access thrombosis,3 and costly procedures may be required to maintain or restore patency.1,4,5 In the United States, the annual cost of procedures related to vascular access has been estimated to exceed $1 billion.1,5
There have been few clinical trials of drug therapy used to reduce the risk of graft dysfunction in patients undergoing hemodialysis, and preventive therapies are lacking. A small clinical trial showed that dipyridamole, with or without aspirin, inhibited thrombosis in newly created hemodialysis grafts.6 Observations of experimental models suggest that dipyridamole acts by inhibiting the proliferation of vascular smooth-muscle cells and the development of stenosis.7–10 Dipyridamole used in combination with aspirin decreases the incidences of progressive stenosis in lower-extremity peripheral vascular disease, late stenosis in coronary-artery bypass grafts, and recurrent strokes.11–13 We performed a randomized, double-blind, placebo-controlled trial involving patients undergoing hemodialysis to determine whether extended-release dipyridamole plus aspirin inhibits stenosis of the vascular access and prolongs primary unassisted patency of newly created arteriovenous grafts.
METHODS
ROLE OF THE SPONSOR
The National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, sponsored the study. Boehringer Ingelheim provided the extended-release dipyridamole plus aspirin (Aggrenox), matching placebo, and financial support but was not involved in the design, analysis, interpretation of the study data, or preparation of the manuscript.
ELIGIBILITY OF PATIENTS
Patients were enrolled at 13 clinical sites in the United States; details of the study design and inclusion and exclusion criteria have been published previously.14 Briefly, patients who had attained the age of majority (those at least 18 years of age, depending on the state) were eligible if they were scheduled to have a new arteriovenous graft placed for the purpose of hemodialysis and were currently undergoing long-term hemodialysis or were expected to undergo it within 6 months after randomization.
Patients were ineligible if they were pregnant or breast-feeding; had an increased risk of bleeding or a known bleeding disorder; had active esophagitis, gastritis, or peptic ulcer disease; had a platelet count of less than 75,000 per cubic millimeter; had advanced liver disease; or required an anticoagulant or antiplatelet agent other than aspirin. Patients with a known allergy or adverse reaction to extended-release dipyridamole plus aspirin or with uncontrolled hypertension were also ineligible.
The institutional review board of each participating clinical center approved the study protocol. Each patient provided written informed consent before enrollment.
STUDY DESIGN AND PROCEDURES
Recruitment began on January 1, 2003, and ended on July 31, 2007; follow-up of patients continued until January 31, 2008. Demographic, clinical, and laboratory data were collected during screening. No more than 2 days after successful access surgery (i.e., placement of a patent graft), patients were randomly assigned to receive treatment with one capsule of dipyridamole plus aspirin or an identical-looking placebo twice daily. Each capsule of dipyridamole plus aspirin contained 200 mg of extended-release dipyridamole plus 25 mg of immediate-release aspirin. Randomization was stratified according to clinical center and access location (forearm or alternative site) with the use of a random permuted-block design. Study personnel and patients were unaware of the treatment assignments. Treatment continued until the occurrence of the primary outcome but was terminated early if the patient was switched to another type of renal-replacement therapy or was transferred to a nonparticipating dialysis unit or if an exclusion criterion developed.
Patients were followed monthly after graft surgery to examine the access site; to record access-related complications, adverse drug reactions, and hospitalizations; and to assess adherence to the study medication regimen by means of a pill count. Follow-up was continued until 1 month after the occurrence of the primary outcome. Blood-flow rates at the access site were measured each month after the patient started hemodialysis, with the use of the ultrasound indicator dilution technique (see the Supplementary Appendix, available with the full text of this article at NEJM.org),15 which provided a uniform studywide approach to detect a decrease in the blood-flow rate indicative of stenosis before it resulted in thrombosis.14
OUTCOMES
The primary outcome was loss of primary unassisted graft patency (i.e., patency without thrombosis or requirement for intervention), defined as the first occurrence of graft thrombosis, an access procedure performed to correct a stenosis of 50% or more of the diameter of the adjacent normal vessel, or other surgical modification of the graft (e.g., for infection). Patients were referred for angiography if the blood-flow rate at the access site declined to less than 600 ml per minute or if the rate declined to less than 1000 ml per minute and the reduction from the baseline rate was more than 25% (see the Supplementary Appendix).14 A sample of angiograms from each clinical center was reviewed in a blinded manner to confirm that the indication for intervention was uniform across study sites.
For patients undergoing regular hemodialysis with the use of a catheter, complete graft failure was defined by failure to use the graft by 12 weeks after placement. For patients not yet undergoing dialysis, loss of graft patency was defined by the absence of both a bruit and thrill.
The major secondary outcomes were cumulative graft failure, defined as the time from randomization to complete loss of the access site for dialysis, death from any cause, and a combined outcome of death from any cause or cumulative graft failure, each of which was ascertained until the administratively defined end date of the study.
