Abstract
A conventional view of development is that cells cooperate to build an organism. However, based on studies of Drosophila, it has been known for years that viable cells can be eliminated by their neighbours through a process termed cell competition. New studies in mammals have revealed that this process is universal and that many factors and mechanisms are conserved. During cell competition, cells with lower translation rates or those with lower levels of proteins involved in signal transduction, polarity and cellular growth can survive in a homogenous environment but are killed when surrounded by cells of higher fitness. Here, we discuss recent advances in the field as well as the mechanistic steps involved in this phenomenon, which have shed light on how and why cell competition exists in developing and adult organisms.
Keywords: Minute, Myc, Apoptosis, Losers, Super-competition, Winners
Introduction
One view of a developing organism is that each cell responds to a preprogrammed genetic plan, which unfolds sequentially to coordinate the patterning and growth of tissues until they reach their final size and cellular constitution. Thus, although cells respond to graded extracellular signals, each cell interprets and computes its coordinates along the tissue axes and executes the appropriate programmes autonomously. However, the analysis of genetically mosaic animals (see Box 1) has revealed that cells are continuously comparing themselves with their immediate neighbours and adjusting their behaviour accordingly. This process was first described in the pioneering work of Gines Morata and Pedro Ripoll (Morata and Ripoll, 1975), who were studying a class of mutants called Minutes (M), which harbour mutations in genes that encode ribosomal proteins (Marygold et al., 2007). Homozygous animals die as a result of cell lethality, but heterozygous animals (M/+) are viable, albeit slow growing. However, when M/+ cells are induced in a wild-type background, these cells are not recovered in the adult. This seminal result indicated that, although M/+ cells in a homotypic environment are able to produce a normal organism, they are eliminated when surrounded by wild-type cells (Fig. 1A,B). The eliminated M/+ cells are commonly referred to as ‘losers’ and the wild-type cells that outcompete them are termed ‘winners’ (see Glossary, Box 2). This context-dependent elimination of a viable cell population was termed ‘cell competition’.
Box 1. Clonal techniques in Drosophila
Growth and proliferation in Drosophila have been studied by making heritably marked cells, called clones, and following the size and number of cells in these clones over time. This approach allows precise control over the time of clone induction and the length of the growth period, thus enabling parameters of growth, such as cell doubling time, to be measured. In epithelial tissues, daughter cells stay in contact with each other, such that clones form coherent patches of cells that can be measured (Garcia-Bellido and Merriam, 1971).
Initially, clonal experiments used chromosomes carrying recessive visible markers that could be made homozygous by X-ray irradiation (Patterson, 1929). Double-strand breaks induced by X-ray can cause crossovers between homologous chromosome arms and, if this occurs after DNA replication (in G2 phase), the segregation of chromosome strands after mitosis can lead to a cell inheriting two copies of the recessive marker. A more recent technique takes advantage of a yeast recombinase enzyme, Flippase, and its recognition site FRT, to induce crossover on specific chromosome arms (Golic, 1991; Xu and Rubin, 1993). Regulation of the developmental time and frequency of the initial recombination step is obtained by using a heat-shock promoter to control the induction of Flippase. However, many studies, particularly those of the eye, make use of a constitutive tissue-specific driver to express Flippase (Newsome et al., 2000), thus continuously generating recombinant clones, leading to large patches of marked tissue that result from the merging of clones induced at different times.
Fig. 1.
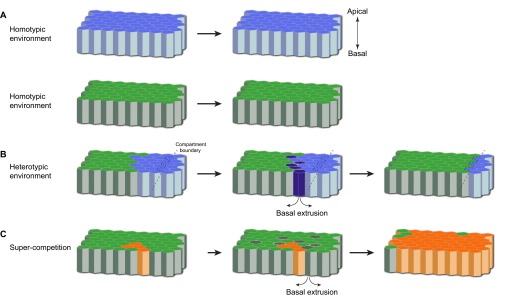
Cell competition. (A) When in a homotypic environment, the cells of two genotypes are viable and produce normal tissues. Blue cells (top) represent less fit cells and green cells (bottom) represent wild-type cells. (B) When these different cells are present in the same tissue (i.e. in a heterotypic environment) competitive interactions take place between them. The less fit cells (blue) are eliminated by apoptosis (dark blue cells), extruded basally (arrows) from the epithelium, and replaced by cells of the fitter type (green). Eventually, the whole compartment (the boundaries of which are indicated by black dashed lines), is colonised by the fitter cell type (green cells). (C) In the case of super-competition, super-competitors (orange) are able to outcompete wild-type cells (green). A clone of super-competitors (orange) induces apoptosis (dark green) and basal extrusion (arrows) of surrounding wild-type cells located up to eight cell diameters away. The subsequent proliferation of super-competitors replaces the outcompeted wild-type cells, resulting in their increased contribution to the final tissue.
Box 2. Glossary
Apicobasal polarity. The organisation of epithelial cells along the axis perpendicular to the epithelial sheet. The side of the cell in contact with the basement membrane is called basal, whereas the side contacting the lumen is apical. Lgl, Dlg and Scrib are basal determinants, whereas Crb is an apical determinant.
Apoptosis. Caspase-dependent programmed cell death, involving cell fragmentation into apoptotic bodies that can be phagocytosed.
Cellular fitness. An as yet unquantifiable concept referring to a quality of a cell, such as the rate of protein synthesis, that cells use to compare themselves with their neighbours.
Cellular growth. The accumulation of mass by a cell. It represents the net rate of protein synthesis in a cell.
Engulfment. The process by which one cell phagocytoses another. In cell competition, the winners have been reported to engulf dying losers.
Loser. A cell that is killed by its neighbours through induction of apoptosis.
Super-competitor. A winner that outcompetes wild-type cells, indicating an increase in fitness over wild type.
Survival factor. A signal that is essential for a cell to live; being deprived of such a signal would cause that cell to undergo apoptosis.
Winner. A cell that kills neighbouring cells that are less fit.
Subsequent work on Minute mutants has expanded our knowledge and established the basic rules for cell competition. Importantly, competition was shown to be dependent on growth rates. There are more than 65 Minute genes that, when disrupted, give rise to a varying severity of growth defects. Classical studies showed that slower growing Minute mutant cells are outcompeted more rapidly than faster growing ones (Simpson, 1979; Simpson and Morata, 1981). Further evidence for the crucial role of differing growth rates in cell competition was the fact that competition between M/+ and wild-type cells could be suppressed by starving the animals (Simpson, 1979). This suggested that, when wild-type cells are made to grow more slowly by nutrient deprivation, their advantage over M/+ cells is abolished. Consistently, competition stops when developmental growth ceases (Simpson and Morata, 1981). Another important aspect of cell competition is that it respects cryptic cell lineage boundaries, called compartments (Garcia-Bellido et al., 1973; Garcia-Bellido et al., 1976). This led to the hypothesis that compartments might be independent units of growth, providing evolution with building blocks that could be changed while insulating the rest of the animal (Crick and Lawrence, 1975). Thus, cell competition causes the clonal make-up of a compartment to change without altering the dimensions of that compartment, suggesting a link between cell competition and size control mechanisms.
As a result of these landmark studies, several features and rules of cell competition were deduced: (1) the viability of a cell and its representation in the final tissue are context dependent; (2) less competitive cells are eliminated; (3) the process of cell competition does not cross compartment boundaries; (4) the competitive status of a cell (i.e. winner or loser) is linked to its relative growth rate; and (5) cell competition does not alter total tissue size. Consequently, cell competition is phenotypically silent; unless the relative contributions of individual cells to the tissue are examined, no change in overall tissue size and morphology can be detected after competitive interactions have occurred. Importantly, it has been shown that this process of cell competition is not limited to Drosophila. Recent work, for example, has identified a mammalian Minute gene called Belly spot and tail (Bst), which encodes Rpl24, that behaves just like a Drosophila Minute, in that Bst/+ cells are outcompeted in chimeras (Oliver et al., 2004). This observation suggests the conservation of cell competition across evolutionary phyla. More recently, two landmark studies have established that cell competition occurs endogenously in the mouse embryo (Clavería et al., 2013; Sancho et al., 2013).
Research in the last decade has challenged these ‘rules’ and has implied that competitive interactions are both more widespread and more varied than previously thought, leading to a broader view of what constitutes cell competition. Here, we discuss the varying types of cell competition and the factors that provoke competitive interactions between cells. We highlight the important findings as well as the controversies in the field and attempt to draw conclusions about the mechanisms and evolutionary significance of this intriguing phenomenon.
