Abstract
Coordinated arterial-venous differentiation is crucial for vascular development and function. The origin of the cardinal vein (CV) in mammals is unknown, while conflicting theories have been reported in chick and zebrafish. Here, we provide the first molecular characterization of endothelial cells (ECs) expressing venous molecular markers, or venous-fated ECs, within the emergent dorsal aorta (DA). These ECs, expressing the venous molecular markers Coup-TFII and EphB4, cohabited the early DA with ECs expressing the arterial molecular markers ephrin B2, Notch and connexin 40. These mixed ECs in the early DA expressed either the arterial or venous molecular marker, but rarely both. Subsequently, the DA exhibited uniform arterial markers. Real-time imaging of mouse embryos revealed EC movement from the DA to the CV during the stage when venous-fated ECs occupied the DA. We analyzed mutants for EphB4, which encodes a receptor tyrosine kinase for the ephrin B2 ligand, as we hypothesized that ephrin B2/EphB4 signaling may mediate the repulsion of venous-fated ECs from the DA to the CV. Using an EC quantification approach, we discovered that venous-fated ECs increased in the DA and decreased in the CV in the mutants, whereas the rest of the ECs in each vessel were unaffected. This result suggests that the venous-fated ECs were retained in the DA and missing in the CV in the EphB4 mutant, and thus that ephrin B2/EphB4 signaling normally functions to clear venous-fated ECs from the DA to the CV by cell repulsion. Therefore, our cellular and molecular evidence suggests that the DA harbors venous progenitors that move to participate in CV formation, and that ephrin B2/EphB4 signaling regulates this aortic contribution to the mammalian CV.
Keywords: Vascular development, Ephrin B2, EphB4, Notch, Coup-TFII, Arterial-venous differentiation, Angiogenesis, Mouse
INTRODUCTION
Proper arterial venous (AV) differentiation is crucial for the establishment of normal vasculature and vascular function. The developing dorsal aorta (DA) and cardinal vein (CV), the first artery and vein pair to form in the body, offer an excellent system in which to study the mechanism of AV differentiation. Endothelial cells (ECs) lining the blood vessel lumen are the key cell type responsible for the initiation of DA and CV development. The DA emerges before the CV, by de novo differentiation and aggregation of angioblasts, the precursors of ECs (Hirakow and Hiruma, 1981; Coffin and Poole, 1988; Drake and Fleming, 2000).
The origin of the CV remains controversial. In chick, Sabin proposed a century ago that longitudinal anastomoses of DA protrusions give rise to the CV, based on her observation of cultured chick embryos (Sabin, 1917). However, later electron microscopic and immunostaining studies refute an aortic origin of the CV, and propose instead that the CV arises in situ by the de novo differentiation of angioblasts from the mesoderm (Hirakow and Hiruma, 1981; Coffin and Poole, 1988).
In zebrafish, it was reported that the AV fate decision is determined in angioblasts in the lateral mesoderm before their migration to form the respective DA and CV (Zhong et al., 2001). Supporting this theory, the DA and CV emerge by direct migration of two separate sources of progenitor cells in two independent waves from the lateral plate mesoderm (Williams et al., 2010; Kohli et al., 2013). However, it has also been reported that the CV forms via segregation from the DA (Herbert et al., 2009). Therefore, whether the DA contributes to the CV remains to be established. One key piece of evidence in support of this model, the presence of ECs with venous molecular identity in the DA, has not been reported in either chick or zebrafish. The mammalian origin of the CV is unknown (Pitulescu and Adams, 2010; Potente et al., 2011).
The identification of genes that are preferentially expressed in either arterial or venous ECs (Rocha and Adams, 2009; Swift and Weinstein, 2009) opens the possibility to identify arterial- and venous-fated ECs in the development of the DA and CV. Efnb2 encodes the transmembrane signaling molecule ephrin B2, the first marker identified in arterial but not venous ECs. Conversely, its tyrosine kinase receptor, EphB4, is expressed in venous but not arterial ECs (Wang et al., 1998; Adams et al., 1999). Although their expression is indicative of AV specification, these genes are not required for arterial or venous fate decisions (Wang et al., 1998).
Notch receptors and ligands are also expressed in arterial but not venous ECs (Villa et al., 2001). They are transmembrane proteins that have been implicated in bi-potential cell fate decisions in multiple systems (Guruharsha et al., 2012). Several lines of evidence indicate that Notch signaling promotes arterial and suppresses venous fates (Swift and Weinstein, 2009). Coup-TFII (Nr2f2 - Mouse Genome Informatics), a member of the orphan nuclear receptor superfamily, is expressed in venous but not arterial ECs (You et al., 2005). Coup-TFII promotes venous and suppresses arterial fates. A Coup-TFII-Notch-ephrin B2/EphB4 axis has been proposed, in which Coup-TFII represses Notch, which in turn upregulates ephrin B2 and downregulates EphB4 (You et al., 2005).
Ephrin B2 and EphB4 mediate crucial cellular processes in the formation of arteries and veins downstream of the transcription factors Notch and Coup-TFII. Although ephrin B2 can bind to multiple EphB class receptors, it is the only known ligand for the EphB4 receptor (Pasquale, 2010). In general, ephrin/Eph signaling mediates cellular behaviors, such as repulsion, motility and adhesion, to sort mixed cell types and set boundaries between different cell types in neuronal, bone and other tissue types (Pasquale, 2010; Klein, 2012). One signature function of ephrin/Eph signaling is to mediate the repulsion of Eph-expressing cells away from ephrin-expressing cells (Pasquale, 2010; Klein, 2012). In vascular development, ephrin B2 and EphB4 are required for the progression from the primitive vascular network into remodeled arteries and veins (Wang et al., 1998; Adams et al., 1999) and to balance the sizes of the DA and CV (Kim et al., 2008). In vitro experiments have suggested that ephrin B2/EphB4 signaling mediates the repulsion of EphB4-overexpressing porcine aortic ECs away from ephrin B2-overexpressing ECs (Füller et al., 2003), but whether this repulsion occurs in ECs in vivo is currently unknown.
Here, we used mouse genetics, high-resolution quantitative microscopy and live imaging technology to analyze the origin of the CV and the function of the ephrin B2/EphB4 signaling pathway in the developing mouse DA and CV. We provide the first molecular evidence that venous precursors originate from the developing DA and relocate to the CV, and that ephrin B2/EphB4 signaling regulates this process in mice. Our molecular evidence validates the historical theory that the CV arises from the DA.
RESULTS
The dorsal aortae contain a heterogeneous population of endothelial cells, with and without arterial identity
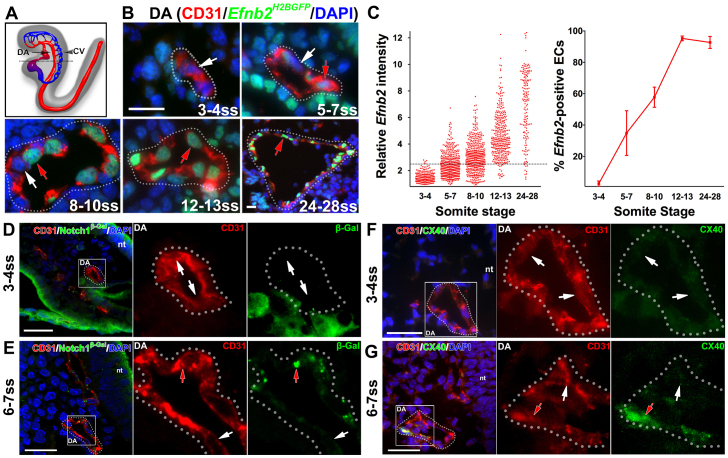
To uncover the emergence of arterial identity in ECs of the developing DA, we investigated the expression pattern of Efnb2. We used the Efnb2H2BGFP reporter mouse, in which a nuclear GFP gene, H2BGFP, is knocked into the Efnb2 locus; thus, the GFP signal reflects Efnb2 expression (Davy and Soriano, 2007) (Fig. 1A,B). The GFP intensity was quantified in individual ECs in the DA between 3 and 28 somite stage (ss; Fig. 1C, top).