STATISTICAL ANALYSIS
Cumulative incidence curves were prepared with the use of the Kaplan–Meier method. The primary and secondary outcomes were analyzed by means of Cox proportional-hazards regression with stratification on the basis of clinical center, access location (forearm or other site), and adjustment, except as otherwise noted, for serum albumin level and use of angiotensin-converting–enzyme inhibitors or angiotensin-receptor blockers at baseline.16,17 Data from each patient were analyzed according to the patient’s randomized treatment assignment, irrespective of medication adherence. Follow-up data with regard to the primary analysis were censored at the administrative end date of the study or at the time of death, a switch to an alternative form of renal-replacement therapy, or the patient’s transfer to a clinical center not involved in the trial, whichever came first. Follow-up data for cumulative graft failure were censored at the time of death or the administrative end date, whichever came first, whereas data for analyses of death and the composite of cumulative graft failure and death were censored at the administrative end date. All reported P values are two-sided and were not adjusted for multiple testing.
Interaction terms between treatment with extended-release dipyridamole plus aspirin and nine prespecified baseline factors were individually added to the primary Cox proportional-hazards model in an exploratory analysis of whether the intervention had different effects on the primary patency outcome among the subgroups defined on the basis of those factors (see the Supplementary Appendix). Subgroups categorized on the basis of continuous variables (age and serum albumin level) were defined on the basis of the median values for these variables.
We aimed to enroll 1056 patients in order to provide a statistical power of 85% to detect a 25% reduction in the incidence of the primary outcome, with a two-sided type I error rate of 5%.14 Assumptions for the power calculations included a predicted rate of loss of primary unassisted patency in the placebo group of 0.54 event per patient-year,17–20 a rate of death or loss to follow-up of 22% per patient-year in the placebo group,21 an annual dropout rate of 15% for the dipyridamole–aspirin group, and a 1% rate of a switch to active treatment in the placebo group. Enrollment was initially planned to occur over a 3-year period, with an additional 1 year of follow-up, but because of slower-than-expected enrollment, the enrollment period was extended to 4.5 years, with 6 additional months of follow-up.
An independent data and safety monitoring board regularly reviewed the progress of the study for safety and efficacy. Five planned interim analyses were performed before the final analysis to provide a guideline as to whether the study should be stopped early because of efficacy or futility. A Lan–DeMets spending function was used, with stopping boundaries derived from the Wang–Tsiatis class and shape parameters of 0 (corresponding to the O’Brien–Fleming stopping rule).22
RESULTS
STUDY POPULATION
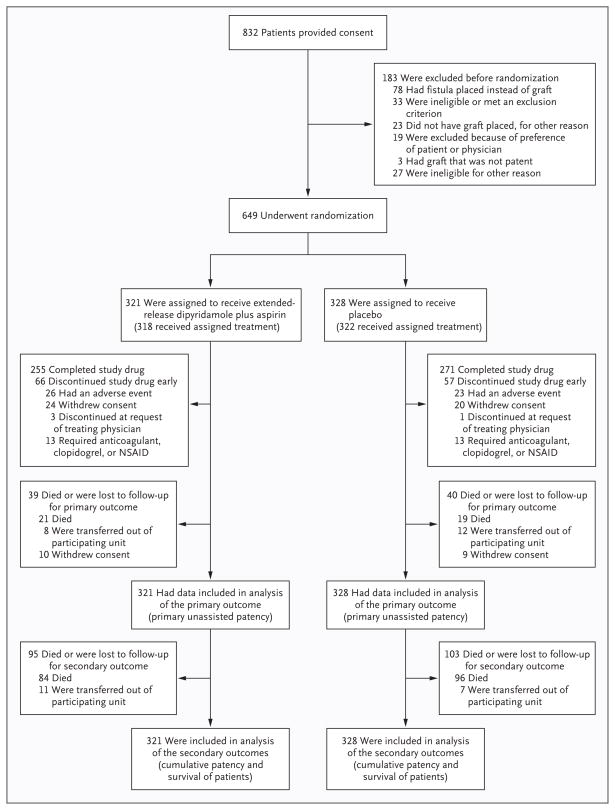
A total of 832 patients provided written informed consent, and 649 were randomly assigned to receive extended-release dipyridamole plus aspirin (321 patients) or placebo (328 patients) (Fig. 1). The most common reason for exclusion before randomization was creation of a fistula instead of placement of a graft. The stopping boundaries for efficacy and futility were not crossed in any of the five interim analyses, and the trial proceeded to its planned final analysis. The recruitment goal was not achieved, primarily because of greater-than-expected numbers of patients with fistulas rather than grafts or ineligibility due to receipt of exclusionary medications or coexisting conditions.
Figure 1. Study Enrollment and Follow-up.
NSAID denotes nonsteroidal antiinflammatory drug.
Baseline characteristics in the two study groups were similar (Table 1), as were graft blood-flow rates at the time of baseline measurement (see the Supplementary Appendix). Among the patients who had undergone randomization, 39 (12%) receiving dipyridamole plus aspirin and 40 (12%) receiving placebo died or were lost to follow-up before the primary outcome was reached (Fig. 1). At the time of study termination, an additional 95 patients (30%) receiving extended-release dipyridamole plus aspirin and 103 (31%) receiving placebo had died or were lost to follow-up for determination of the secondary outcomes of cumulative graft failure and death. All patients who underwent randomization were included in their assigned groups for analysis of the primary and secondary outcomes.
Table 1.