The many types and regulators of cell competition
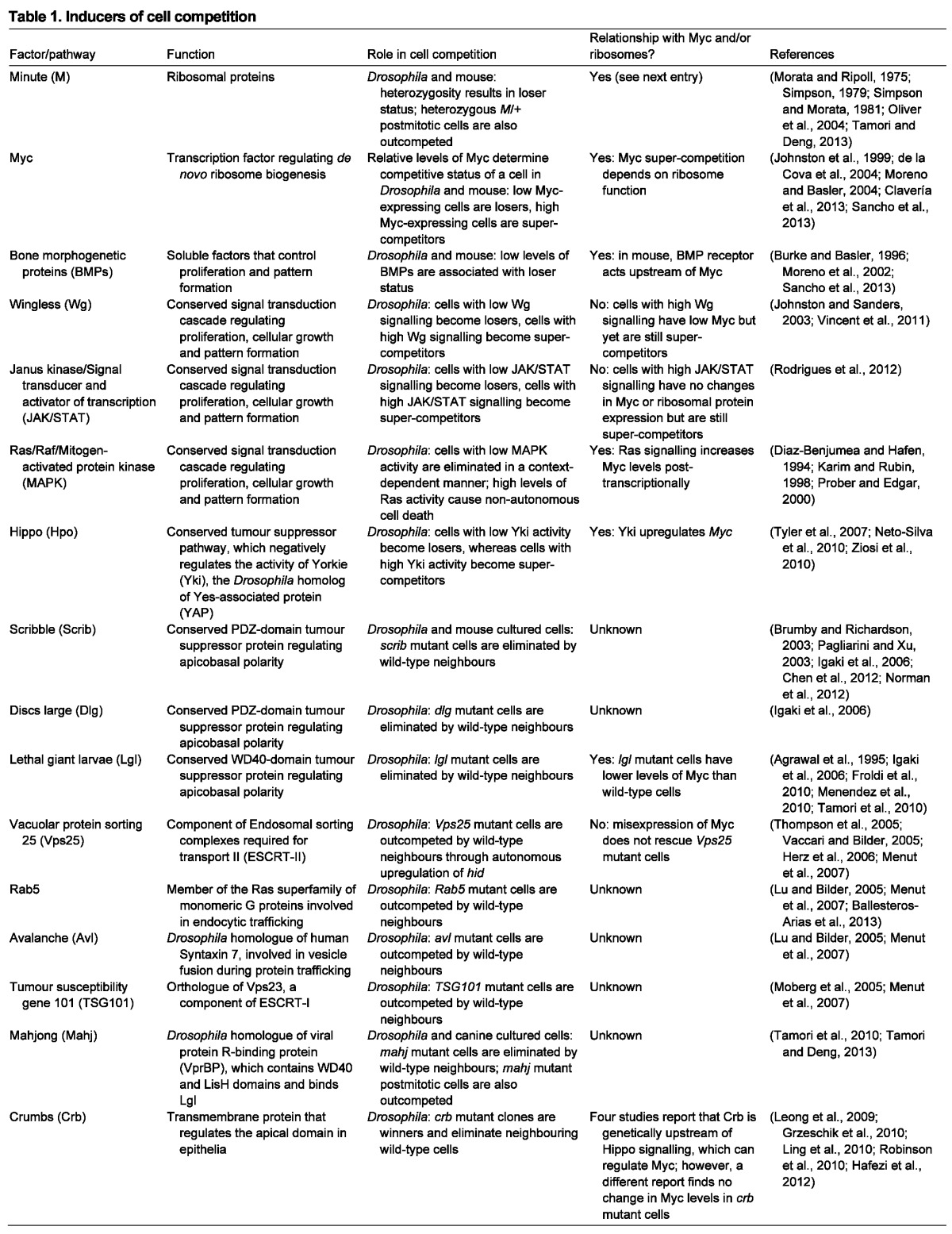
For over three decades only Minute mutants were known to cause cell competition, but within the last decade the field has exploded. Many factors have been shown to regulate cell competition and here we group them into three broad classes (Myc, signal transduction, polarity) that are discussed below (Table 1).
Table 1.
Inducers of cell competition
Myc and the discovery of ‘super-competition’
In classical cell competition, wild-type cells always outcompete the slowly growing M/+ cells through induction of programmed cell death (Moreno et al., 2002), or apoptosis (see Glossary, Box 2). Thus, the losers have reduced cellular fitness (see Glossary, Box 2), and their elimination and replacement by wild-type cells through competition could be viewed as restoring a diminished function (i.e. growth) to the tissue. Studies of the sole Drosophila homologue of Myc [also referred to as dmyc or diminutive (dm)], an essential growth regulator, showed that Myc mutant cells are outcompeted (Table 1) (Johnston et al., 1999). By contrast, if cells express higher levels of Myc than their neighbours, they become winners and outcompete wild-type cells (Fig. 1C) (de la Cova et al., 2004; Moreno and Basler, 2004). Thus, in cell competition, the relative amount of Myc gene product determines whether a cell is the winner or the loser. In the case of Myc-overexpressing cells, competition no longer eliminates unfit cells, as wild-type cells are now the losers. This was the first demonstration of super-competitors (see Glossary, Box 2) (Abrams, 2002), which bear gain-of-function mutations that are potentially harmful to the organism and expand at the expense of wild-type cells.
Myc is a key regulator of cellular growth (see Glossary, Box 2) due to its control of de novo ribosome biogenesis (Grewal et al., 2005), and the ability of Myc to increase rates of protein translation is crucial to its competitive effects. Indeed, cells that overexpress Myc lose their ability to outcompete wild-type cells when they are heterozygous for a Minute mutation (Moreno and Basler, 2004). This suggests a common thread between Myc and Minute genes, and indicates that competition depends on ribosomes and growth regulation. However, an intriguing difference between Minute- and Myc-dependent competition exists: Myc-overexpressing winners are able to kill wild-type cells at a distance [up to eight cell diameters away (de la Cova et al., 2004)], whereas most cell death of M/+ losers occurs adjacent to winners (Simpson and Morata, 1981; Moreno et al., 2002; Li and Baker, 2007). This suggests that Minute-induced cell competition requires cell-cell contact, whereas Myc-dependent competition does not.
In fact, experimental evidence from cell culture studies supports the existence of secreted signals mediating cell competition. These studies have shown that apoptosis of losers with lower levels of Myc can be induced in cell cultures in which cell contact is prevented (Senoo-Matsuda and Johnston, 2007). Furthermore, when Myc-overexpressing cells are co-cultured with wild-type cells, the resulting conditioned medium can induce cell death when incubated with wild-type cells. The response to this medium depends on the level of Myc expressed by the cell: cell death is observed in the case of cells expressing low levels of Myc, whereas increased proliferation is seen if the cells express high levels of Myc (Senoo-Matsuda and Johnston, 2007). Although these results have been questioned by another laboratory (Portela et al., 2010), they raise the hypothesis that competition can be mediated through soluble secreted factors. Furthermore, they suggest that each cell interprets these factors differently according to its level of Myc. In other words, naïve cells ‘know’ if they are to be winners or losers.
Recent exciting studies have shown that Myc-dependent cell competition and super-competition are not limited to Drosophila but also exist in early mouse embryos (Clavería et al., 2013; Sancho et al., 2013). Clavería et al. made mosaic mouse embryos with cells expressing differing levels of Myc and found that cells with lower Myc levels were eliminated. Sancho et al. studied Myc indirectly and arrived at a similar conclusion: they found that when cells lacking the bone morphogenetic protein (BMP) receptor Bmpr1a are juxtaposed with wild-type cells, they have decreased levels of Myc and are subsequently eliminated (Table 1) (Sancho et al., 2013). Thus, the same rules apply to mouse Myc as to Drosophila Myc: cells measure Myc content relative to their neighbours, and the cells with lower Myc levels (even if they express wild-type levels) are eliminated, although the overall size of the embryo remains unchanged (Clavería et al., 2013). Notably, these two papers differ in the required proximity of the loser to the winner. Clavería et al. observed cell death only in low-level Myc losers immediately adjacent to winners. By contrast, Sancho et al. conducted similar experiments to the Drosophila cell culture studies (Senoo-Matsuda and Johnston, 2007) and found that cells lacking Bmpr1a are outcompeted in trans-well plates where there is no direct contact between winners and losers. Nevertheless, both studies show that, in the absence of genetic manipulation, endogenous cell death in the mouse embryo occurs in cells with lower Myc expression (Clavería et al., 2013; Sancho et al., 2013). This provides for the first time a tantalising hint that cell competition may occur in vivo, under normal physiological conditions.
Signalling pathways and survival factors
There has been a growing realisation that cell competition occurs downstream of many important developmental signalling pathways, leading to a re-interpretation of the idea of a survival signal (see ‘survival factor’, Glossary, Box 2). Such a signal is a secreted factor that cells need absolutely in order to stay alive; being deprived of it causes a cell to die (Raff, 1992). Numerous ligands that activate conserved signalling pathways have been proposed to act as survival factors. For example, Wnt/Wingless (Wg) fits the criterion for such a factor (Table 1). In the Drosophila wing disc, cells that cannot transduce Wg do not grow and are eliminated through cell death (Giraldez and Cohen, 2003; Johnston and Sanders, 2003). However, this elimination was shown recently to be context dependent, as Wg-deficient cells juxtaposed with less fit cells are able to contribute to an entire compartment (Vincent et al., 2011). Conversely, cells with a mutation conferring constitutive Wg signalling become super-competitors and trigger apoptosis in neighbouring wild-type cells. Similar observations were made for the JAK/STAT signalling pathway (Table 1), whereby loss of Stat92E, the sole STAT gene in Drosophila, causes cells to grow poorly and be eliminated by apoptosis (Rodrigues et al., 2012). Preventing the death of these cells or placing them in a context in which they have a growth advantage over their neighbours rescues their growth, suggesting context-dependent elimination, a hallmark of cell competition. Furthermore, hyperactivating JAK/STAT signalling not only causes autonomous overgrowth, but also induces cell death in wild-type cells several cell diameters away (Rodrigues et al., 2012). The Ras/Raf/MAPK pathway has also been known for many years as crucial for cell survival because clonal reduction in this signalling pathway leads to cell death (Table 1). This was presumed to be due to the autonomous role of MAPK in promoting survival or blocking apoptosis (Diaz-Benjumea and Hafen, 1994; Bergmann et al., 1998; Kurada and White, 1998; Yang and Baker, 2001; Yang and Baker, 2003). However, an alternative explanation is that MAPK signal-deficient cells are outcompeted by wild-type neighbours. Indeed, the elimination of ras (Ras85D) mutant cells can be reversed by juxtaposing them with M/+ growth-compromised cells (Prober and Edgar, 2000). Moreover, expressing an activated form of Ras induces apoptosis in wild-type cells located several cell diameters away (Karim and Rubin, 1998), suggesting that sustained activation of the MAPK pathway also induces super-competition. Finally, dysregulation of the Hippo (Hpo) pathway, a central and conserved regulator of proliferation and survival (Table 1) (Pan, 2010), can rescue the loss of M/+ cells (Tyler et al., 2007). Hyperactivation of Yorkie (Yki), a downstream effector of the Hippo pathway, leads to autonomous overgrowth, and these cells become super-competitors, inducing apoptosis in neighbouring wild-type cells (Tyler et al., 2007; Neto-Silva et al., 2010; Ziosi et al., 2010).