Fig. 1.
The dorsal aorta transiently contained both arterial marker-positive and -negative endothelial cells. (A-C) Arterial marker Efnb2 expression in cross-sections of the developing DA using the Efnb2H2BGFP/+ reporter. (A) Schematic lateral view of the DA (arrow) and the CV (arrowhead) at E8.5. The dotted black line indicates the level of cross-sections in B. (B) At 3-4 ss, the DA was composed of ECs without detectable Efnb2 expression. At 5-7 ss and 8-10 ss, both Efnb2-positive (red arrows) and -negative (white arrows) ECs were detected. By 12-13 ss, as well as at 24-28 ss, most ECs in the DA were Efnb2 positive. DA is outlined by a dotted line. (C) The intensity of Efnb2 expression in individual ECs was quantified and the average percentage of Efnb2-positive ECs ± s.e.m. was plotted. A relative Efnb2 intensity above 2.5, marked with a dotted line, was considered positive. (D-G) Expression of other arterial markers in cross-sections of the developing DA. (D,E) Notch1+/lacZ embryo stained for β-gal (reporter for Notch1) at 3-4 ss (D) and 6-7 ss (E). ECs in the DA were primarily negative for Notch1 expression (white arrows) at 3-4 ss. At 6-7 ss, Notch1-positive (red arrow) and -negative ECs were present in the DA. (F,G) Cx40 staining at 3-4 ss (F) and 6-7 ss (G). ECs in the DA were primarily negative for Cx40 expression (white arrows) at 3-4 ss, but at 6-7 ss, both Cx40-positive (red arrows) and -negative ECs were present in the DA. CD31 (red), arterial markers (green), DAPI (blue). Boxed areas are magnified in the right-hand panels. CV, cardinal vein; DA, dorsal aorta; nt, neural tube. Scale bars: 25 μm.
At 3-4 ss, few ECs (2.1±1.2%) in the DA were positive for Efnb2 expression (Fig. 1C, bottom; supplementary material Table S1). At 5-7 ss, 34.9±14.2% and at 8-10 ss, 57.8±6.4% of ECs in the DA were positive for Efnb2 expression. Therefore, by 10 ss, some ECs in the DA were positive for Efnb2 expression and others were negative. By 12-13 ss, virtually all ECs (95.2±1.5%) in the DA were positive for Efnb2 expression. This uniform Efnb2 expression was maintained thereafter, e.g. at 24-28 ss (92.7±3.8%). We did detect a few Efnb2-negative ECs at these late stages, which might be due to the nature of our detection methodology.
ECs in the DA that do not express Efnb2 may reflect uncommitted cells or cells that are early in the arterial fate specification pathway. To determine this, we assayed expression of Notch1, using a Notch1lacZ reporter, in which the lacZ gene is knocked into the Notch1 locus; thus, the β-galactosidase reflects Notch1 expression (supplementary material Fig. S1A-C). Most ECs were Notch1 negative in the DA at 3-4 ss as detected by either X-Gal staining or immunofluorescence staining of β-galactosidase (β-gal; Fig. 1D), even though we detected high expression levels in the presomitic mesoderm and endocardium at this stage (supplementary material Fig. S1D). Similar to EfnB2, both Notch1-positive and -negative ECs were found in the DA at 6-7 ss (Fig. 1E; supplementary material Fig. S1E).
Finally, we examined the expression pattern of a third arterial marker, connexin 40 (Cx40; Gja5 - Mouse Genome Informatics). We first verified that Cx40 was indeed arterial specific at 25 ss, when arterial identity was established in the DA (supplementary material Fig. S2). However, we did not detect Cx40-positive ECs in the DA at 3-4 ss (Fig. 1F), but did find both Cx40-positive and -negative ECs at 6-7 ss (Fig. 1G). We therefore confirmed that the developing DA contains both arterial marker-positive and -negative ECs (supplementary material Fig. S3). Thus, the DA evolved through an intermediate stage containing ECs with and without arterial identity.
The dorsal aortae contain a heterogeneous population of endothelial cells, with and without venous identity
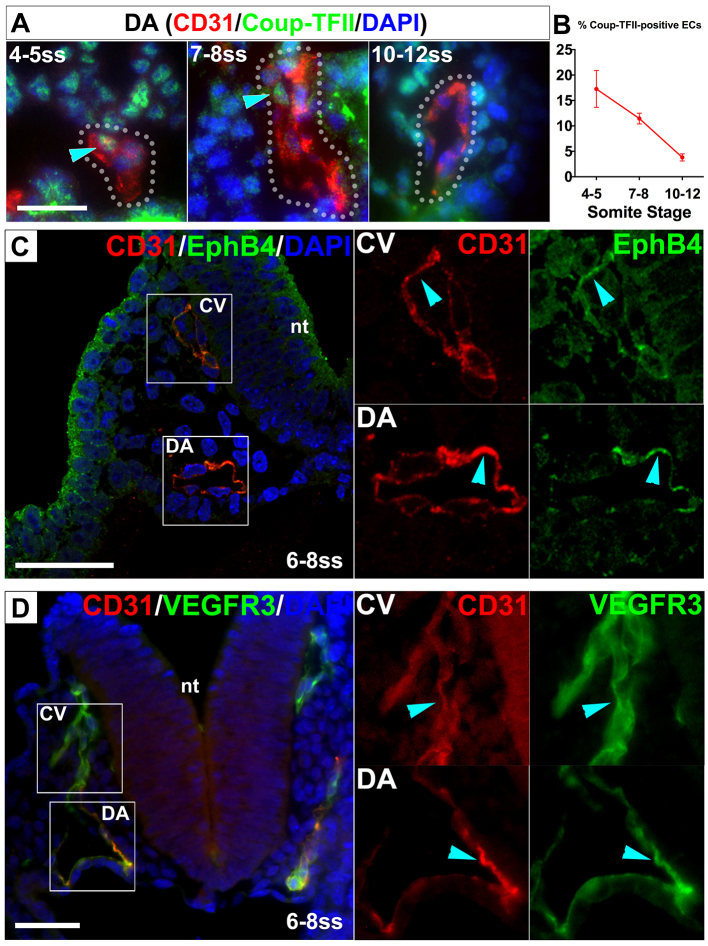
Because some ECs in the DA did not express arterial markers, we asked whether there were ECs in the DA that exhibit venous lineage identity. To address this question, we examined the expression pattern of the venous-specific marker nuclear Coup-TFII (You et al., 2005). We performed immunostaining for Coup-TFII (Fig. 2A), followed by quantification of the staining intensity in the nuclei of individual ECs. We found that 17.3±3.6% of ECs were Coup-TFII positive in the DA at 4-5 ss, 11.4±1.1% at 7-8 ss and 3.8±0.7% by 10-12 ss (Fig. 2B; supplementary material Table S2). In addition, we visually scored individual ECs as either Coup-TFII positive or negative and achieved similar results. Therefore, the DA contained not only cells uncommitted to arterial fate, but also a significant subset of ECs expressing the venous marker Coup-TFII. The Coup-TFII expression also suggests a heterogeneous stage.