Baseline Characteristics of the Patients, According to Study Group.*
| Characteristic | Extended-Release Dipyridamole plus Aspirin (N = 321) | Placebo (N = 328) |
|---|---|---|
| Age (yr) | 59.1±13.5 | 57.5±14.9 |
| Male sex (%) | 41 | 38 |
| Black race (%)† | 72 | 70 |
| Body-mass index‡ | 30.8±8.6 | 30.5±8.2 |
| Blood pressure (mm Hg) | ||
| Systolic | 144±26 | 143±24 |
| Diastolic | 78±14 | 77±15 |
| Diabetes mellitus (%) | 66 | 60 |
| Cardiovascular disease (%) | 39 | 42 |
| Cerebrovascular disease (%) | 15 | 16 |
| Peripheral arterial disease (%) | 17 | 15 |
| Venous thromboembolic disease (%) | 3 | 2 |
| Aspirin use (%) | 40 | 44 |
| Use of ACE inhibitor or ARB (%) | 51 | 56 |
| Current tobacco use (%) | 14 | 17 |
| Hemoglobin (g/dl) | 11.9±1.7 | 11.6±1.7 |
| Serum albumin (g/dl) | 3.7±0.5 | 3.7±0.5 |
| No. of previous arteriovenous fistulas or grafts placed (%)§ | ||
| 0 | 48 | 49 |
| 1 | 26 | 25 |
| ≥2 | 25 | 25 |
| Current access through central venous catheter (%) | 63 | 66 |
| Hemodialysis initiated before graft placement (%) | 71 | 74 |
| Duration of hemodialysis before randomization (mo)¶ | 23.3±28.6 | 28.3±43.2 |
Plus–minus values are means ±SD. Data were compared between the two study groups with the use of t-tests for continuous variables and chi-square tests for categorical variables (P>0.05 for all comparisons). Cardiovascular disease was defined by a history of myocardial infarction, angina, coronary-artery angioplasty, coronary-artery bypass grafting, or congestive heart failure. Cerebrovascular disease was defined by a history of stroke, transient ischemic attack, or carotid endarterectomy. Peripheral arterial disease was defined by a history of nontraumatic amputation, angioplasty or bypass surgery of the leg or foot, or claudication. Venous thromboembolic disease was defined by a history of deep-vein thrombosis or pulmonary embolism. ACE denotes angiotensin-converting enzyme, and ARB angiotensin-receptor blocker.
Race was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The number of previous arteriovenous fistulas or grafts was unknown for two patients receiving dipyridamole plus aspirin and three patients receiving placebo.
The duration of hemodialysis before enrollment was calculated for the patients who started hemodialysis before randomization: 229 patients receiving dipyridamole plus aspirin and 244 patients receiving placebo. Ten patients receiving dipyridamole plus aspirin and four patients receiving placebo had been undergoing long-term maintenance dialysis for an unknown length of time before randomization but had stopped the therapy for a variety of reasons (e.g., renal transplantation); the duration of this previous dialysis that had been stopped before randomization was not included in the calculation of dialysis duration.
Most patients (94%) received grafts composed of expanded polytetrafluoroethylene; 5% of patients received grafts made of another synthetic material, and 1% received a graft composed of biologic material (e.g., a cryopreserved saphenous vein). Grafts were placed in the forearm (in 49% of patients), the upper arm (in 44%), the leg (in 6%), the chest (in 1%), or another location (in <1%). There were no significant differences on the basis of graft composition or location between the two study groups.
DELIVERY OF STUDY DRUG AND ADHERENCE
The median time from surgical placement of the graft to the initiation of the study medication was 2.1 hours. The study drug was discontinued before ascertainment of the primary outcome in 66 patients (21%) receiving dipyridamole plus aspirin and in 57 (17%) receiving placebo (Fig. 1). The rate of adherence to the study medication regimen, as estimated from pill counts, was 83% in both groups.
EFFICACY
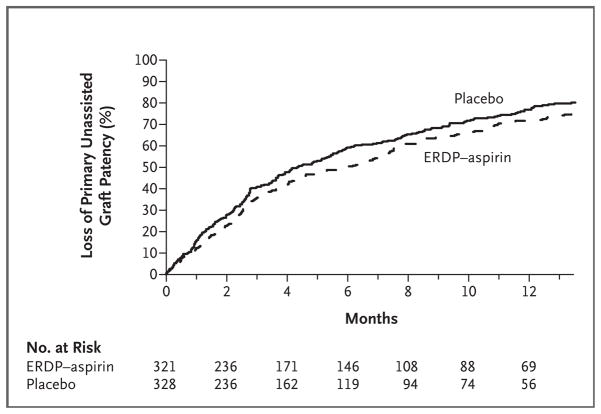
The incidence of primary unassisted patency at 1 year was 23% (95% confidence interval [CI], 18 to 28) in the placebo group and 28% (95% CI, 23 to 34) in the dipyridamole–aspirin group, an absolute difference of 5 percentage points (Fig. 2). Thus, treatment with dipyridamole plus aspirin significantly prolonged the duration of primary unassisted graft patency as compared with placebo. After adjustment for the prespecified factors, the hazard ratio for loss of primary unassisted graft patency in the dipyridamole–aspirin group, as compared with the placebo group, was 0.82 (95% CI, 0.68 to 0.98; P = 0.03) (Table 2), for a relative reduction in the rate of loss of primary unassisted graft patency of 18%. The corresponding unadjusted hazard ratio was 0.81 (95% CI, 0.68 to 0.97; P = 0.02).