These studies raised the possibility that most signalling pathways described as survival factors are actually required to maintain the competitiveness of cells. For example, in Drosophila and in mammals, autonomous lack of BMP reception causes cells to be eliminated in a process consistent with cell competition (Burke and Basler, 1996; Sancho et al., 2013). Likewise, loss of Insulin/PI3 kinase (PI3K) pathway signalling leads to elimination (Böhni et al., 1999; Verdu et al., 1999). However, ectopic activation of PI3K (or misexpression of a growth regulatory Cyclin/Cyclin-dependent kinase complex) does not lead to super-competition (de la Cova et al., 2004). These studies suggest that the model of signalling pathways as rheostats of competition (i.e. too little results in loser status and too much results in winner status) is too simplistic. Rather, a cell needs these signalling pathways to avoid being outcompeted but becoming a winner and inflicting competitive stress on your neighbours is more complicated.
Do all of these pathways converge on a common regulator of cellular fitness? Several pathways have been shown to modulate Myc levels. For example, clear evidence exists to confirm that the competitive nature of Hpo pathway mutant cells depends on upregulation of Myc. The pathway effector Yki directly regulates Myc transcription and this is essential for cells with sustained Yki activation to outcompete neighbours (Neto-Silva et al., 2010; Ziosi et al., 2010). Similarly, studies in the early mouse embryo have shown that Myc is a central regulator of cell competition. In the case of Bmpr1a mutant cells, loser status was associated with lower Myc levels, which presumably accounts for their outcompetition in the presence of wild-type cells (Sancho et al., 2013). Ras also regulates Myc expression in Drosophila wing discs, and ras mutant clones are partially rescued by expression of Myc (Prober and Edgar, 2000).
By contrast, both JAK/STAT and Wg pathways were shown to act independently of Myc (Vincent et al., 2011; Rodrigues et al., 2012), suggesting that cell competition can measure differences other than simply ribosomal function. In fact, as Wg signalling represses Myc expression (Duman-Scheel et al., 2004; Vincent et al., 2011), the winners in Wg-induced competition are actually the cells with lower Myc. This indicates that, although the lower levels of Myc in these cells should in fact render them losers, the sustained Wg signalling alters their status. Moreover, sustained JAK/STAT pathway activation can partially rescue Myc null losers, suggesting that there are parallel pathways for measuring cellular fitness (Rodrigues et al., 2012). It is important to note that, unlike classical cell competition mutants, these signalling pathways when disrupted lead to visible growth phenotypes and do not respect size control mechanisms. Thus, there are likely to be many types of cell competition.
Polarity genes and neoplastic tumour suppressors
The study of a class of genes called neoplastic tumour suppressors revealed an unexpected link between cell polarity and cell competition (Gateff, 1978; Bilder, 2004). Mutants for these genes were identified by their neoplastic appearance, that is to say growths of larval tissues that had lost both epithelial structure and the ability to differentiate. Many genes in this class were found to encode proteins that are essential components of complexes required for the proper apicobasal polarity (see Glossary, Box 2) of epithelial cells, and include Scribble (Scrib), Discs large (Dlg, also known as Dlg1) and Lethal giant larvae [Lgl, also known as L(2)gl] (Bilder, 2004). Although there are some differences between these genes (see Table 1), here we consider them together.
Cells mutant for any of these three genes are viable - and in fact proliferate faster and cause overgrowths - when in a homotypic environment and exhibit high Yki activity (Bilder, 2004; Grzeschik et al., 2010; Doggett et al., 2011; Sun and Irvine, 2011; Chen et al., 2012). By contrast, when polarity-deficient clones are confronted with wild-type cells, they are eliminated. This occurs through activation of Jun N-terminal kinase (JNK) signalling, which suppresses Yki activity (Table 2) (Woods and Bryant, 1991; Agrawal et al., 1995; Brumby and Richardson, 2003; Pagliarini and Xu, 2003; Uhlirova et al., 2005; Igaki et al., 2006; Grzeschik et al., 2007; Grzeschik et al., 2010; Tamori et al., 2010; Chen et al., 2012). However, polarity-deficient clones can survive when given a competitive advantage (for example, when induced in a M/+ background), and high levels of Yki activity and Myc protein are observed within them (Agrawal et al., 1995; Froldi et al., 2010; Menéndez et al., 2010; Chen et al., 2012). The context-specific behaviour of these cells is a hallmark of cell competition, and similar competitive outcomes have been observed following loss of Scrib in mammalian cells (Norman et al., 2012). In this type of competition, the losers are actually the faster proliferating cells that possess tumourigenic potential. This is in contrast to the situation observed in Myc- and Minute-induced competition, in which the winner is the cell with the fastest growth rate. Thus, in the context of polarity-deficient cells, cell competition acts in a tumour-suppressing role.
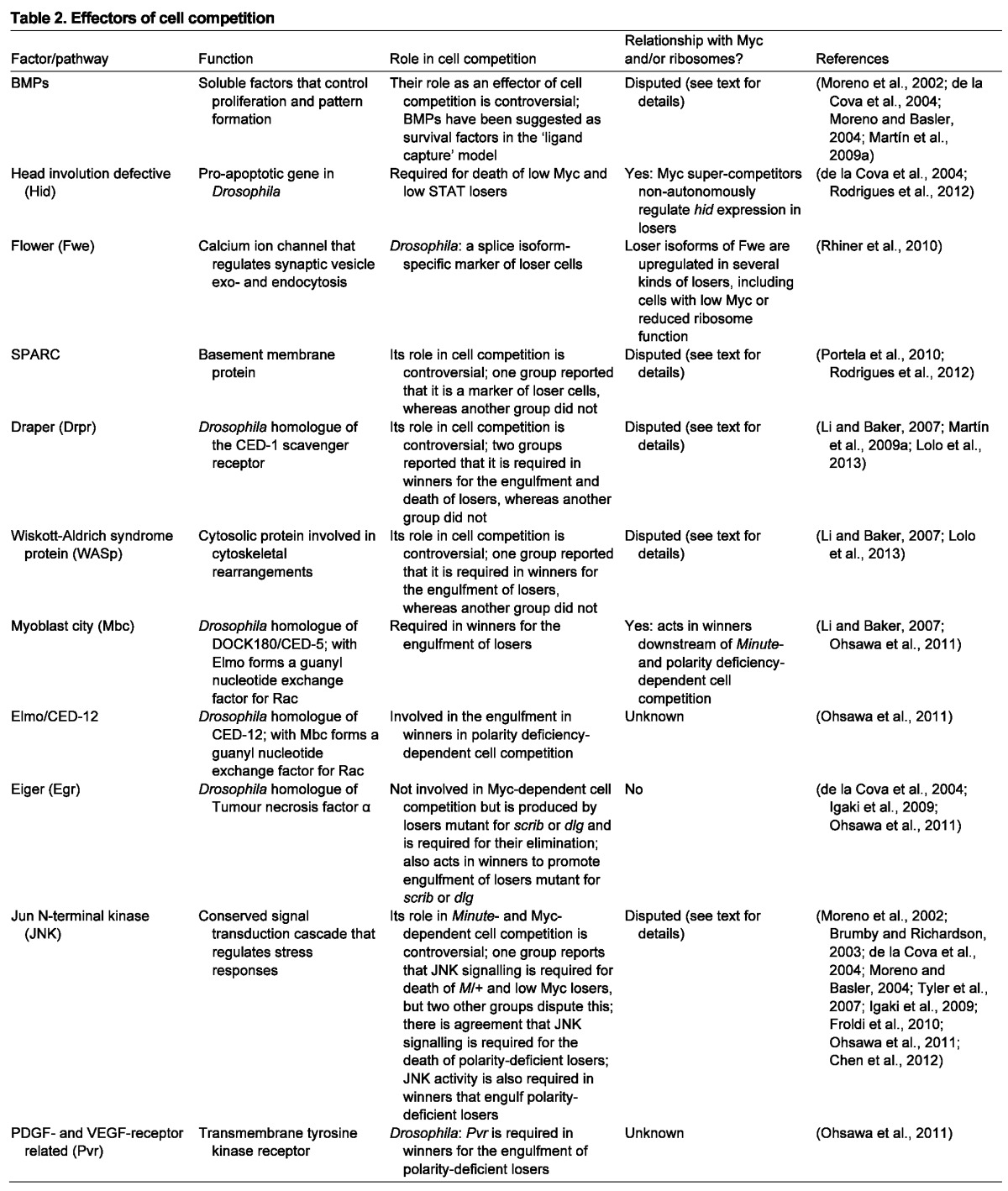
Table 2.
Effectors of cell competition
Strikingly, a reversal of the loser status of polarity-deficient cells can be observed if they co-express an active form of Ras (RasV12) (Brumby and Richardson, 2003; Pagliarini and Xu, 2003). That RasV12 can rescue these cells from being outcompeted is likely to be due to the fact that RasV12 leads to hyperactivity of Yki and that both Ras and Yki regulate Myc expression (Prober and Edgar, 2000; Menéndez et al., 2010; Neto-Silva et al., 2010; Ziosi et al., 2010). In contrast to studies of classical cell competition, in which discrete clones are generated and then analysed after a defined period of time, many studies of polarity-deficient cells employ techniques that continuously generate clones (see Box 1), resulting in large merged patches of mutant tissue (Brumby and Richardson, 2003; Pagliarini and Xu, 2003; Igaki et al., 2006; Igaki et al., 2009; Cordero et al., 2010; Grzeschik et al., 2010; Doggett et al., 2011; Chen et al., 2012). In fact, a careful analysis of discrete RasV12 lgl mutant clones found that these cells are still outcompeted (Menéndez et al., 2010). However, if enough clones are present in the tissue, they merge to form large microenvironments that are impervious to cell competition and that sustain them even in the presence of wild-type cells (Menéndez et al., 2010; Chen et al., 2012).