Fig. 2.
The dorsal aorta transiently contained both venous marker-positive and -negative endothelial cells. (A,B) Cross-sections of the DA immunostained for the venous marker Coup-TFII. (A) Coup-TFII (green) was detected in some ECs (blue arrowheads) in the DA by 4-8 ss but only in a few ECs at 10-12 ss. (B) Quantification of Coup-TFII-positive ECs in the DA. Error bars represent s.e.m. (C,D) Venous marker expression was detected in a subset of ECs in the DA. Cross-sections of the DA and CV stained for CD31 (red), the venous markers EphB4 and Vegfr3 (green), and DAPI (blue) in 6-8 ss embryos. EphB4- (C) and Vegfr3- (D) positive ECs (blue arrowheads) were present in the DA as well as in the CV. Boxed areas are magnified in the right-hand panels. CV, cardinal vein; DA, dorsal aorta; nt, neural tube. Scale bars: 25 μm.
To validate the venous identity of ECs in the DA, we also examined the expression of two other venous markers. EphB4-positive ECs were present in the DA at 6-8 ss (Fig. 2C; supplementary material Fig. S4). Similarly, we found ECs expressing the putative venous marker Vegfr3 (Flt4 - Mouse Genome Informatics) in the DA at 6-8 ss (Fig. 2D). Thus, our data demonstrate that the DA contains a subset of ECs exhibiting venous identity. These findings, together with our data on arterial expression in the DA, demonstrate that when the DA contains ECs with and without arterial identity, the DA also contains ECs with venous identity.
The heterogeneous population of endothelial cells exhibited either arterial or venous identity
To determine whether the ECs in the DA with venous identity also expressed markers of arterial identity, we performed immunofluorescence staining for Coup-TFII in Efnb2H2BGFP embryos. In general, the Coup-TFII-positive ECs in both the DA and the CV were negative for Efnb2 (Fig. 3A). We further quantified the expression of both Coup-TFII and Efnb2 in ECs between 4 and 12 ss (Fig. 3B). We found that 99.1% of all ECs in the DA did not co-express Coup-TFII and Efnb2. Of the 16.1% Coup-TFII-positive ECs in the DA, 94.4% were Efnb2 negative.
Fig. 3.
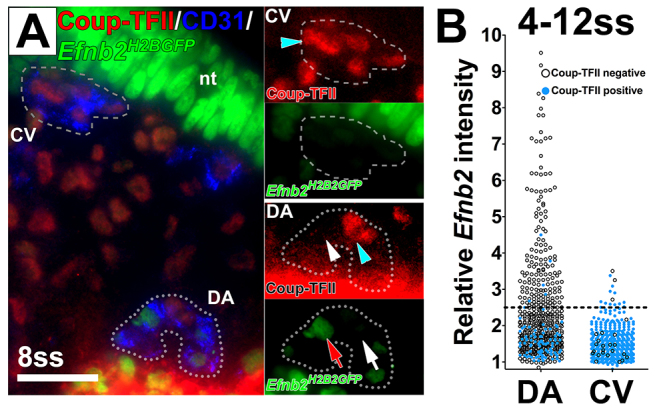
A heterogeneous population of endothelial cells in the dorsal aorta and cardinal vein expressed either the arterial marker Efnb2 or the venous marker Coup-TFII. (A) Cross-section of an Efnb2H2BGFP/+ embryo stained for CD31 (blue) and Coup-TFII (red). Insets show magnified views of the DA and CV. Coup-TFII (blue arrowheads) and Efnb2 (red arrow) were not detected in the same EC in either the DA or the CV. White arrow indicates Efnb2-negative ECs and white arrowhead Coup-TFII-negative ECs. (B) Quantification of Efnb2 intensity in Coup-TFII-stained Efnb2H2BGFP/+ embryos at 4-12 ss. Relative Efnb2 intensity above 2.5 was considered positive (dotted line). Blue circles indicate Coup-TFII-positive ECs and black circles indicate Coup-TFII-negative ECs. The Efnb2 intensity was below 2.5 for most Coup-TFII-positive ECs. nt: neural tube. CV, cardinal vein; DA, dorsal aorta; nt, neural tube. Scale bar: 25 μm.
Despite the rarity (2-5%) of weakly Efnb2-positive ECs in the CV, we found, similar to the DA, that 97.3% of all ECs in the CV did not co-express Coup-TFII and Efnb2. Of the Coup-TFII-positive ECs, 97.1% were Efnb2 negative. Therefore, most individual ECs in the DA or CV did not co-express arterial and venous markers and instead exhibited either arterial or venous identity. This observation further supports our finding that venous-fated cells reside in the DA.
Endothelial cells move from the dorsal aorta to the cardinal vein
We have identified a previously uncharacterized population of ECs with venous molecular identity in the developing DA that decreases over time. One hypothesis as to the disappearance of these cells is that venous marker-expressing ECs move from the DA to participate in CV formation, as proposed in chick (Sabin, 1917) and zebrafish (Herbert et al., 2009). We and others have reported transient vascular connections between the DA and CV (Gerety and Anderson, 2002; Kim et al., 2008; Walls et al., 2008), which are present around this developmental stage, when venous-fated ECs are present in the DA. We hypothesize that these connections between the DA and CV are possible physical conduits for EC movement from the DA to the CV.
To test directly the hypothesis that ECs move from the DA to CV, we used a transgenic endothelial reporter, Tie2-Cre;mT/mG, in which membrane-targeted GFP (mG) (Muzumdar et al., 2007) is expressed after Tie2-Cre-mediated excision. Tie2-Cre is active in ECs and hematopoietic cells (Braren et al., 2006). At this stage, the hematopoietic cells are primarily primitive red blood cells, which are easily distinguishable from ECs by morphology. Thus, we were able to identify ECs based on Tie2-Cre activity (GFP) and morphology. To meet the challenge of imaging vascular structures deep within a living mouse embryo over time, we modified the static ex utero whole-embryo culture method pioneered by Jones et al. (Jones et al., 2002). These techniques allowed us to keep the DA and CV in focus in five of 35 cultured embryos for continuous imaging for up to 12 hours. Imaging was started after turning of the embryo, at ∼10-12 ss. All five embryos showed ECs moving from the DA to the CV (Fig. 4; supplementary material Movie 1). We validated this finding using 4D two-photon imaging (supplementary material Movie 2). In these experiments, for the connections that were in focus, we captured at least one relocation event per embryo. In addition, we saw signs of movement in connections that were out of focus. Given the technical difficulties in imaging ECs deep in mouse embryos, this result probably understates the frequency of EC movement events. We did not observe ECs moving from the CV to the DA. Thus, our results demonstrate definitively and for the first time that ECs do indeed relocate from the DA to the CV during normal mouse embryo development.
Fig. 4.