Figure 2. Kaplan–Meier Estimates of the Cumulative Incidence of Loss of Primary Unassisted Graft Patency, According to Study Group.
The median duration of patency was 5.8 months (95% confidence interval [CI], 4.3 to 7.1) in the extended-release dipyridamole (ERDP)–aspirin group and 4.3 months (95% CI, 3.6 to 5.4) in the placebo group.
Table 2.
Incidences of Primary and Secondary Outcomes, According to Study Group.*
| Outcome | Extended-Release Dipyridamole plus Aspirin (N = 321) | Placebo (N = 328) | Hazard Ratio (95% CI) |
|---|---|---|---|
| number (percent) | |||
| Primary outcomes | |||
|
| |||
| Loss of primary unassisted patency | 256 (80) | 274 (84) | 0.82 (0.68–0.98)† |
|
| |||
| Thrombosis | 127 (40) | 139 (42) | 0.84 (0.65–1.08) |
|
| |||
| With stenosis ≥50%, on angiography | 69 (21) | 79 (24) | 0.74 (0.53–1.04) |
|
| |||
| With stenosis <50%, on angiography | 5 (2) | 3 (1) | 1.59 (0.38–6.70) |
|
| |||
| Angiography not performed | 53 (17) | 57 (17) | 0.95 (0.64–1.39) |
|
| |||
| Angioplasty | |||
|
| |||
| Stenosis ≥50%, no thrombosis | 93 (29) | 103 (31) | 0.70 (0.52–0.95) |
|
| |||
| Stenosis <50%, no thrombosis | 1 (0.3) | 4 (1) | 0.29 (0.03–2.79) |
|
| |||
| Procedure performed for infection | 21 (7) | 14 (4) | 1.54 (0.75–3.17) |
|
| |||
| Procedure performed for other reason‡ | 10 (3) | 6 (2) | 1.75 (0.58–5.30) |
|
| |||
| Failure to use graft by wk 12 in patients with catheter for access | 4 (1) | 8 (2) | 0.48 (0.14–1.59) |
|
| |||
| Stenosis ≥50%, with or without thrombosis | 162 (50) | 182 (55) | 0.72 (0.57–0.90)§ |
|
| |||
| Secondary outcomes | |||
|
| |||
| Cumulative graft failure | 161 (50) | 173 (53) | 0.95 (0.76–1.19) |
|
| |||
| Death | 105 (33) | 115 (35) | 1.00 (0.76–1.31) |
|
| |||
| Cumulative graft failure or death | 208 (65) | 218 (66) | 1.01 (0.83–1.24) |
Data were compared between the two study groups with the use of a Cox proportional-hazards model with the prespecified adjustments for serum albumin level and use or nonuse of an angiotensin-converting–enzyme inhibitor or angiotensin-receptor blocker. The unadjusted hazard ratio for the primary outcome of loss of primary unassisted patency in the dipyridamole–aspirin group was 0.81 (95% confidence interval [CI], 0.68 to 0.97; P = 0.02).
P = 0.03.
Procedures performed for other reasons were as follows: in the dipyridamole–aspirin group, access ligation for hand ischemia (in three patients), angioplasty or access ligation for central vein stenosis (three patients), surgical revision for pseudoaneurysm without stenosis (two patients), access ligation for arm edema without stenosis (one patient), and ligation for uncontrolled bleeding without angiography (one patient); and in the placebo group, access ligation for hand ischemia (in four patients), angioplasty or access ligation for central vein stenosis (one patient), and surgical revision for pseudo-aneurysm without stenosis (one patient).
P = 0.005.
The hazard ratio for loss of primary unassisted graft patency in the dipyridamole–aspirin group, as compared with the placebo group, was 0.76 (95% CI, 0.60 to 0.96; P = 0.02) among patients not receiving aspirin at baseline and 0.92 (95% CI, 0.68 to 1.24; P = 0.57) among those receiving aspirin at baseline (see Table 1 in the Supplementary Appendix). However, there was no significant interaction between baseline use of aspirin and the assigned study treatment (P = 0.33). There was no significant heterogeneity in the efficacy of the two study treatments across other predefined subgroups (Table 1 in the Supplementary Appendix).
The reasons for loss of graft patency are shown in Table 2. At the time of study termination, the primary outcome had occurred in 80% of patients receiving dipyridamole plus aspirin and 84% of those receiving placebo. Thrombosis was the most common overall cause of the primary outcome, occurring in 40% of patients receiving dipyridamole plus aspirin and in 42% of those receiving placebo. Angiography was performed in 59% of all patients with thrombosis, and stenosis of 50% or more was found in more than 90% of these patients. Overall, clinically significant stenosis (≥50%) with or without a preceding thrombosis was documented in 50% of patients receiving extended-release dipyridamole plus aspirin and 55% receiving placebo, corresponding to an overall 28% reduction in the rate of stenosis from treatment with dipyridamole plus aspirin (hazard ratio, 0.72; 95% CI, 0.57 to 0.90; P = 0.005).
The median duration of cumulative graft patency was 22.5 months (95% CI, 20.0 to 28.2) in the placebo group. Treatment with dipyridamole plus aspirin, administered until the primary outcome was reached, did not significantly decrease the incidence of cumulative graft failure, death, or the composite of these two outcomes (Table 2).