It is interesting that genes encoding components of the endosomal trafficking machinery also behave as neoplastic tumour suppressors in Drosophila and induce competitive interactions when mutated. Cells lacking TSG101, Vps25, Rab5 or avalanche (also known as Syntaxin 7) (see Table 1) are eliminated by wild-type neighbours (Lu and Bilder, 2005; Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005; Herz et al., 2006; Menut et al., 2007; Ballesteros-Arias et al., 2013). However, when given a growth advantage, these mutant cells survive, lose apicobasal polarity and produce overgrown tissue resembling tumours. Like RasV12 lgl mutant clones (Menéndez et al., 2010), the density of Rab5 clones strongly influences their elimination: when of sufficient size, they can escape cell competition (Ballesteros-Arias et al., 2013). Intriguingly, overexpression of Myc does not rescue Vps25 mutant cells whereas ectopic activation of Yki does, suggesting that in this instance of cell competition Yki acts independently of Myc (Herz et al., 2006).
Other polarity factors and their associated proteins that are not neoplastic tumour suppressors can also induce cell competition in mosaic tissue. Loss of Mahjong (Mahj), a binding partner of Lgl (see Table 1), leads to competitive elimination in Drosophila and mammals (Tamori et al., 2010). mahj-deficient cells are killed through JNK-induced apoptosis and, in mammalian cells, this can be blocked by a JNK inhibitor. Importantly, Mahj acts downstream of Lgl; the elimination of lgl mutant clones is largely suppressed when they overexpress Mahj (Tamori et al., 2010). Loss of the apical determinant Crumbs (Crb) also leads to clonal overgrowth (Chen et al., 2010; Ling et al., 2010; Richardson and Pichaud, 2010; Robinson et al., 2010), and one group has shown that this results from crb mutant cells acquiring super-competitive abilities that lead to apoptosis in neighbouring wild-type cells (Hafezi et al., 2012). Several groups report that loss of Crb leads to upregulation of Yki target genes, and that reducing the genetic dose of yki can suppress Crb-dependent overgrowth (Chen et al., 2010; Grzeschik et al., 2010; Ling et al., 2010; Robinson et al., 2010; Hafezi et al., 2012). This strongly suggests that Crb-induced cell competition is mediated through inactivation of Hpo signalling.
Mechanisms of cell competition
As highlighted above, cell competition depends on the elimination of losers by cell death. When cell death is inhibited in losers, for example by expressing the pan-caspase inhibitor P35 (Hay et al., 1994), it not only rescues their poor growth and removal, but it also abolishes the growth advantage of winners (Moreno and Basler, 2004; Li and Baker, 2007; Martín et al., 2009a). There is, however, some debate as to whether the death of losers is essential for the increased proliferation of winners (Martín et al., 2009a). Here, we consider cell death as central to the process of cell competition and, as such, as its defining feature. There are two alternative models of how cells are removed during cell competition: the ligand capture model, which involves a passive fight for a survival factor; and the comparative fitness model, in which cells communicate with each other to sense their differences and elicit competitive outcomes (Fig. 2).
Fig. 2.
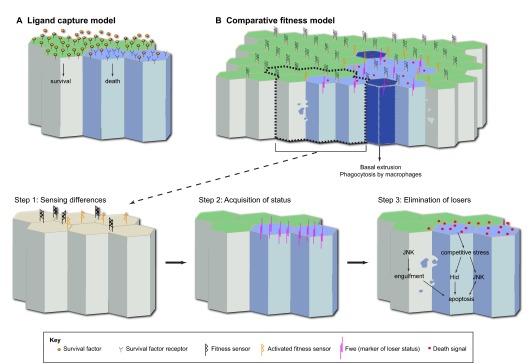
Potential models of competitive interactions and death. (A) In the ligand capture model, winners (green) capture more survival factor (orange circles) and deprive losers (blue) of it. The lack of a survival signal causes cells to die. (B) In the comparative fitness model, cells sense differences between themselves, leading to competitive interactions. This process can be divided into three sequential steps: sensing differences (step 1), acquisition of status (step 2) and elimination of losers (step 3). Briefly, winners (green) kill losers (light blue) and the dying losers (dark blue) are engulfed by winners and/or are basally extruded and phagocytosed by circulating macrophages (arrow). In step 1, differences in fitness are sensed across a field of naïve epithelial cells (beige) that contains cells of variable fitness. For simplicity, we have drawn a hypothetical surface sensor protein (black). Although many scenarios can be imagined, we propose that the sensor makes homotypic interactions between neighbouring cells (indicated by interlocking black shapes). If cells are different, these interactions may not occur, causing the sensor to become activated (orange, shown here in presumptive winner cells). When this receptor is activated, competitive interactions are induced. In step 2, cells acquire winner (green) or loser (blue) status and begin to express markers of their status. For example, Fwe isoforms (magenta) begin to be expressed in losers. Note that, although no markers of winners have been identified, there is evidence that winners know their status (Senoo-Matsuda and Johnston, 2007). In step 3, extracellular death signals (red) induce death in losers. We do not distinguish between winners producing the death signal that kills losers and losers producing the death signal, resulting in cellular suicide. Ultimately, this competitive stress leads to Hid expression and JNK activation in losers, resulting in apoptosis. Winners themselves might also phagocytose losers and cause their death, via the activation of several phagocytosis proteins downstream of JNK activation.
The ligand capture model
If cellular survival depends absolutely on limiting amounts of secreted survival factors (Raff, 1992), a cell that captures more of this factor, for instance by having more receptors on its surface or by increasing its endocytosis rate, would restrict its neighbours’ access to the survival factor, effectively killing them (Fig. 2A). Such mechanisms have been proposed for culling excess neurons that are produced during development (Raff, 1992). Cell competition has been proposed to work in this way in the Drosophila wing disc, where Decapentaplegic (Dpp), a Drosophila BMP homologue, is proposed to play the role of a survival factor (Moreno et al., 2002; Moreno and Basler, 2004). Dpp is expressed in a stripe of anterior cells next to the anterior-posterior compartment boundary and diffuses to form a gradient, with the highest concentration of Dpp thus occurring in the centre of the disc (Posakony et al., 1990; Teleman and Cohen, 2000). The ligand capture model predicts that competition for Dpp, and hence the chance of becoming a loser, increases the further a cell is from the Dpp source.
Some observations support this model. For example, preventing endocytosis by expressing a dominant-negative form of Rab5 leads to downregulation of BMP signal reception (Moreno et al., 2002). Furthermore, markers of diminished BMP signalling are observed in losers of Minute- and Myc-induced competition (Moreno et al., 2002; Moreno and Basler, 2004; Tyler et al., 2007). Losers that have reduced Myc can be rescued by increasing the expression of positive regulators of Dpp signal transduction or by knocking down negative regulators of the pathway (Moreno and Basler, 2004; Ziv et al., 2009).
However, two other laboratories report no change in Dpp signalling in M/+ losers or in losers with lower levels of Myc (de la Cova et al., 2004; Martín et al., 2009a). Several other observations also contradict the ligand capture model. First, cells lacking the Dpp receptor Thickveins (Tkv) are eliminated when surrounded by wild-type cells but survive in a Minute background (Burke and Basler, 1996). The fact that tkv mutant cells can survive when surrounded by less fit cells suggests that the survival of cells lacking BMP signalling depends on the fitness of their neighbours and not on an autonomous process. Second, whereas the ligand capture model predicts that the cells furthest away from the signal source would be most vulnerable to loss of signal reception, tkv mutant cells are in fact eliminated preferentially in the centre of the disc, close to the source of Dpp (Burke and Basler, 1996). This indicates that the requirement for BMP signal reception is not absolute but depends on comparison of relative signalling levels with immediate neighbours. Finally, it has been shown in mammalian models that BMP ligands are not limiting for cell competition (Sancho et al., 2013). Indeed, if BMP were a limiting survival factor then the intensity of competition would be modulated by BMP levels; however, varying the concentration of BMPs in competitive co-cultures did not alter the intensity of the competition (Sancho et al., 2013).
The comparative fitness model: sensing and elimination pathways
The ligand capture model thus does not fully explain all aspects of cell competition, and instead it appears that the relative fitness of cells might be the parameter that matters. However, what fitness is and how it is measured remain unanswered questions. When cell competition was initially identified, it was thought to be related to proliferation rates (Simpson and Morata, 1981). However, subsequent work has shown that proliferation and cellular growth are separable (Neufeld et al., 1998). Myc seemed to link cellular growth to competition via ribosome biogenesis (de la Cova et al., 2004; Moreno and Basler, 2004), a common thread with Minute genes. However, Wg- and JAK/STAT pathway-induced competition exhibit no evidence of accelerated growth through increased ribosome biogenesis, and not all growth regulators (i.e. PI3K) can induce cell competition (de la Cova et al., 2004; Vincent et al., 2011; Rodrigues et al., 2012). Therefore, it might be that cells sense some common change in fitness downstream of many different kinds of insult; alternatively, there might be different kinds of competition and no universal measure of fitness.
Nevertheless, it is possible to break down the events that occur during cell competition into a minimum of three steps (Fig. 2B). First, neighbouring cells must recognise differences between them. Second, these differences are translated into winner or loser status. Third, winners eliminate the losers and replace them. Below, we discuss each of these steps, although it should be noted that much more is known about the third step than about the first two.
Sensing differences and acquiring status
In the simplest model, each cell regulates the amount of a secreted or membrane ‘sensor’ protein that allows cells to compare their relative fitness with that of their neighbours (Fig. 2B). [We note that this is hypothetical and undoubtedly an oversimplification of the actual situation.] Crb is one of only a handful of transmembrane proteins that have been linked to cell competition. As described above, wild-type cells adjacent to crb mutant super-competitors become losers and die (Hafezi et al., 2012), which is consistent with the requisite properties for a sensor molecule. However, Crb-overexpressing losers cause the death of neighbouring wild-type cells (Hafezi et al., 2012), which is inconsistent with the prediction of a sensor molecule. It has recently been suggested that mechanical stress, as opposed to ‘sensing’ through cell surface markers, could be an initial step in cell competition (Vincent et al., 2013). Specifically, this model proposes that differences in growth rates cause planar elongation of the losers at the clone border with winners. In a non-competitive situation, planar elongation has been shown to lead to delamination from the epithelium (Marinari et al., 2012).