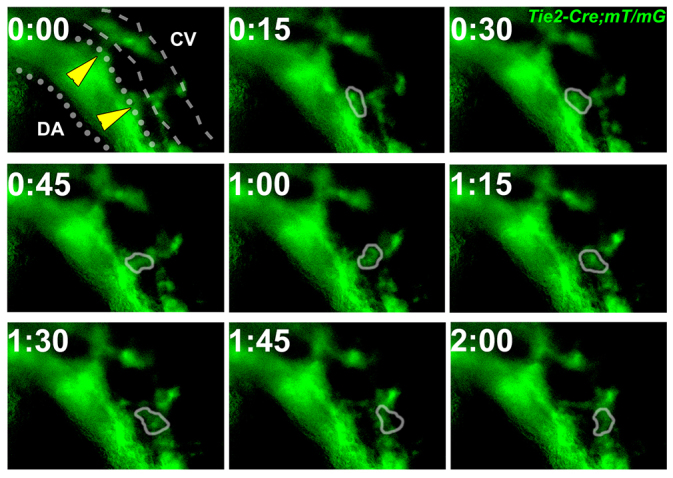
Endothelial cells moved from the dorsal aorta to the cardinal vein. Time-lapse imaging of EC movement in a Tie2-Cre;mT/mG embryo at 10 ss. Membrane-targeted GFP (green) is expressed by cells of the Tie2 lineage. During a 2-hour time frame, GFP-positive cell(s) with EC morphology (circled) moved from the DA (dotted line) to the CV (dashed line). Yellow arrowheads indicate connections between the DA and CV. CV, cardinal vein; DA, dorsal aorta.
Efnb2 and EphB4 are required for the morphogenesis of the dorsal aorta and cardinal vein from the heterogeneous arterial venous stage
We have previously reported that Efnb2-/- and EphB4-/- mutants exhibit a larger DA and a concomitantly smaller CV (Kim et al., 2008), and we hypothesize that these genes are required in EC distribution between these vessels. Given our observations of venous-fated cells in the DA and of EC migration from DA to CV during the heterogeneous stage, we predict that these genes are required at this stage. If true, then a larger DA and smaller CV phenotype would emerge in the Efnb2-/- and EphB4-/- mutants from the heterogeneous AV stage.
To ascertain the onset of Efnb2-/- and EphB4-/- DA and CV phenotype, we used high-resolution 3D two-photon microscopy to analyze the sizes of the DA and CV in CD31 (Pecam1)-stained whole-mount embryos. At 8-10 ss, we did not detect obvious signs of a larger DA in the Efnb2-/- or EphB4-/- mutants compared with controls (Fig. 5A,B,E,F; supplementary material Movies 3, 4). By 11-13 ss, Efnb2-/- and EphB4-/- mutants exhibited an obviously larger DA and smaller CV compared with controls (Fig. 5C,D,G,H; supplementary material Movies 5, 6). Therefore, the emergence of the hypothesized phenotype did indeed occur in the Efnb2-/- and EphB4-/- mutants during the heterogeneous AV stage.
Fig. 5.
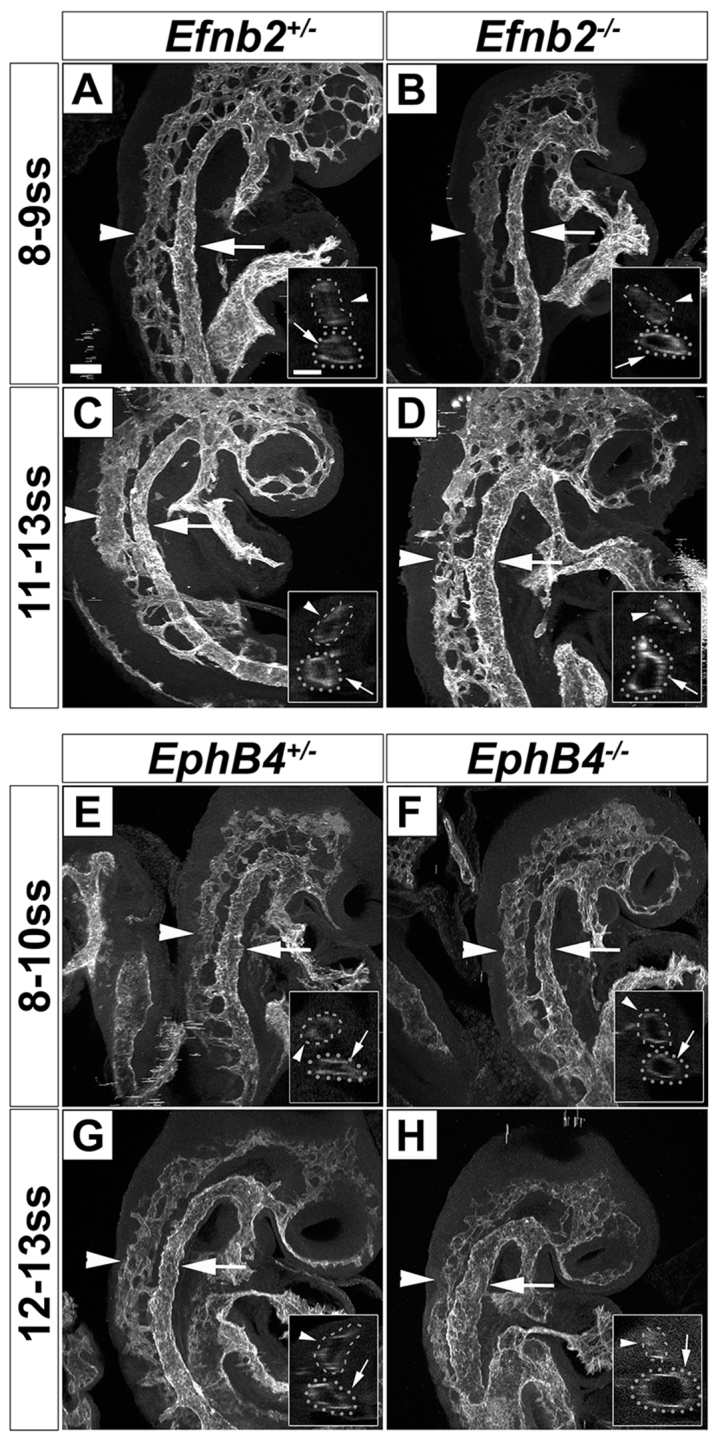
Efnb2 and EphB4 are required for the morphogenesis of the dorsal aorta and cardinal vein from the heterogeneous AV stage. (A-D) A larger DA and a smaller CV were first detected at 11-13 ss in Efnb2-/- mutants. Two-photon optical sectioning microscopy of CD31-stained embryos at 8-9 and 11-13 ss depicting the DA (arrows) and CV (arrowheads). At 8-9 ss, the DA and CV sizes in Efnb2-/- mutants (B) were comparable to controls (A). At 11-13 ss, Efnb2-/- mutants (D) had a bigger DA and a smaller CV compared with controls (C). Insets show an optical projection of selected slices depicting the DA and CV. (E-H) A larger DA and a smaller CV were first detected at 12-13 ss in EphB4-/- mutants. Two-photon optical sectioning microscopy of CD31-stained embryos at 8-10 and 12-13 ss depicting the DA (arrows) and CV (arrowheads). At 8-10 ss, the DA and CV sizes in EphB4-/- mutants (F) were comparable to controls (E). At 12-13 ss, EphB4-/- mutants (H) had a bigger DA and a smaller CV compared with controls (G). Both left and right sides of four embryos of each genotype were analyzed. CV, cardinal vein; DA, dorsal aorta. Scale bars: 100 μm.