ADVERSE EVENTS
Serious adverse events occurred in 175 patients receiving dipyridamole plus aspirin (55%, or 1.19 events per patient-year) and in 174 patients receiving placebo (53%, or 1.47 events per patient-year; hazard ratio for dipyridamole plus aspirin, 0.93; 95% CI, 0.75 to 1.15). Table 3 lists the percentages of patients in whom selected subcategories of serious adverse events occurred and the event rate per patient-year. There was no significant difference between the two study groups with regard to any adverse-event category. Bleeding occurred in 37 patients receiving dipyridamole plus aspirin (12%, or 0.20 event per patient-year) and in 40 patients receiving placebo (12%, or 0.24 event per patient-year) (hazard ratio for dipyridamole plus aspirin, 0.86; 95% CI, 0.55 to 1.35).
Table 3.
Rates of Adverse Events, According to Study Group.*
| Serious Adverse Event | Extended-Release Dipyridamole plus Aspirin (N = 321) | Placebo (N = 328) | Hazard Ratio for Event Rate (95% CI)† | ||||
|---|---|---|---|---|---|---|---|
| No. of Patients (%) | No. of Events | Event Rate | No. of Patients (%) | No. of Events | Event Rate | ||
| no./patient-yr | no./patient-yr | ||||||
|
| |||||||
| Any | 175 (55) | 331 | 1.19 | 174 (53) | 360 | 1.47 | 0.93 (0.75–1.15) |
|
| |||||||
| Bleeding‡ | 37 (12) | 42 | 0.20 | 40 (12) | 50 | 0.24 | 0.86 (0.55–1.35) |
|
| |||||||
| Intermediate or minor | 23 (7) | 24 | 0.12 | 26 (8) | 33 | 0.15 | |
|
| |||||||
| Major | 6 (2) | 7 | 0.03 | 9 (3) | 9 | 0.05 | |
|
| |||||||
| Life-threatening | 9 (3) | 9 | 0.05 | 8 (2) | 8 | 0.05 | |
|
| |||||||
| Fatal | 2 (1) | 2 | 0.01 | 0 | 0 | 0.00 | |
|
| |||||||
| Hospitalization | 172 (54) | 280 | 1.17 | 171 (52) | 301 | 1.44 | 0.93 (0.75–1.15) |
|
| |||||||
| For ischemic heart disease | 19 (6) | 23 | 0.10 | 18 (5) | 23 | 0.11 | |
|
| |||||||
| For congestive heart failure | 19 (6) | 25 | 0.10 | 20 (6) | 31 | 0.12 | |
|
| |||||||
| For arrhythmia | 8 (2) | 9 | 0.04 | 10 (3) | 12 | 0.06 | |
|
| |||||||
| For cerebrovascular disease | 5 (2) | 6 | 0.03 | 3 (1) | 6 | 0.02 | |
|
| |||||||
| For peripheral vascular disease | 13 (4) | 15 | 0.07 | 6 (2) | 7 | 0.04 | |
|
| |||||||
| For event at site of study graft | 62 (19) | 71 | 0.34 | 48 (15) | 52 | 0.29 | |
|
| |||||||
| For event at other vascular access site | 14 (4) | 14 | 0.07 | 15 (5) | 16 | 0.09 | |
|
| |||||||
| Death | 17 (5) | 17 | 0.09 | 13 (4) | 13 | 0.07 | 1.20 (0.58–2.47) |
A patient-level analysis was used for event rates, which were calculated as the ratio of the number of first serious adverse event of each type to the total number of patient-years of follow-up until the first serious adverse event of this type occurred in each patient.
P>0.15 for each type of serious adverse event.
Intermediate or minor bleeding events were those that were not classified as major, life-threatening, or fatal. Major bleeding was defined as confirmed retroperitoneal, intraarticular, intraocular, or intracranial bleeding or any bleeding that led to a drop in the hemoglobin level of 2 g per deciliter and required hospitalization or transfusion. Life-threatening bleeding was defined as any bleeding that led to a drop in the hemoglobin level of 5 g per deciliter or more, required emergency surgical intervention, caused a symptomatic intracranial hemorrhage, or required a transfusion of more than 4 units of packed red cells or whole blood. Fatal bleeding was any bleeding that caused or precipitated death.
DISCUSSION
Our study of patients receiving a new arteriovenous graft for hemodialysis access showed that treatment with dipyridamole plus aspirin, as compared with placebo, resulted in a significant but modest decrease in the cumulative incidence of loss of primary unassisted graft patency (absolute reduction, 5 percentage points; relative reduction, 18%) and in the incidence of clinically significant graft stenosis. The frequency of bleeding or other serious adverse events was not increased with active treatment. The burden of graft failure was substantial, with over three fourths of patients requiring an intervention to maintain patency or to treat another access site complication within the first year after graft placement.