After differences between neighbouring cells have been perceived, cells upregulate markers of their status (Fig. 2B). Expression profiling has identified two proteins, SPARC and Flower (Table 2), that might be markers of losers during cell competition (Portela et al., 2010; Rhiner et al., 2010). SPARC is an extracellular matrix protein that was shown to be expressed in a variety of losers (Portela et al., 2010). However, using identical assays, our group did not observe SPARC upregulation in multiple types of losers during cell competition (Rodrigues et al., 2012), a contradiction we are at a loss to explain. flower (fwe), named after its mutant phenotype in which flowery neuromuscular junction boutons are found (Yao et al., 2009), encodes a Ca2+ channel and has several splice isoforms. One is expressed ubiquitously, whereas the other two, named FweLose-A and FweLose-B, are found only in loser cells and mark them for elimination (Fig. 2B, step 2) (Rhiner et al., 2010). Wg regulates expression of the FweLose-B isoform during developmental cell death in the eye disc (Merino et al., 2013). In the future it will be important to determine whether this regulatory relationship exists in cell competition. Since Fwe expression only occurs after cells have sensed that they are losers, it cannot account for the initial sensing interaction between different cells.
Inducing and translating cell death signals: the role of JNK
Cell death pathways have been well characterised in Drosophila (Steller, 2008) and depend on three pro-apoptotic proteins: Hid (also known as Wrinkled), Grim and Reaper. Numerous papers have reported markers of apoptosis (cleaved Caspase 3 and TUNEL assay) in losers (Moreno et al., 2002; de la Cova et al., 2004; Moreno and Basler, 2004; Uhlirova et al., 2005; Li and Baker, 2007; Senoo-Matsuda and Johnston, 2007; Tyler et al., 2007; Froldi et al., 2010; Menéndez et al., 2010; Rhiner et al., 2010; Ziosi et al., 2010; Vincent et al., 2011; Hafezi et al., 2012; Lolo et al., 2012; Rodrigues et al., 2012; Clavería et al., 2013; Sancho et al., 2013). In addition, induction of hid is observed non-autonomously in losers when Myc is overexpressed (Table 2) (de la Cova et al., 2004), highlighting that Myc super-competitors kill wild-type cells at a distance. Reducing the genetic dose of hid dominantly suppresses super-competition downstream of Myc and of STAT (de la Cova et al., 2004; Senoo-Matsuda and Johnston, 2007; Rodrigues et al., 2012). These findings suggest that the outcome of competitive intercellular communication culminates in transcription-dependent apoptosis (Fig. 2B, step 3).
One such possible activator of death in losers is JNK, which has been implicated in cell death following many kinds of stresses (Igaki, 2009). One group found markers of JNK activation in both Myc and Minute losers, and they reported that blocking the JNK pathway using a dominant-negative form of the Drosophila JNK Basket (Bsk) in these cells rescues their viability and growth (Moreno et al., 2002; Moreno and Basler, 2004). However, two groups did not observe JNK activity in Myc losers and, more importantly, found that competitive cell death in Myc or Minute losers was not suppressed in animals globally deficient for JNK signalling (i.e. mutants for Jun/AP-1 jun, JNK bsk, the JNK kinase hemipterous, or the JNK kinase kinase misshapen) (de la Cova et al., 2004; Tyler et al., 2007). JNK activity was also not observed in JAK/STAT-dependent competition (Rodrigues et al., 2012). Therefore, the role of JNK in Minute-, Myc- and JAK/STAT-dependent competition appears to be minor.
By contrast, numerous groups agree that JNK contributes significantly to the elimination of polarity-deficient losers (Brumby and Richardson, 2003; Uhlirova et al., 2005; Herz et al., 2006; Igaki et al., 2006; Igaki et al., 2009; Leong et al., 2009; Cordero et al., 2010; Menéndez et al., 2010; Tamori et al., 2010; Chen et al., 2012; Ballesteros-Arias et al., 2013). However, it is not clear whether JNK signalling is responsible for the death of these cells, or whether JNK simply dictates their status as losers. When JNK is inhibited, polarity-deficient cells become winners and upregulate Yki activity (Chen et al., 2012). Yki, in turn, is a well-known regulator of cell survival, acting via direct transcriptional activation of the anti-apoptotic gene diap1 (also known as thread) (Huang et al., 2005). There is evidence to suggest that the role of JNK during the elimination of these cells is not restricted to the induction of cell death. When JNK signalling is blocked in polarity-deficient cells, they overgrow. However, when caspase activation is blocked, they do not, presumably because JNK is still active and anti-tumourigenic (Brumby and Richardson, 2003; Igaki et al., 2009). The examination of Rab5-deficient cells, which exhibit polarity defects, has revealed that JNK is genetically downstream of caspase activation, suggesting that JNK activation is a consequence and not a cause of cell death (Ballesteros-Arias et al., 2013). When the latter is considered with the observation that JNK signalling contributes to the tumourigenic potential of polarity-deficient cells that escape elimination due to ectopic Ras or sufficient clone density (Uhlirova and Bohmann, 2006; Cordero et al., 2010; Ballesteros-Arias et al., 2013), the role of JNK in these cells becomes opaque and is probably indirectly linked to cell death. In summary, the evidence linking JNK signalling in multiple types of losers to induction of cell death awaits further clarification.
Eliminating cells: the role of engulfment
It is striking that in Minute- and polarity deficient-dependent competition, cell death is observed predominantly, if not exclusively, in losers that are in immediate contact with winners (Li and Baker, 2007; Froldi et al., 2010; Tamori et al., 2010; Ohsawa et al., 2011). This contrasts with competition induced by Myc and other signalling pathways, where cell death can be seen at a distance from the winners (Karim and Rubin, 1998; de la Cova et al., 2004; Ziosi et al., 2010; Rodrigues et al., 2012). Further investigation revealed fragments of dead M/+ losers within neighbouring winners (Fig. 2B) (Li and Baker, 2007), suggesting that the losers had been engulfed by the winners, which is to say that the winners phagocytose the dying losers (see ‘engulfment’ in Glossary, Box 2). This was unexpected and different from other situations, in which dying cells are basally extruded from the epithelium and often removed by macrophages (Abrams et al., 1993; Tepass et al., 1994; Franc et al., 1999; Gibson and Perrimon, 2005; Shen and Dahmann, 2005). Li and Baker identified several regulators of engulfment, including draper (drpr), WASp, myoblast city (mbc) and Rac1 (Table 2). When the functions of these genes are removed from winners, the death and engulfment of losers is blocked and the growth advantage of winners is reduced (Li and Baker, 2007; Martín et al., 2009a). In Drosophila and C. elegans, Drpr and its homologue CED-1 are required for the engulfment of apoptotic cells by non-professional phagocytes (Zhou et al., 2001; Etchegaray et al., 2012). Similarly, in competition induced by loss of polarity genes, fragmentation and engulfment of losers by their winner neighbours was observed and captured by live imaging (Ohsawa et al., 2011). Moreover, this group reported that JNK signalling in the winners enables their engulfing ability, through upregulation of PDGF- and VEGF-receptor related (Pvr), and requires the intracellular effectors Mbc and CED-12 (also known as ELMO) (Table 2).
Recently, another group obtained dramatically different results for similar experiments. Lolo and colleagues found that neither drpr nor WASp function is required for cell death in losers or for winner clone growth in Minute- or polarity-induced competition (Lolo et al., 2012). Instead, they report that losers are basally extruded from the epithelium and then phagocytosed by circulating macrophages. Similar observations of haemocytes being recruited to sites of competition have been made by other groups (Pastor-Pareja et al., 2008; Cordero et al., 2010). At present it is difficult to reconcile these two findings, which might be explained by differing experimental conditions or genetic backgrounds, and the significance of engulfment is still under debate. Although engulfment of losers with lower levels of Myc has been observed in both Drosophila and mouse (Li and Baker, 2007; Clavería et al., 2013), the contribution of this engulfment to loser clone disadvantage has not been quantified.
In conclusion, the effector mechanisms at work during cell competition are as yet unclear, as there is no consensus on the identity of a viable sensing mechanism or a cell death pathway. In addition, there are other cellular behaviours that must be accounted for after cell competition occurs, such as the increased growth of winners to make up for the space left by the losers. Li and Baker report that the growth of winners is dependent upon the death of losers (Li and Baker, 2007), a phenomenon also observed in JAK/STAT pathway-induced cell competition (Rodrigues et al., 2012). The increased growth of winners might be due to engulfment, oriented divisions to invade the space vacated by dying losers (Li et al., 2009), or death-derived compensatory proliferation signals (reviewed by Martín et al., 2009b). However, Morata and colleagues report that the growth rate of winners is not affected by the death of losers and the large size of winner clones is simply due to their endogenous growth rate (Martín et al., 2009a).
How universal is cell competition?
Is cell competition a conserved phenomenon? Is it relevant to normal development or disease progression? Does it have any therapeutic benefits that could be exploited? Although four decades of work in flies and the use of the Minute mutants convinced Drosophila researchers of the importance of cell competition, it is only recently that this process has piqued the interest of the broader community. As mentioned above, cell competition has been experimentally induced by introducing differences in the expression of Bst, a mouse Minute (Oliver et al., 2004), Scrib or Mahj in cultured cells (Tamori et al., 2010; Norman et al., 2012), and of Myc in mouse embryonic stem cells (ESCs) and embryos (Clavería et al., 2013; Sancho et al., 2013). Furthermore, within the last decade, the therapeutic potential of cell competition has been explored. A prime example is that of stem cell competition (see Box 3). Exploiting the mechanisms of cell competition might help to ensure better colonisation of the host by donor cells following bone marrow transplantation, thus ensuring that a higher proportion of the injected cells engraft in the host at the expense of the endogenous, dysfunctional stem cells (e.g. Bondar and Medzhitov, 2010). Similarly, it has been shown that cell competition can occur in the liver following transplantation of rapidly proliferating embryonic hepatocytes. These cells replace the endogenous liver cells by inducing their cell death (Oertel et al., 2006). Harnessing this process to replace diseased liver cells is an attractive alternative to full organ transplants.