In EphB4-/- mutants, Coup-TFII-positive ECs increase in the DA and concomitantly decrease in the CV
The temporal coincidence of venous-fated cells in the DA, EC migration from DA to CV, and onset of Efnb2-/- and EphB4-/- mutant phenotypes, in addition to the known role of EphB4 mediating the repulsion of EphB4-expressing cells away from ephrin B2-expressing cells in vitro (Füller et al., 2003), led us to hypothesize that ephrin B2/EphB4 signaling mediates segregation of venous-fated ECs from the DA to the CV formation during the heterogeneous AV stage. According to this model, the larger EphB4-/- mutant DA contains extra venous-fated ECs that would otherwise relocate to the CV, and the smaller CV results from the absence of these ECs.
To test our hypothesis, we quantified the Coup-TFII-positive and total ECs in EphB4-/- embryos at 11-15 ss, near the end of the heterogeneous stage of DA development. This is after the onset of a large DA and small CV phenotype in the EphB4-/- embryos (Fig. 5G,H), but before the embryos develop severe developmental defects, which might lead to secondary defects and therefore interfere with data interpretation. We stained cross-sections of mutant EphB4-/- and control EphB4+/- embryos for both the EC marker CD31 and the venous marker Coup-TFII and detected an increase of Coup-TFII-positive ECs in the larger EphB4-/- DA (Fig. 6A,B). We then measured the signal intensity for Coup-TFII and counted CD31-positive ECs, as shown in Fig. 2.
Fig. 6.
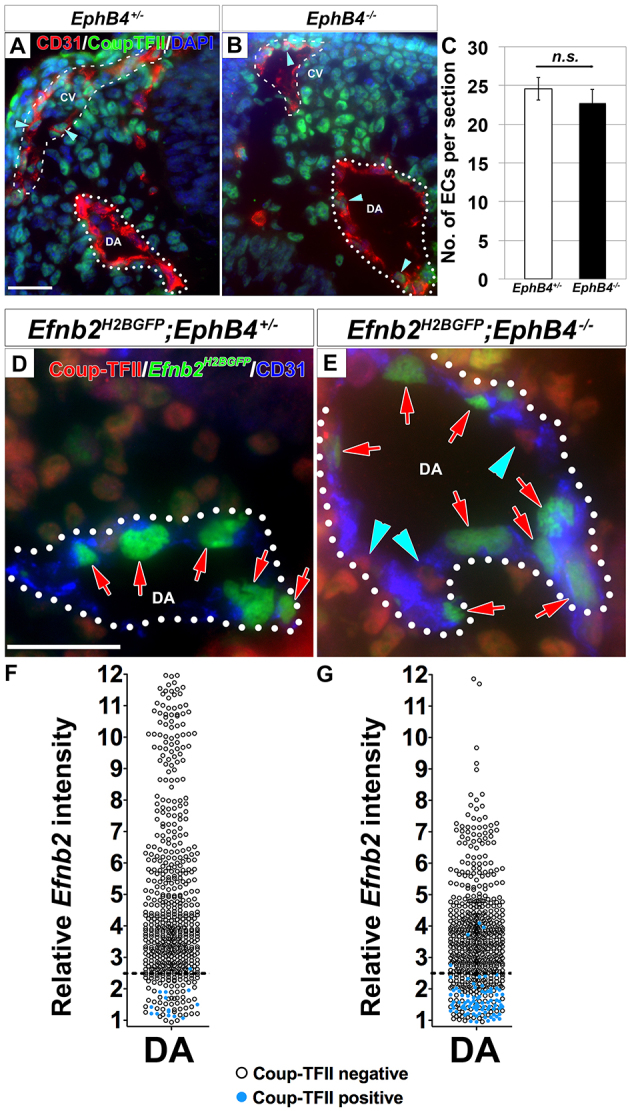
Coup-TFII-positive endothelial cells mislocalized to the larger dorsal aorta at the expense of the concomitantly smaller cardinal vein in EphB4-/- mutants. (A-C) Coup-TFII-positive ECs were detected in the DA in EphB4-/- mutants at 11-15 ss. Cross-sections of the DA (dotted line) and CV (dashed line) in 14 ss EphB4+/- (A) and EphB4-/- (B) embryos stained for Coup-TFII (green) and CD31 (red). EphB4-/- embryos exhibited an increased number of Coup-TFII-positive ECs in the larger DA (arrowheads), and a decreased number of Coup-TFII-positive ECs (arrowheads) in the smaller CV. This was accompanied by a similar increase in total number of ECs in the DA and similar decrease in the CV (see Table 1). There was no significant difference in total ECs in the DA and CV per section between EphB4+/- and EphB4-/- embryos (C). EphB4+/-: 24.6±1.5 ECs; EphB4-/-: 22.7±1.8 ECs; P=0.45, n=5. Error bars represent s.e.m. (D-G) Coup-TFII-positive ECs in the DA of EphB4-/- embryos did not express Efnb2. Cross-sections of the DA (dotted line) from 14 ss Efnb2H2BGFP/+;EphB4+/- control (D) and Efnb2H2BGFP/+;EphB4-/- mutant (E) embryos stained for Coup-TFII (red) and CD31 (blue). Coup-TFII-positive ECs (blue arrowheads) of the DA in the mutant did not co-express Efnb2 (red arrows). The Efnb2 intensity was quantified in Coup-TFII-stained EphB4+/- (F) and EphB4-/- (G) embryos at 11-14 ss. Relative Efnb2 intensity above 2.5 (dotted line) was considered positive. Blue circles indicate Coup-TFII-positive ECs and black circles indicate Coup-TFII-negative ECs. The Efnb2 intensity was below 2.5 for almost all Coup-TFII-positive ECs. DAPI (blue). CV, cardinal vein; DA, dorsal aorta. Scale bars: 25 μm.
We did not detect a change in the average number of ECs per section in the DA and CV combined between the control and mutant (Fig. 6C). Thus, we interpret changes in the number of cells in each vessel as changes in distribution between them and present them as proportion of total ECs (Table 1). ECs were distributed equally in the DA and CV in EphB4+/- controls. In EphB4-/- mutants, we found an average 7.9±0.9% change in the distribution of ECs (increase in the DA and decrease in the CV). The total number of Coup-TFII-positive ECs in the DA and CV combined (DA+CV) was unchanged in the mutant demonstrating that the overall number of venous ECs is unperturbed.
Table 1.