Currently, the principal approach to maintaining the patency of grafts for hemodialysis access is the use of procedural interventions (e.g., angioplasty) to treat stenosis or thrombosis after it develops.23 The need for repeated procedures to maintain patency and the need for temporary alternatives to vascular access, such as central venous catheters, contribute greatly to the cost and morbidity associated with hemodialysis.1,4,5 Despite the enormous burden, there have been few clinical trials conducted to address this problem.24
The improvement in graft patency seen in our study is similar to that in a previous, single-center trial of dipyridamole to prevent thrombosis in newly placed arteriovenous grafts.6 Clinical trials of dipyridamole plus aspirin have also shown a reduction in the incidences of late stenosis in coronary-artery bypass grafts, progression of peripheral vascular disease, and recurrent stroke.11–13,25,26
In contrast, several previous randomized trials of antithrombotic therapies have failed to demonstrate a significant improvement in the patency of arteriovenous grafts in patients undergoing hemodialysis.27,28 The Department of Veterans Affairs (VA) Cooperative Trial of aspirin plus clopidogrel in patients undergoing hemodialysis who had an established graft was terminated early owing to an increased risk of bleeding.27 That trial failed to show a benefit of the drug, as compared with placebo, for the prevention of thrombosis (hazard ratio, 0.81; 95% CI, 0.49 to 1.40). Similarly, a clinical trial of low-dose warfarin showed an increased risk of bleeding without a reduction in the risk of arteriovenous graft thrombosis.28
The earlier trials raised concern that the low-dose aspirin in dipyridamole plus aspirin might cause excess bleeding.27,28 Accordingly, we excluded patients at high risk for bleeding. The rate of bleeding among our patients assigned to receive placebo was half that observed in the VA Cooperative Trial.27 Moreover, the risk of bleeding was not further increased by treatment with dipyridamole plus aspirin. An increased risk of bleeding with extended-release dipyridamole plus aspirin may have been masked by the short duration of use of the drug, the frequent use of aspirin that was not part of the active study treatment, or the exclusion of patients at high risk for bleeding. The results are reassuring regarding the safety of dipyridamole plus aspirin in the selected population, but caution must be exercised in extrapolating the results to the general population of patients undergoing dialysis.
Approximately three fourths of the study patients had a loss of graft patency at 1 year, a rate that was substantially higher than the 54% rate originally predicted. This greater-than-expected loss of graft patency may reflect the characteristics of the study patients. Many of the patients were women, many were black, and many had diabetes or a history of vascular disease — all risk factors for vascular access failure.4,20,29,30 The frequency of each of these risk factors was higher in our study population than in the general hemodialysis population in the United States31,32 and was substantially higher than that among patients in the Dialysis Access Consortium (DAC) Fistula Trial (ClinicalTrials.gov number, NCT00067119), a trial performed concurrently with our study at a subgroup of the clinical centers.33 The preponderance of patients at high risk for graft failure in our study population may reflect the ongoing national trend toward the use of autogenous fistula placement, with a corresponding decrease in the rate of graft placement.34 A fistula is used in patients who have suitable vascular anatomy, whereas a graft is used by default in other patients. Monthly blood-flow monitoring was performed to comply with clinical practice guidelines and focus the primary study outcome on the detection of stenosis rather than thrombosis.14 Although flow monitoring might have increased the rate of loss of primary patency, only 24% of all cases of the primary outcome were precipitated by flow monitoring, and flow monitoring probably played a minor role in the observed high rate of loss of graft patency.
Although the ability of dipyridamole plus aspirin to inhibit graft failure was significant, the clinical effect of this therapy was modest. On the basis of the Kaplan–Meier estimates, this effect can be expressed as an aggregate delay of 6 weeks in the median time to loss of primary patency in all patients or as a reduction in the number of patients with primary graft failure by 1 patient for every 20 patients treated for 1 year. The efficacy of dipyridamole plus aspirin may be greater in the subgroup of patients not already receiving aspirin; however, our study did not have sufficient power to address this question. Treatment with dipyridamole plus aspirin costs between $500 and $2,200 per year per person, and formal cost-effectiveness analysis is needed before clinical-practice recommendations can be made.
This study has certain limitations. We did not meet the enrollment goal, despite the addition of recruiting sites and extension of the recruitment period. The adverse effect of enrolling fewer patients than planned was partially offset by the higher-than-anticipated rate of graft failure, such that the statistical power of the study was maintained. However, the smaller-than-planned group of patients may limit the applicability of the subgroup analyses. In addition, because the study drug was stopped after the primary outcome was reached, the possible benefit of continued treatment with dipyridamole plus aspirin on cumulative graft patency could not be evaluated.
In conclusion, our study shows that treatment with extended-release dipyridamole plus aspirin results in a significant but clinically modest improvement in primary unassisted graft patency. As is consistent with the known antiproliferative effects of extended-release dipyridamole plus aspirin, the results suggest that strategies targeting neointimal hyperplasia may be an important area for future research.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (under cooperative agreements U01DK058981, U01DK058982, U01DK058973, U01DK058985, U01DK058978, U01DK058968, U01DK058986, and U01DK058966) and Boehringer Ingelheim Pharmaceuticals, which also provided the extended-release dipyridamole plus aspirin and matched placebo.