Box 3. Stem cell competition
The engraftment of transplanted hematopoietic stem cells (HSCs), which must displace host HSCs to persist, resembles cell competition (Micklem et al., 1968). Recently, the stress mediator p53 was found to decrease the competitiveness of colonising HSCs, suggesting that stress-induced senescence affects their competitive ability (Bondar and Medzhitov, 2010; Marusyk et al., 2010). However, stem cell competition does not rely on the death of loser stem cells (Bondar and Medzhitov, 2010). Instead, winners displace other stem cells from their niche, leading to differentiation of the losers, which no longer contribute to the stem cell pool. In this way, stem cell competition leads to the same outcome as cell competition, in that the tissue is eventually clonal. It is notable that several types of stem cells in Drosophila also compete for niche space (Zhang and Kalderon, 2001; Nystul and Spradling, 2007; Jin et al., 2008; Issigonis et al., 2009; Rhiner et al., 2009).
A clue into the mechanism underlying stem cell competition has come from studies in the mouse intestine, where intestinal crypts become monoclonal with time (Griffiths et al., 1988; Winton et al., 1988). This monoclonality implies that individual stem cells can be lost and replaced by their neighbours. A combination of experimental and modelling approaches revealed that this competition among intestinal stem cells follows neutral drift dynamics (Lopez-Garcia et al., 2010; Snippert et al., 2010; Klein and Simons, 2011). Similar observations have been made in the Drosophila midgut (de Navascués et al., 2012). Thus, stem cell competition can be understood as a constantly occurring neutral process, and future work will need to identify the underlying mechanisms that allow one stem cell to outcompete another.
Most of the work described above was carried out by making genetically mosaic tissues or cultures composed of mutant and wild-type cells, and this mixing of two different genotypes might be viewed as somewhat artificial. However, there are several instances of naturally occurring mosaicism in mammals (see Poduri et al., 2013), and cell competition might come into play in these instances. One situation of note is that of random X chromosome inactivation in female mammals, which results in mosaicism. Elegant work has shown that the X-linked gene OCNC1 (also known as CNGA2) is not essential for cell survival in hemizygous males (Zhao and Reed, 2001). However, in heterozygous females, X-inactivation leads to patches of OCNC1 mutant cells that are eliminated. Finally, it is worth highlighting the fact that stochastic and epigenetic differences between cells of the same genotype (i.e. in the absence of mosaicism) can exist and can lead to cell competition (reviewed by Khare and Shaulsky, 2006). Such random differences in expression levels have been shown to account for the probability of individual cells dying after ligand-dependent induction of apoptosis in cultured cells (Spencer et al., 2009).
In fact, two groundbreaking studies have recently suggested that cell competition occurs between genetically identical cells during normal mouse embryo development (Clavería et al., 2013; Sancho et al., 2013). Both studies reported that Myc levels are intrinsically heterogeneous between cells in the early mouse embryo and that cell death is correlated with lower levels of Myc expression. This indicates for the first time that competition may occur naturally in developing animals as a result of stochastic variations in gene expression. Similarly random variations in signalling levels between cells in the Drosophila eye disc can contribute to endogenous cell death. For example, uniformly increasing MAPK signalling can suppress endogenous cell death, implicating differences in MAPK signal transduction in the death of wild-type cells (Yang and Baker, 2003). Consistent with this, the clonal loss of BMP reception in mammals leads to reduced Myc expression and consequent outcompetition (Sancho et al., 2013). Thus, it is possible that much of the endogenous apoptosis observed during development is a consequence of competitive interactions triggered by stochastic differences in gene expression or in signal transduction between neighbouring cells.
Despite its occurrence across species and in many tissues, not all cell types are capable of cell competition. It was shown early on, for instance, that Drosophila larval histoblasts that give rise to the adult abdominal cuticle are not subject to Minute-induced cell competition (Morata and Ripoll, 1975). Furthermore, where competition does occur, there are temporal constraints. For example, Minute-induced cell competition in Drosophila wing discs ceases at the end of larval growth (Simpson and Morata, 1981), and Myc-induced competition in the mouse embryo takes place between older epiblast cells but not younger ESCs (Clavería et al., 2013; Sancho et al., 2013). Intriguingly, cell competition can occur in postmitotic follicle cells in the adult Drosophila ovary. When M/+ or mahj mutant cells are generated, the winner cells increase their size and accelerate endoreplication, revealing a new mechanism for maintaining epithelial integrity (Tamori and Deng, 2013).
Why does cell competition exist?
The conservation of cell competition between invertebrates and mammals suggests that it has important evolutionary purposes. Although these are not yet clear, one proposal is that cell competition is a mechanism of size control and, in particular, that it reduces size variability. Indeed, Drosophila wings in which cell death has been blocked show greater variability in size distribution than controls, although mean wing size is not affected (de la Cova et al., 2004). However, another study disputes that cell competition is a mechanism of size control and argues that the final contribution of a cell to the tissue is purely a consequence of its intrinsic proliferation rate (Martín et al., 2009a). The latter might be true for Minute-induced competition, but in the case of Myc- and STAT-induced competition there is clear evidence for the growth rate of neighbouring cells being affected by the extrinsic process of cell competition (de la Cova et al., 2004; Senoo-Matsuda and Johnston, 2007; Rodrigues et al., 2012).
An issue of interest is the relationship between cell competition and cancer progression, and there is now evidence that competition can function as both a tumour promoter and suppressor. In the case of Minute mutants, it was proposed that competition maintains tissue fitness by removing cells with growth defects from an organism (Morata and Ripoll, 1975). Obviously, this paradigm does not apply to super-competition, in which wild-type cells are removed and the winner has a propensity to excess growth (de la Cova et al., 2004; Moreno and Basler, 2004; Tyler et al., 2007; Ziosi et al., 2010; Vincent et al., 2011; Rodrigues et al., 2012). The phenomenon of super-competition has been compared to a clinical observation called field cancerisation, in which multiple tumour foci appear within the same tissue, suggesting that a progenitor has spread an oncogenic mutation across a large area (Slaughter et al., 1953). The existence of super-competitors suggests that cell competition can promote tumourigenesis. Conversely, in the case of polarity deficient models, cell competition eliminates cells with oncogenic potential (Froldi et al., 2010; Menéndez et al., 2010; Tamori et al., 2010; Ohsawa et al., 2011; Chen et al., 2012; Norman et al., 2012; Ballesteros-Arias et al., 2013). Taken together with the fact that carcinogenesis, occurring mostly in post-reproductive adult life, is unlikely to be under much evolutionary selective pressure, the contradictory roles of cell competition argue against tumour prevention being the reason why it has been preserved through evolution.
One might speculate that cell competition is a remnant of social adaptation of unicellular organisms to multicellular life. For example, the social amoebae Dictyostelium discoideum must cooperate to build a stalk for sporulation; some amoebae take advantage of others to spread their genetic material within the spores but do not contribute to the building of the stalk (Strassmann and Queller, 2011). In a phenomenon reminiscent of cell competition, some of these ‘cheaters’ only cheat when in chimeras but cooperate when they are in animals derived from their same genotype (Santorelli et al., 2008). Recent exciting work has explored this idea of ‘cheating’ in mouse induced pluripotent stem cells (iPSCs) and chimeric embryos. These studies uncovered a network of ‘cheater’ genes that control the relative contribution of cells to the final organism (Dejosez et al., 2013). Although the potential links between cell competition and ‘cheating’ are provocative, future research will be required to establish whether the mechanisms are shared.
Conclusions
Despite the controversies in the field, certain themes have emerged. There is consensus that Minutes, Myc, Scrib and Lgl are conserved inducers of cell competition and that disparities in their levels between neighbouring cells trigger a sequential series of intercellular communications, culminating in the caspase-dependent death of losers. However, the exact effector mechanisms at play during cell competition remain unclear. Nonetheless, the recent groundbreaking studies showing that cell competition occurs in mammalian embryonic development will no doubt fuel fruitful investigations in the future and lead to a better understanding of the communication between neighbouring, competing cells.
Acknowledgments
We thank H. D. Ryoo and three anonymous reviewers for helpful comments on the manuscript. We apologise to colleagues whose work was not included owing to space constraints.
Footnotes
Competing interests
The authors declare no competing financial interests.
Funding
Work in the E.A.B. laboratory is supported by National Institutes of Health grants and an NYSTEM grant to E.A.B. Deposited in PMC for release after 12 months.