Distribution of Coup-TFII-positive endothelial cells in 11-15 ss EphB4-/- mutants
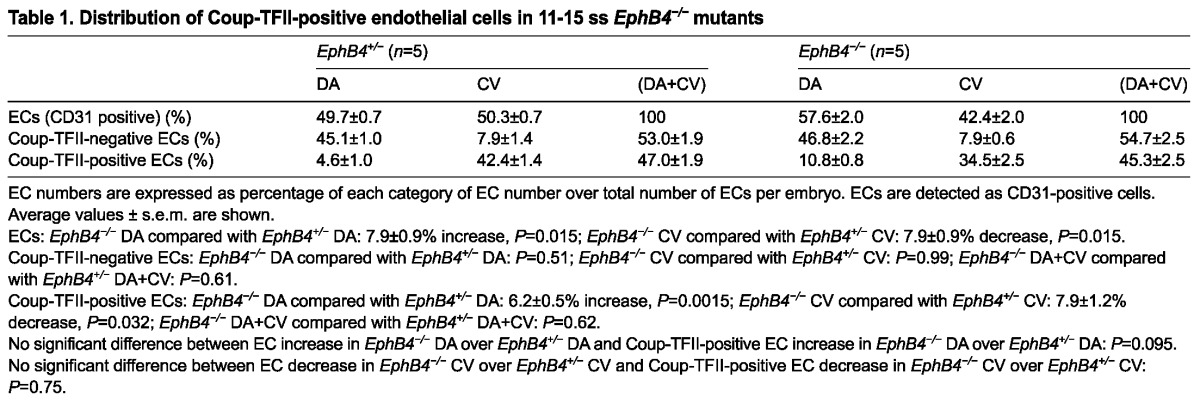
We did not detect a difference between control and mutant in Coup-TFII-negative ECs in the DA, CV, or combined (DA+CV). However, we did detect a 6.2±0.5% increase in the Coup-TFII-positive ECs in the mutant DA and a similar (7.9±1.2%) decrease in the mutant CV. Importantly, the increase in Coup-TFII-positive ECs in the DA was similar to the increase in total ECs in the DA in the mutant, and the decrease in Coup-TFII-positive ECs was similar to the decrease in total ECs in the CV in the mutant. Therefore, in the EphB4-/- mutant, the increase in Coup-TFII-positive ECs in the DA is responsible for the increase in the overall number of ECs in the DA and the accompanying decrease in the ECs in the CV, thereby resulting in a larger DA and smaller CV. The additional venous ECs in the DA in the EphB4 mutant represent venous ECs of arterial origin. Therefore, we estimate that ∼15% (7.9/50.3%) of ECs in the CV originate from the DA as a result of EphB4 functions up until 11-15 ss. However, this estimate may under-represent the total number of CV ECs that come from the DA throughout development, at other time points or via other signaling pathways.
To ascertain whether the Coup-TFII-positive ECs that we observed in the EphB4 mutant DA exhibit arterial identity, we examined the Coup-TFII-positive ECs in the mutant DA for Efnb2 expression. We stained Efnb2H2BGFP;EphB4-/- mutant and Efnb2H2BGFP;EphB4+/- control embryos at 11-14 ss for Coup-TFII and CD31. ECs were individually scored as either Coup-TFII positive or negative. As in wild type (Fig. 3), we found that Efnb2 expression and Coup-TFII staining did not overlap in general in the Efnb2H2BGFP;EphB4-/- mutant (Fig. 6D,E). We further quantified the expression of Efnb2 in the ECs of the EphB4-/-;Efnb2H2BGFP embryos by measuring GFP expression, and found that most (92±4.8%) of the Coup-TFII-positive ECs in the DA were negative for Efnb2 (Fig. 6G). Thus, the extra Coup-TFII-positive ECs in the enlarged mutant DA did not express Efnb2.
In sum, our observations of a transient population of venous-fated ECs in the DA, movement of ECs from the DA to the CV in wild-type embryos, onset of the DA phenotype in Efnb2-/- and EphB4-/- mutants, and EC quantification data on EphB4-/- mutants suggest that Coup-TFII-expressing ECs are mislocalized in the DA of these mutants because they failed to relocate from the DA to the CV during the heterogeneous AV stage. Thus, EphB4 signaling is a crucial regulator of the cell movements that regulate an arterial contribution to the CV.
DISCUSSION
Our findings provide the first cellular and molecular insights into the aortic origin of the CV in mammals (Fig. 7). By imaging the mouse embryo at cellular resolution, we demonstrate that venous-fated progenitors relocate from the DA to the CV. Our findings reveal that the mammalian DA develops through an intermediate stage when it harbors a heterogeneous population of ECs with arterial or venous molecular identity. These venous-fated ECs transiently inhabit the DA before relocating to the CV. Supporting this finding, our time-lapse imaging in live mouse embryos show movement of ECs from the DA to the CV at this stage. We also show that ephrin B2/EphB4 signaling is a molecular regulator in this process, essential to sort these venous-fated ECs from the DA to the CV. This venous-fated EC population in the DA is crucial for the coordinated morphogenesis of the DA and CV.
Fig. 7.
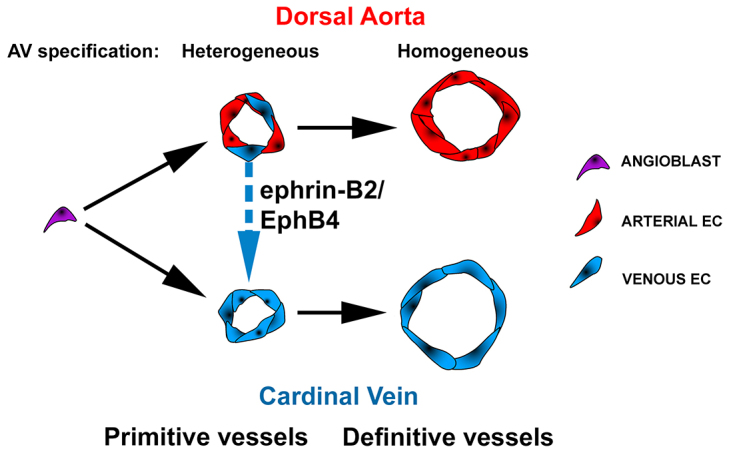
Proposed model depicting the heterogeneous AV stage of dorsal aorta and cardinal vein development. Angioblasts initially assemble into the primitive DA (pDA). Subsequently, heterogeneous populations of ECs expressing arterial or venous molecular markers (arterial- or venous-fated) co-habitate the pDA. Venous-fated ECs in the pDA segregate from the pDA and participate in the formation of the CV, in a process requiring ephrin B2/EphB4 signaling, leading to the definitive DA uniformly exhibiting arterial specification.
A transient population of venous-fated ECs in the developing aorta suggests an arterial origin for the CV
Using sensitive fluorescence imaging at single-cell resolution, we analyzed AV identities of the developing DA. We demonstrate that arterial markers including Notch1, ephrin B2 and Cx40 are virtually undetectable at 3-4 ss, when the DA emerges, consistent with a previous report using whole-mount RNA in situ hybridization (Chong et al., 2011). Following this initial stage, a subset of DA ECs gains arterial marker expression, whereas others gain venous marker expression. Subsequently, the DA ECs uniformly express arterial markers. Our finding of ECs expressing venous molecular markers in the developing DA resembles that of the ECs expressing lymphatic markers in the developing CV (Wigle and Oliver, 1999), key evidence for the venous origin of lymphatic vessels. By analogy, we provide key evidence for an arterial origin for the CV.
Resolution of the heterogeneous stage occurs via ephrin B2/EphB4-mediated movement, not change in AV specification, of ECs
Using in vivo time-lapse microscopy, we provide direct evidence revealing trafficking of ECs from the DA to the CV in cultured mouse embryos around the heterogeneous AV stage. Time-lapse imaging of EC migration in the DA and CV deep within live mouse embryos is especially challenging, both with regards to imaging resolution and embryo survival. Despite a 2% success rate, we detected EC movements from the DA to the CV in all embryos successfully imaged.
We previously reported that Efnb2-/- and EphB4-/- mutants develop a larger DA and a smaller CV (Kim et al., 2008), raising an important question regarding whether ephrin B2/EphB4 signaling regulates the distribution of ECs from the DA to the CV. Here, we hypothesized that ephrin B2/EphB4 signaling regulates the segregation of venous-fated ECs from the DA to the CV during the heterogeneous AV stage of DA development. The onset of the larger DA and smaller CV phenotype in the Efnb2-/- and EphB4-/- mutants at this very stage supports the hypothesis that these signaling molecules function at this stage of development.