Dr. Dixon reports receiving consulting fees from Proteon Therapeutics and Pervasis Therapeutics, lecture fees from Renal Advantage, and grant support from Proteon Therapeutics; Dr. Beck, grant support from Boehringer Ingelheim Pharmaceuticals; Dr. Delmez, consulting fees from Abbott Laboratories and Genzyme and grant support from Genzyme; Dr. Allon, consulting fees from Angiotech; Dr. Dember, consulting fees from Proteon Therapeutics and Bellus Health (formerly Neurochem) and grant support from Neurochem; Dr. Himmelfarb, consulting fees from Boehringer Ingelheim Pharmaceuticals; Dr. Fenves, lecture fees from Novartis; Dr. Kaufman, consulting fees from Proteon Therapeutics; Dr. Rahman, lecture fees from Boehringer Ingelheim Pharmaceutical, GlaxoSmithKline, Merck, Novartis, and Ortho Biotech; and Dr. Feldman, consulting fees from GE Healthcare for providing expert testimony and grant support from Amgen–Research Triangle Institute and GE Healthcare. Dr. Kusek reports holding equity in Eli Lilly, Pfizer, and DeCode Genetics.
APPENDIX
Members of the DAC Graft Study Group were as follows: Boston University Medical Center — L. Dember, J. Kaufman, M. Hawley, A. Lauer, P. LeSage, R. Nathan, E. Holmberg; Baystate Medical Center — G. Braden, M. Ryan, A. Berkowitz; Charleston Area Medical Center — A. Rahman, B. Lucas, Jr., R. Santos, B. Reyes; Duke University Medical Center — A. Greenberg, M. Berkoben, E. Kovalik, J. Lawson, J. Middleton, S, Schwab, D. Schumm, S. Adams, K. Gitter, T. Cantaffa, A. Quarles; Emory University — J. Work, S. Rhodes; Maine Medical Center — J. Himmelfarb, J. Whiting, J. Kane, S. Freedman, R. Violette, H. Cyr-Alves, K. Garrison; Saint Louis University — K. Martin, P Schmitz, V. Jenkins; Tyler Nephrology Associates — J. Cotton, Jr., E. Husband; University of Alabama at Birmingham — M. Allon, M. Robbin, M. Lockhart, B. Casey, J. Newsome; University of Iowa — B. Dixon, B. Franzwa, L. Hunsicker, J. Hoballah, D. Katz, W. Sharp, T. Kresowik, Y. Wu, S Rayhill; Renal Core Associates —— T. Pflederer, K. DuPage, K. Welch, F. Darras, A. Banqero, B. Ketel, A. Wounded Arrow, C. Grant, J. Deeb, L. Pyszka, Covenant Medical Center — M. Slavin, D. Wedeking; University of Texas Southwestern Medical Center — M. Vazquez, I. Davidson, R. Toto, L. Littmon, C. Ying, T. Lightfoot, H. Quinones, R. Saxena, P. Clagett, J. Valentine, B. Dolmatch, J. Thompson; Baylor University Medical Center — A. Fenves, G. Pearl; Vanderbilt University Medical Center — A. Ikizler, P. Egbert; Vascular Surgery Associates — J. McNeil, D. Holmes, W. Freiberger; Washington University in St. Louis — J. Delmez, D. Windus, D. Coyne, M. Rothstein, S. Shenoy, R. Creaghan, B. Lluka; National Institute of Diabetes and Digestive and Kidney Diseases — J. Kusek, C. Meyers; Steering Committee Chair — H. Feldman (University of Pennsylvania); Data Coordinating Center (Cleveland Clinic Foundation) — G. Beck, J. Gassman, T. Greene, B. Hu, S. Bi, A. Liu, M. Radeva, L. Tuason, B. Weiss; Data and Safety Monitoring Board — N. Levin (chair), A. Besarab, G. Chertow, M. Diener-West, T. Louis, W. McClellan, C. Stehman–Breen.
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7:523–35. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System. 2006 Annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 3.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–27. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 4.Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO. Vascular access survival and incidence of revisions: a comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg. 2001;34:694–700. doi: 10.1067/mva.2001.117890. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Renal Data System. 2007 Annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 6.Sreedhara R, Himmelfarb J, Lazarus JM, Hakim RM. Anti-platelet therapy in graft thrombosis: results of a prospective, randomized, double-blind study. Kidney Int. 1994;45:1477–83. doi: 10.1038/ki.1994.192. [DOI] [PubMed] [Google Scholar]
- 7.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Adenosine inhibits growth of human aortic smooth muscle cells via A2B receptors. Hypertension. 1998;31:516– 21. doi: 10.1161/01.hyp.31.1.516. [DOI] [PubMed] [Google Scholar]
- 8.Ingerman-Wojenski CM, Silver MJ. Model system to study interaction of platelets with damaged arterial wall. II. Inhibition of smooth muscle cell proliferation by dipyridamole and AH-P719. Exp Mol Pathol. 1988;48:116–34. doi: 10.1016/0014-4800(88)90050-0. [DOI] [PubMed] [Google Scholar]
- 9.Singh JP, Rothfuss KJ, Wiernicki TR, et al. Dipyridamole directly inhibits vascular smooth muscle cell proliferation in vitro and in vivo: implications in the treatment of restenosis after angioplasty. J Am Coll Cardiol. 1994;23:665–71. doi: 10.1016/0735-1097(94)90752-8. [DOI] [PubMed] [Google Scholar]