References
- Abrams J. M. (2002). Competition and compensation: coupled to death in development and cancer. Cell 110, 403–406 [DOI] [PubMed] [Google Scholar]
- Abrams J. M., White K., Fessler L. I., Steller H. (1993). Programmed cell death during Drosophila embryogenesis. Development 117, 29–43 [DOI] [PubMed] [Google Scholar]
- Agrawal N., Kango M., Mishra A., Sinha P. (1995). Neoplastic transformation and aberrant cell-cell interactions in genetic mosaics of lethal(2)giant larvae (lgl), a tumor suppressor gene of Drosophila. Dev. Biol. 172, 218–229 [DOI] [PubMed] [Google Scholar]
- Ballesteros-Arias L., Saavedra V., Morata G. (2013). Cell competition may function either as tumour-suppressing or as tumour-stimulating factor in Drosophila. Oncogene (in press). doi: 10.1038/onc.2013.407 [DOI] [PubMed]
- Bergmann A., Agapite J., McCall K., Steller H. (1998). The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell 95, 331–341 [DOI] [PubMed] [Google Scholar]
- Bilder D. (2004). Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18, 1909–1925 [DOI] [PubMed] [Google Scholar]
- Böhni R., Riesgo-Escovar J., Oldham S., Brogiolo W., Stocker H., Andruss B. F., Beckingham K., Hafen E. (1999). Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97, 865–875 [DOI] [PubMed] [Google Scholar]
- Bondar T., Medzhitov R. (2010). p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6, 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby A. M., Richardson H. E. (2003). scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769–5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R., Basler K. (1996). Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development 122, 2261–2269 [DOI] [PubMed] [Google Scholar]
- Chen C. L., Gajewski K. M., Hamaratoglu F., Bossuyt W., Sansores-Garcia L., Tao C., Halder G. (2010). The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. USA 107, 15810–15815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Schroeder M. C., Kango-Singh M., Tao C., Halder G. (2012). Tumor suppression by cell competition through regulation of the Hippo pathway. Proc. Natl. Acad. Sci. USA 109, 484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavería C., Giovinazzo G., Sierra R., Torres M. (2013). Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500, 39–44 [DOI] [PubMed] [Google Scholar]
- Cordero J. B., Macagno J. P., Stefanatos R. K., Strathdee K. E., Cagan R. L., Vidal M. (2010). Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev. Cell 18, 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H., Lawrence P. A. (1975). Compartments and polyclones in insect development. Science 189, 340–347 [DOI] [PubMed] [Google Scholar]
- de la Cova C., Abril M., Bellosta P., Gallant P., Johnston L. A. (2004). Drosophila myc regulates organ size by inducing cell competition. Cell 117, 107–116 [DOI] [PubMed] [Google Scholar]
- de Navascués J., Perdigoto C. N., Bian Y., Schneider M. H., Bardin A. J., Martínez-Arias A., Simons B. D. (2012). Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 31, 2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejosez M., Ura H., Brandt V. L., Zwaka T. P. (2013). Safeguards for cell cooperation in mouse embryogenesis shown by genome-wide cheater screen. Science 341, 1511–1514 [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Hafen E. (1994). The sevenless signalling cassette mediates Drosophila EGF receptor function during epidermal development. Development 120, 569–578 [DOI] [PubMed] [Google Scholar]
- Doggett K., Grusche F. A., Richardson H. E., Brumby A. M. (2011). Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Dev. Biol. 11, 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel M., Johnston L. A., Du W. (2004). Repression of dMyc expression by Wingless promotes Rbf-induced G1 arrest in the presumptive Drosophila wing margin. Proc. Natl. Acad. Sci. USA 101, 3857–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray J. I., Timmons A. K., Klein A. P., Pritchett T. L., Welch E., Meehan T. L., Li C., McCall K. (2012). Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells. Development 139, 4029–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc N. C., Heitzler P., Ezekowitz R. A., White K. (1999). Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science 284, 1991–1994 [DOI] [PubMed] [Google Scholar]
- Froldi F., Ziosi M., Garoia F., Pession A., Grzeschik N. A., Bellosta P., Strand D., Richardson H. E., Pession A., Grifoni D. (2010). The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biol. 8, 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A., Merriam J. R. (1971). Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev. Biol. 24, 61–87 [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Ripoll P., Morata G. (1973). Developmental compartmentalisation of the wing disk of Drosophila. Nat. New Biol. 245, 251–253 [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Ripoll P., Morata G. (1976). Developmental compartmentalization in the dorsal mesothoracic disc of Drosophila. Dev. Biol. 48, 132–147 [DOI] [PubMed] [Google Scholar]
- Gateff E. (1978). Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200, 1448–1459 [DOI] [PubMed] [Google Scholar]
- Gibson M. C., Perrimon N. (2005). Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science 307, 1785–1789 [DOI] [PubMed] [Google Scholar]
- Giraldez A. J., Cohen S. M. (2003). Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development 130, 6533–6543 [DOI] [PubMed] [Google Scholar]
- Golic K. G. (1991). Site-specific recombination between homologous chromosomes in Drosophila. Science 252, 958–961 [DOI] [PubMed] [Google Scholar]
- Grewal S. S., Li L., Orian A., Eisenman R. N., Edgar B. A. (2005). Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat. Cell Biol. 7, 295–302 [DOI] [PubMed] [Google Scholar]
- Griffiths D. F., Davies S. J., Williams D., Williams G. T., Williams E. D. (1988). Demonstration of somatic mutation and colonic crypt clonality by X-linked enzyme histochemistry. Nature 333, 461–463 [DOI] [PubMed] [Google Scholar]
- Grzeschik N. A., Amin N., Secombe J., Brumby A. M., Richardson H. E. (2007). Abnormalities in cell proliferation and apico-basal cell polarity are separable in Drosophila lgl mutant clones in the developing eye. Dev. Biol. 311, 106–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik N. A., Parsons L. M., Allott M. L., Harvey K. F., Richardson H. E. (2010). Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573–581 [DOI] [PubMed] [Google Scholar]
- Hafezi Y., Bosch J. A., Hariharan I. K. (2012). Differences in levels of the transmembrane protein Crumbs can influence cell survival at clonal boundaries. Dev. Biol. 368, 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay B. A., Wolff T., Rubin G. M. (1994). Expression of baculovirus P35 prevents cell death in Drosophila. Development 120, 2121–2129 [DOI] [PubMed] [Google Scholar]
- Herz H. M., Chen Z., Scherr H., Lackey M., Bolduc C., Bergmann A. (2006). vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development 133, 1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Wu S., Barrera J., Matthews K., Pan D. (2005). The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122, 421–434 [DOI] [PubMed] [Google Scholar]
- Igaki T. (2009). Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis 14, 1021–1028 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pagliarini R. A., Xu T. (2006). Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16, 1139–1146 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pastor-Pareja J. C., Aonuma H., Miura M., Xu T. (2009). Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 16, 458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issigonis M., Tulina N., de Cuevas M., Brawley C., Sandler L., Matunis E. (2009). JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science 326, 153–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Kirilly D., Weng C., Kawase E., Song X., Smith S., Schwartz J., Xie T. (2008). Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell 2, 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. A., Sanders A. L. (2003). Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat. Cell Biol. 5, 827–833 [DOI] [PubMed] [Google Scholar]
- Johnston L. A., Prober D. A., Edgar B. A., Eisenman R. N., Gallant P. (1999). Drosophila myc regulates cellular growth during development. Cell 98, 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F. D., Rubin G. M. (1998). Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development 125, 1–9 [DOI] [PubMed] [Google Scholar]
- Khare A., Shaulsky G. (2006). First among equals: competition between genetically identical cells. Nat. Rev. Genet. 7, 577–584 [DOI] [PubMed] [Google Scholar]
- Klein A. M., Simons B. D. (2011). Universal patterns of stem cell fate in cycling adult tissues. Development 138, 3103–3111 [DOI] [PubMed] [Google Scholar]
- Kurada P., White K. (1998). Ras promotes cell survival in Drosophila by downregulating hid expression. Cell 95, 319–329 [DOI] [PubMed] [Google Scholar]
- Leong G. R., Goulding K. R., Amin N., Richardson H. E., Brumby A. M. (2009). Scribble mutants promote aPKC and JNK-dependent epithelial neoplasia independently of Crumbs. BMC Biol. 7, 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Baker N. E. (2007). Engulfment is required for cell competition. Cell 129, 1215–1225 [DOI] [PubMed] [Google Scholar]
- Li W., Kale A., Baker N. E. (2009). Oriented cell division as a response to cell death and cell competition. Curr. Biol. 19, 1821–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C., Zheng Y., Yin F., Yu J., Huang J., Hong Y., Wu S., Pan D. (2010). The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl. Acad. Sci. USA 107, 10532–10537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolo F. N., Casas-Tintó S., Moreno E. (2012). Cell competition time line: winners kill losers, which are extruded and engulfed by hemocytes. Cell Rep. 2, 526–539 [DOI] [PubMed] [Google Scholar]
- Lolo F. N., Casas Tintó S., Moreno E. (2013). How winner cells cause the demise of loser cells: cell competition causes apoptosis of suboptimal cells: their dregs are removed by hemocytes, thus preserving tissue homeostasis. BioEssays 35, 348–353 [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C., Klein A. M., Simons B. D., Winton D. J. (2010). Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825 [DOI] [PubMed] [Google Scholar]
- Lu H., Bilder D. (2005). Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7, 1232–1239 [DOI] [PubMed] [Google Scholar]
- Marinari E., Mehonic A., Curran S., Gale J., Duke T., Baum B. (2012). Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545 [DOI] [PubMed] [Google Scholar]
- Martín F. A., Herrera S. C., Morata G. (2009a). Cell competition, growth and size control in the Drosophila wing imaginal disc. Development 136, 3747–3756 [DOI] [PubMed] [Google Scholar]
- Martín F. A., Peréz-Garijo A., Morata G. (2009b). Apoptosis in Drosophila: compensatory proliferation and undead cells. Int. J. Dev. Biol. 53, 1341–1347 [DOI] [PubMed] [Google Scholar]