Furthermore, we observed that a coordinated increase in DA size and decrease in CV size in the EphB4-/- mutant was accompanied by a similar change in the distribution of venous cells. Because ephrin B2/EphB4 signaling is known to mediate the repulsion of EphB4-positive cells away from ephrin B2-positive cells (Füller et al., 2003), the most straightforward model that explains these observations is one in which venous progenitors in the DA, which normally express EphB4, fail to be repulsed away from ephrin B2-positive neighboring cells in the DA to join the CV.
One alternative to this segregation model is a transdifferentiation model, in which these venous marker-expressing ECs remain in the DA but lose their venous marker expression. Although the expression data alone may be consistent with either the segregation or transdifferentiation model, our observations of cell movement from the DA to the CV and the change in the distribution of venous-fated ECs that accounts for the concomitant changes in DA and CV size in EphB4-/- mutants, provide strong evidence in favor of the segregation model.
For the transdifferentiation model to be a valid explanation of the Efnb2 and EphB4 knockout phenotypes, it would require that loss of these genes either (1) increases the proliferation of venous marker-expressing ECs in the DA or (2) changes the fates of ECs in the DA from arterial to venous, while increasing the proliferation of ECs in the DA. In addition, both of these choices require a coordinated decrease in proliferation of ECs in the CV. This explanation seems highly unlikely, as a function for ephrin B2/EphB4 signaling in cell proliferation and cell fate change has not been previously reported. Thus, we believe that our segregation model fits the existing data better.
Novel insight on the development of the cardinal veins in mammals
Our findings that (1) ECs expressing venous markers reside temporarily in the DA, (2) ECs move from the DA to the CV, and (3) venous-fated ECs are retained in the DA and missing in the CV in EphB4-/- mutants, provide strong evidence that the mammalian CV has a DA origin. Our cellular and molecular evidence in mice also advance the controversial concept of an aortic origin of the CV. In both zebrafish and chick, the CV has been proposed to form from the DA (Sabin, 1917; Herbert et al., 2009). However, conflicting reports have refuted this theory and proposed instead that the CV arises by in situ differentiation of ECs (Hirakow and Hiruma, 1981; Coffin and Poole, 1988). Importantly, the presence of ECs with venous molecular identity in the DA was not reported in chick or fish. Here, we have provided such crucial evidence of venous precursors in the emergent DA, as well as confirmed EC movement from the DA to the CV in mammals. It is therefore likely that ECs that move from the DA to participate in CV formation is an evolutionarily conserved process.
Regarding the sources of the CV, we favor the idea that some CV ECs come from the DA, and some do not. Our quantification data suggest that ∼15% of the ECs of the CV up to 11-15 ss originate from the DA. These data suggest that the venous-fated ECs from the DA are only one of the sources of CV ECs.
In summary, this study provides new insight on the origin of the CV and highlights the likelihood of aortic and extra-aortic sources, which helps to explain the historical controversies regarding this process. It has been shown that the CV transiently contains a subpopulation of precursor ECs that give rise to the lymphatic vasculature (Wigle and Oliver, 1999; Karkkainen et al., 2004). Thus, the DA harbors ECs that form the CV, which in turn harbors ECs that give rise to lymphatic vessels. Overall, our study conceptually advances the understanding of the molecular and cellular mechanisms that underlie vascular development in mammals. Furthermore, our molecular characterization and quantification methods provide a useful framework by which to analyze AV differentiation in other model organisms, angiogenesis events, and various vascular mutants.
MATERIALS AND METHODS
Mice
Efnb2-H2BGFP (Davy and Soriano, 2007), Efnb2-tauLacZ (Wang et al., 1998), EphB4-tauLacZ (Gerety et al., 1999), Tie2-Cre (Braren et al., 2006) and mTmG (Muzumdar et al., 2007) transgenic lines were described previously. The Notch1+/lacZ mice, were generated from a BayGenomics clone by Dr Alexander Chervonsky (University of Chicago, IL, USA) and kindly provided by Dr Thomas Gridley (Jackson Laboratory). Mice used were in a mixed genetic background. All animals were treated in accordance with the guidelines of the University of California San Francisco (UCSF) Institutional Animal Care and Use Committee.
Whole-mount immunofluorescence staining and histology
Embryo harvest, whole-mount specimen processing, and immunostaining were performed as described (Braren et al., 2006). Primary antibodies used were: goat anti-connexin 40 (1:2000; sc-20466, Santa Cruz Biotechnology); rat anti-CD31 (1:100; 553370, BD Biosciences); rabbit anti-βgal (1:200; 55976, MP Biomedicals); mouse anti-Coup-TFII (1:100; PP-H7147-00, R&D Systems), goat anti-EphB4 (1:50; AF446, R&D Systems) and goat anti-Vegfr3 (1:100; AF743, R&D Systems). Secondary antibodies were: Alexa Fluor 488 donkey anti-rat IgG (1:1000), Alexa Fluor 488 donkey anti-goat IgG (1:1000) and Alexa Fluor 488 and 568 donkey anti-rabbit IgG (1:1000) (all from Invitrogen); Alexa Fluor 488-conjugated streptavidin (1:1000; MP Biomedicals); and FITC, Cy3 and DyLight 649 donkey anti-rat IgG (1:1000; Jackson ImmunoResearch Laboratories). Rehydrated paraffin sections of stained embryos were washed and then mounted with Vectashield mounting medium containing DAPI from Vector Laboratories.
Quantification of Efnb2H2BGFP and Coup-TFII expression
Embryos were fixed in 4% paraformaldehyde for 15 minutes, embedded in OCT, and sectioned (8 μm). Sections were blocked in 5% donkey serum and 0.2% Triton X-100 in PBS for 1 hour at room temperature (RT). The sections were then incubated with primary antibody in blocking solution at 4°C overnight, followed by secondary antibody incubation for 1 hour at RT, and mounted with DAPI-containing Vectashield (Vector Laboratories). Images were captured using a Zeiss Axiovert2 Plus microscope equipped with a Sensicam CCD camera and Slidebook software (Intelligent Imaging Innovations). The same exposure time was used for all slides stained with a given antibody. ECs in the DA and CV between the top of the aortic arch and the point at which the CV merges with the common cardinal vein were analyzed.
For Efnb2H2BGFP quantification, captured images were exported as 16-bit TIFF files and imported into ImageJ. In the DA and CV, DAPI-positive nuclei in CD31-positive cells were encircled and the GFP intensity was determined. To control for variation between embryos, the intensity for each cell was normalized against the average value of the five lowest GFP-expressing cells in either the DA or the CV in the corresponding embryo. A normalized GFP intensity of 2.5 or higher was scored positive.
For Coup-TFII quantification, images were exported as described above. An EC was determined to be positive or negative, either by visual inspection, or by measuring the nuclear Coup-TFII intensity using ImageJ. To determine the Coup-TFII intensity, we first performed rolling ball background subtraction to control for background variations within the section. The nuclear staining intensity in ECs was then measured. To control for background variation between sections, the intensity for each cell was normalized against the average value of five Coup-TFII negative cells in that section. A normalized nuclear Coup-TFII intensity of three or higher was scored positive.