- 10.Faxon DP, Sanborn TA, Haudenschild CC, Ryan TJ. Effect of antiplatelet therapy on restenosis after experimental angioplasty. Am J Cardiol. 1984;53:72C–76C. doi: 10.1016/0002-9149(84)90751-3. [DOI] [PubMed] [Google Scholar]
- 11.Chesebro JH, Fuster V, Elveback LR, et al. Effect of dipyridamole and aspirin on late vein-graft patency after coronary bypass operations. N Engl J Med. 1984;310:209–14. doi: 10.1056/NEJM198401263100401. [DOI] [PubMed] [Google Scholar]
- 12.Hess H, Mietaschk A, Deichsel G. Drug-induced inhibition of platelet function delays progression of peripheral occlusive arterial disease: a prospective double-blind arteriographically controlled trial. Lancet. 1985;1:415–9. doi: 10.1016/s0140-6736(85)91144-4. [DOI] [PubMed] [Google Scholar]
- 13.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 14.Dixon BS, Beck GJ, Dember LM, et al. Design of the Dialysis Access Consortium (DAC) Aggrenox Prevention of Access Stenosis Trial. Clin Trials. 2005;2:400–12. doi: 10.1191/1740774505cn110oa. [DOI] [PubMed] [Google Scholar]
- 15.Depner TA, Krivitski NM. Clinical measurement of blood flow in hemodialysis access fistulae and grafts by ultrasound dilution. ASAIO J. 1995;41:M745–M749. doi: 10.1097/00002480-199507000-00112. [DOI] [PubMed] [Google Scholar]
- 16.Gradzki R, Dhingra RK, Port FK, Roys E, Weitzel WF, Messana JM. Use of ACE inhibitors is associated with prolonged survival of arteriovenous grafts. Am J Kidney Dis. 2001;38:1240–4. doi: 10.1053/ajkd.2001.29220. [DOI] [PubMed] [Google Scholar]
- 17.Miller PE, Carlton D, Deierhoi MH, Redden DT, Allon M. Natural history of arteriovenous grafts in hemodialysis patients. Am J Kidney Dis. 2000;36:68–74. doi: 10.1053/ajkd.2000.8269. [DOI] [PubMed] [Google Scholar]
- 18.Cinat ME, Hopkins J, Wilson SE. A prospective evaluation of PTFE graft patency and surveillance techniques in hemodialysis access. Ann Vasc Surg. 1999;13:191–8. doi: 10.1007/s100169900241. [DOI] [PubMed] [Google Scholar]
- 19.Hodges TC, Fillinger MF, Zwolak RM, Walsh DB, Bech F, Cronenwett JL. Longitudinal comparison of dialysis access methods: risk factors for failure. J Vasc Surg. 1997;26:1009–19. doi: 10.1016/s0741-5214(97)70014-4. [DOI] [PubMed] [Google Scholar]
- 20.Woods JD, Turenne MN, Strawderman RL, et al. Vascular access survival among incident hemodialysis patients in the United States. Am J Kidney Dis. 1997;30:50–7. doi: 10.1016/s0272-6386(97)90564-3. [DOI] [PubMed] [Google Scholar]
- 21.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–9. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 22.Wang SK, Tsiatis AA. Approximately optimal one-parameter boundaries for group sequential trials. Biometrics. 1987;43:193–9. [PubMed] [Google Scholar]
- 23.Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2:786–800. doi: 10.2215/CJN.00860207. [DOI] [PubMed] [Google Scholar]
- 24.Himmelfarb J. Chronic kidney disease and the public health: gaps in evidence from interventional trials. JAMA. 2007;297:2630–3. doi: 10.1001/jama.297.23.2630. [DOI] [PubMed] [Google Scholar]
- 25.Dörffler-Melly J, Koopman MM, Prins MH, Büller HR. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev. 2005;1:CD002071. doi: 10.1002/14651858.CD002071.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–73. doi: 10.1016/S0140-6736(06)68734-5. [Erratum, Lancet 2007;369:274.] [DOI] [PubMed] [Google Scholar]
- 27.Kaufman JS, O’Connor TZ, Zhang JH, et al. Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol. 2003;14:2313–21. doi: 10.1097/01.asn.0000081661.10246.33. [DOI] [PubMed] [Google Scholar]
- 28.Crowther MA, Clase CM, Margetts PJ, et al. Low-intensity warfarin is ineffective for the prevention of PTFE graft failure in patients on hemodialysis: a randomized controlled trial. J Am Soc Nephrol. 2002;13:2331–7. doi: 10.1097/01.asn.0000027356.16598.99. [DOI] [PubMed] [Google Scholar]
- 29.Saran R, Dykstra DM, Wolfe RA, Gillespie B, Held PJ, Young EW. Association between vascular access failure and the use of specific drugs: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2002;40:1255–63. doi: 10.1053/ajkd.2002.36895. [DOI] [PubMed] [Google Scholar]
- 30.Yevzlin AS, Conley EL, Sanchez RJ, Young HN, Becker BN. Vascular access outcomes and medication use: a USRDS study. Semin Dial. 2006;19:535–9. doi: 10.1111/j.1525-139X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 31.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–9. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Reddan D, Klassen P, Frankenfield DL, et al. National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol. 2002;13:2117–24. doi: 10.1097/01.asn.0000022422.79790.a8. [DOI] [PubMed] [Google Scholar]
- 33.Dember LM, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299:2164–71. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spergel LM, Ravani P, Asif A, Roy-Chaudhury P, Besarab A. Autogenous arteriovenous fistula options. J Nephrol. 2007;20:288–98. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.