- Marusyk A., Porter C. C., Zaberezhnyy V., DeGregori J. (2010). Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 8, e1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marygold S. J., Roote J., Reuter G., Lambertsson A., Ashburner M., Millburn G. H., Harrison P. M., Yu Z., Kenmochi N., Kaufman T. C., et al. (2007). The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 8, R216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menéndez J., Pérez-Garijo A., Calleja M., Morata G. (2010). A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc. Natl. Acad. Sci. USA 107, 14651–14656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menut L., Vaccari T., Dionne H., Hill J., Wu G., Bilder D. (2007). A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics 177, 1667–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino M. M., Rhiner C., Portela M., Moreno E. (2013). ‘Fitness fingerprints’ mediate physiological culling of unwanted neurons in Drosophila. Curr. Biol. 23, 1300–1309 [DOI] [PubMed] [Google Scholar]
- Micklem H. S., Clarke C. M., Evans E. P., Ford C. E. (1968). Fate of chromosome-marked mouse bone marrow cells tranfused into normal syngeneic recipients. Transplantation 6, 299–302 [PubMed] [Google Scholar]
- Moberg K. H., Schelble S., Burdick S. K., Hariharan I. K. (2005). Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell 9, 699–710 [DOI] [PubMed] [Google Scholar]
- Morata G., Ripoll P. (1975). Minutes: mutants of drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211–221 [DOI] [PubMed] [Google Scholar]
- Moreno E., Basler K. (2004). dMyc transforms cells into super-competitors. Cell 117, 117–129 [DOI] [PubMed] [Google Scholar]
- Moreno E., Basler K., Morata G. (2002). Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759 [DOI] [PubMed] [Google Scholar]
- Neto-Silva R. M., de Beco S., Johnston L. A. (2010). Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev. Cell 19, 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld T. P., de la Cruz A. F., Johnston L. A., Edgar B. A. (1998). Coordination of growth and cell division in the Drosophila wing. Cell 93, 1183–1193 [DOI] [PubMed] [Google Scholar]
- Newsome T. P., Asling B., Dickson B. J. (2000). Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127, 851–860 [DOI] [PubMed] [Google Scholar]
- Norman M., Wisniewska K. A., Lawrenson K., Garcia-Miranda P., Tada M., Kajita M., Mano H., Ishikawa S., Ikegawa M., Shimada T., et al. (2012). Loss of Scribble causes cell competition in mammalian cells. J. Cell Sci. 125, 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul T., Spradling A. (2007). An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1, 277–285 [DOI] [PubMed] [Google Scholar]
- Oertel M., Menthena A., Dabeva M. D., Shafritz D. A. (2006). Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology 130, 507–520, quiz 590 [DOI] [PubMed] [Google Scholar]
- Ohsawa S., Sugimura K., Takino K., Xu T., Miyawaki A., Igaki T. (2011). Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev. Cell 20, 315–328 [DOI] [PubMed] [Google Scholar]
- Oliver E. R., Saunders T. L., Tarlé S. A., Glaser T. (2004). Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development 131, 3907–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini R. A., Xu T. (2003). A genetic screen in Drosophila for metastatic behavior. Science 302, 1227–1231 [DOI] [PubMed] [Google Scholar]
- Pan D. (2010). The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja J. C., Wu M., Xu T. (2008). An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis. Model. Mech. 1, 144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. T. (1929). The production of mutations in somatic cells of Drosophila melanogaster by means of X-rays. J. Exp. Zool. 53, 327–372 [Google Scholar]
- Poduri A., Evrony G. D., Cai X., Walsh C. A. (2013). Somatic mutation, genomic variation, and neurological disease. Science 341, 1237758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela M., Casas-Tinto S., Rhiner C., López-Gay J. M., Domínguez O., Soldini D., Moreno E. (2010). Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev. Cell 19, 562–573 [DOI] [PubMed] [Google Scholar]
- Posakony L. G., Raftery L. A., Gelbart W. M. (1990). Wing formation in Drosophila melanogaster requires decapentaplegic gene function along the anterior-posterior compartment boundary. Mech. Dev. 33, 69–82 [DOI] [PubMed] [Google Scholar]
- Prober D. A., Edgar B. A. (2000). Ras1 promotes cellular growth in the Drosophila wing. Cell 100, 435–446 [DOI] [PubMed] [Google Scholar]
- Raff M. C. (1992). Social controls on cell survival and cell death. Nature 356, 397–400 [DOI] [PubMed] [Google Scholar]
- Rhiner C., Díaz B., Portela M., Poyatos J. F., Fernández-Ruiz I., López-Gay J. M., Gerlitz O., Moreno E. (2009). Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche. Development 136, 995–1006 [DOI] [PubMed] [Google Scholar]
- Rhiner C., López-Gay J. M., Soldini D., Casas-Tinto S., Martín F. A., Lombardía L., Moreno E. (2010). Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev. Cell 18, 985–998 [DOI] [PubMed] [Google Scholar]
- Richardson E. C., Pichaud F. (2010). Crumbs is required to achieve proper organ size control during Drosophila head development. Development 137, 641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. S., Huang J., Hong Y., Moberg K. H. (2010). Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr. Biol. 20, 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A. B., Zoranovic T., Ayala-Camargo A., Grewal S., Reyes-Robles T., Krasny M., Wu D. C., Johnston L. A., Bach E. A. (2012). Activated STAT regulates growth and induces competitive interactions independently of Myc, Yorkie, Wingless and ribosome biogenesis. Development 139, 4051–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho M., Di-Gregorio A., George N., Pozzi S., Sánchez J. M., Pernaute B., Rodríguez T. A. (2013). Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev. Cell 26, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santorelli L. A., Thompson C. R., Villegas E., Svetz J., Dinh C., Parikh A., Sucgang R., Kuspa A., Strassmann J. E., Queller D. C., et al. (2008). Facultative cheater mutants reveal the genetic complexity of cooperation in social amoebae. Nature 451, 1107–1110 [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N., Johnston L. A. (2007). Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc. Natl. Acad. Sci. USA 104, 18543–18548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Dahmann C. (2005). Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science 307, 1789–1790 [DOI] [PubMed] [Google Scholar]
- Simpson P. (1979). Parameters of cell competition in the compartments of the wing disc of Drosophila. Dev. Biol. 69, 182–193 [DOI] [PubMed] [Google Scholar]
- Simpson P., Morata G. (1981). Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Dev. Biol. 85, 299–308 [DOI] [PubMed] [Google Scholar]
- Slaughter D. P., Southwick H. W., Smejkal W. (1953). Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6, 963–968 [DOI] [PubMed] [Google Scholar]
- Snippert H. J., van der Flier L. G., Sato T., van Es J. H., van den Born M., Kroon-Veenboer C., Barker N., Klein A. M., van Rheenen J., Simons B. D., et al. (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 [DOI] [PubMed] [Google Scholar]
- Spencer S. L., Gaudet S., Albeck J. G., Burke J. M., Sorger P. K. (2009). Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459, 428–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H. (2008). Regulation of apoptosis in Drosophila. Cell Death Differ. 15, 1132–1138 [DOI] [PubMed] [Google Scholar]
- Strassmann J. E., Queller D. C. (2011). Evolution of cooperation and control of cheating in a social microbe. Proc. Natl. Acad. Sci. USA 108 Suppl. 2, 10855–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Irvine K. D. (2011). Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev. Biol. 350, 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y., Deng W. M. (2013). Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Dev. Cell 25, 350–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y., Bialucha C. U., Tian A. G., Kajita M., Huang Y. C., Norman M., Harrison N., Poulton J., Ivanovitch K., Disch L., et al. (2010). Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 8, e1000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman A. A., Cohen S. M. (2000). Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103, 971–980 [DOI] [PubMed] [Google Scholar]
- Tepass U., Fessler L. I., Aziz A., Hartenstein V. (1994). Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 120, 1829–1837 [DOI] [PubMed] [Google Scholar]
- Thompson B. J., Mathieu J., Sung H. H., Loeser E., Rørth P., Cohen S. M. (2005). Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell 9, 711–720 [DOI] [PubMed] [Google Scholar]
- Tyler D. M., Li W., Zhuo N., Pellock B., Baker N. E. (2007). Genes affecting cell competition in Drosophila. Genetics 175, 643–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M., Bohmann D. (2006). JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25, 5294–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M., Jasper H., Bohmann D. (2005). Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc. Natl. Acad. Sci. USA 102, 13123–13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T., Bilder D. (2005). The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell 9, 687–698 [DOI] [PubMed] [Google Scholar]
- Verdu J., Buratovich M. A., Wilder E. L., Birnbaum M. J. (1999). Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell Biol. 1, 500–506 [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Kolahgar G., Gagliardi M., Piddini E. (2011). Steep differences in wingless signaling trigger Myc-independent competitive cell interactions. Dev. Cell 21, 366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. P., Fletcher A. G., Baena-Lopez L. A. (2013). Mechanisms and mechanics of cell competition in epithelia. Nat. Rev. Mol. Cell Biol. 14, 581–591 [DOI] [PubMed] [Google Scholar]
- Winton D. J., Blount M. A., Ponder B. A. (1988). A clonal marker induced by mutation in mouse intestinal epithelium. Nature 333, 463–466 [DOI] [PubMed] [Google Scholar]
- Woods D. F., Bryant P. J. (1991). The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66, 451–464 [DOI] [PubMed] [Google Scholar]
- Xu T., Rubin G. M. (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 [DOI] [PubMed] [Google Scholar]
- Yang L., Baker N. E. (2001). Role of the EGFR/Ras/Raf pathway in specification of photoreceptor cells in the Drosophila retina. Development 128, 1183–1191 [DOI] [PubMed] [Google Scholar]
- Yang L., Baker N. E. (2003). Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev. Cell 4, 359–369 [DOI] [PubMed] [Google Scholar]
- Yao C. K., Lin Y. Q., Ly C. V., Ohyama T., Haueter C. M., Moiseenkova-Bell V. Y., Wensel T. G., Bellen H. J. (2009). A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell 138, 947–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kalderon D. (2001). Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599–604 [DOI] [PubMed] [Google Scholar]
- Zhao H., Reed R. R. (2001). X inactivation of the OCNC1 channel gene reveals a role for activity-dependent competition in the olfactory system. Cell 104, 651–660 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Hartwieg E., Horvitz H. R. (2001). CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104, 43–56 [DOI] [PubMed] [Google Scholar]
- Ziosi M., Baena-López L. A., Grifoni D., Froldi F., Pession A., Garoia F., Trotta V., Bellosta P., Cavicchi S., Pession A. (2010). dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genet. 6, e1001140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv O., Suissa Y., Neuman H., Dinur T., Geuking P., Rhiner C., Portela M., Lolo F., Moreno E., Gerlitz O. (2009). The co-regulator dNAB interacts with Brinker to eliminate cells with reduced Dpp signaling. Development 136, 1137–1145 [DOI] [PubMed] [Google Scholar]