For EC quantification of Coup-TFII-positive ECs in EphB4-/- mutant, 11-15 ss embryos were processed and stained as described above. ECs in the DA and CV were determined to be Coup-TFII positive or negative, and the percentages of Coup-TFII-positive or -negative ECs, compared with total number of ECs, were determined in each embryo.
Whole-mount X-Gal staining
X-Gal staining was performed as previously described (Carpenter et al., 2005) with the following modifications. Embryos were fixed for 15 minutes and incubated in staining solution for 8 hours at 37°C. After staining, embryos were fixed in formalin, dehydrated, embedded in agar and paraffin, and sectioned (5 μm).
Two-photon microscopy and 3D analysis
Images were obtained using a locally constructed two-photon microscope (Kim et al., 2012). Prior to imaging, mouse embryos were cleared in methylsalicylate after serial dehydration in 25, 50, 75, 90 and 100% ethanol at 30-minute intervals. Whole-mount embryos were imaged by acquiring stacks of planar images with 1 μm spacing along the optical axis. The data were reconstructed in three dimensions and analyzed with Imaris software (Bitplane Scientific Software).
Time-lapse imaging
Embryos (9-10 ss) were dissected in 37°C high-glucose DMEM/F12 supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin, and placed in preheated 37°C high-glucose DMEM/F12 supplemented with 50% rat serum, 15 mM HEPES and 1% Penicillin-Streptomycin. To prevent motion during the time-lapse acquisition, embryos were stabilized in 3% low-melting agarose. Embryos were cultured for up to 24 hours in a humidified 5% CO2 mixture at 37°C using a time-lapse imaging system from Intelligent Imaging Innovations or a custom-built two-photon microscope (Kim et al., 2012). One frame was recorded every 5 minutes for up to 20 hours.
Statistical analysis
Values are expressed as mean ± s.e.m. The two-tailed Student’s t-test was used for comparisons between two groups and the one-way ANOVA for multiple groups. P<0.05 was considered significant.
Acknowledgments
We thank Drs Alexander Chervonsky (University of Chicago) and Thomas Gridley (Jackson Laboratories) for the Notch1+/lacZ mice; Huiqing Hu, Gloria Lu, Sissi Liu and Dr Marc Bolliger for technical assistance; Pamela Derish and Sara Peyrot for editorial advice; and the members of our laboratory for helpful discussions.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
R.A.W., Y.H.K., H.L. and E.B.J. developed concepts; R.A.W., H.L., Y.H.K. and E.B.J. designed experiments; H.L., Y.H.K., E.B.J., Y.K., S.G.G. and T.N.K. performed experiments; R.A.W., H.L., E.B.J. and S.G.G. prepared the manuscript.
Funding
This work was supported by the National Institutes of Health (NIH) [R01 HL075033 to R.A.W.; F32 HL097400 to E.B.J.; T32 HL007544 to S.G.G.]; a grant from the Foundation for Accelerated Vascular Research; the University of California San Francisco Howard Hughes Medical Institute Biomedical Research Support Program (to R.A.W.); a Swedish Research Council postdoctoral fellowship (to H.L.); and an American Heart Association postdoctoral fellowship (to H.L.). Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.101808/-/DC1
References
- Adams R. H., Wilkinson G. A., Weiss C., Diella F., Gale N. W., Deutsch U., Risau W., Klein R. (1999). Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13, 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braren R., Hu H., Kim Y. H., Beggs H. E., Reichardt L. F., Wang R. (2006). Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J. Cell Biol. 172, 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B., Lin Y., Stoll S., Raffai R. L., McCuskey R., Wang R. (2005). VEGF is crucial for the hepatic vascular development required for lipoprotein uptake. Development 132, 3293–3303 [DOI] [PubMed] [Google Scholar]
- Chong D. C., Koo Y., Xu K., Fu S., Cleaver O. (2011). Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev. Dyn. 240, 2153–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. D., Poole T. J. (1988). Embryonic vascular development: immunohistochemical identification of the origin and subsequent morphogenesis of the major vessel primordia in quail embryos. Development 102, 735–748 [DOI] [PubMed] [Google Scholar]
- Davy A., Soriano P. (2007). Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev. Biol. 304, 182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C. J., Fleming P. A. (2000). Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 95, 1671–1679 [PubMed] [Google Scholar]
- Füller T., Korff T., Kilian A., Dandekar G., Augustin H. G. (2003). Forward EphB4 signaling in endothelial cells controls cellular repulsion and segregation from ephrinB2 positive cells. J. Cell Sci. 116, 2461–2470 [DOI] [PubMed] [Google Scholar]
- Gerety S. S., Anderson D. J. (2002). Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development 129, 1397–1410 [DOI] [PubMed] [Google Scholar]
- Gerety S. S., Wang H. U., Chen Z. F., Anderson D. J. (1999). Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol. Cell 4, 403–414 [DOI] [PubMed] [Google Scholar]
- Guruharsha K. G., Kankel M. W., Artavanis-Tsakonas S. (2012). The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert S. P., Huisken J., Kim T. N., Feldman M. E., Houseman B. T., Wang R. A., Shokat K. M., Stainier D. Y. (2009). Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 326, 294–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakow R., Hiruma T. (1981). Scanning electron microscopic study on the development of primitive blood vessels in chick embryos at the early somite-stage. Anat. Embryol. (Berl.) 163, 299–306 [DOI] [PubMed] [Google Scholar]
- Jones E. A., Crotty D., Kulesa P. M., Waters C. W., Baron M. H., Fraser S. E., Dickinson M. E. (2002). Dynamic in vivo imaging of postimplantation mammalian embryos using whole embryo culture. Genesis 34, 228–235 [DOI] [PubMed] [Google Scholar]
- Karkkainen M. J., Haiko P., Sainio K., Partanen J., Taipale J., Petrova T. V., Jeltsch M., Jackson D. G., Talikka M., Rauvala H., et al. (2004). Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 [DOI] [PubMed] [Google Scholar]
- Kim Y. H., Hu H., Guevara-Gallardo S., Lam M. T., Fong S. Y., Wang R. A. (2008). Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development 135, 3755–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. N., Goodwill P. W., Chen Y., Conolly S. M., Schaffer C. B., Liepmann D., Wang R. A. (2012). Line-scanning particle image velocimetry: an optical approach for quantifying a wide range of blood flow speeds in live animals. PLoS ONE 7, e38590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. (2012). Eph/ephrin signalling during development. Development 139, 4105–4109 [DOI] [PubMed] [Google Scholar]
- Kohli V., Schumacher J. A., Desai S. P., Rehn K., Sumanas S. (2013). Arterial and venous progenitors of the major axial vessels originate at distinct locations. Dev. Cell 25, 196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L., Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. (2010). Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer 10, 165–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu M. E., Adams R. H. (2010). Eph/ephrin molecules - a hub for signaling and endocytosis. Genes Dev. 24, 2480–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M., Gerhardt H., Carmeliet P. (2011). Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 [DOI] [PubMed] [Google Scholar]
- Rocha S. F., Adams R. H. (2009). Molecular differentiation and specialization of vascular beds. Angiogenesis 12, 139–147 [DOI] [PubMed] [Google Scholar]
- Sabin F. R. (1917). Origin and development of the primitive vessels of the chick and of the pig. Contrib. Embryol. 6, 61–124 [Google Scholar]
- Swift M. R., Weinstein B. M. (2009). Arterial-venous specification during development. Circ. Res. 104, 576–588 [DOI] [PubMed] [Google Scholar]
- Villa N., Walker L., Lindsell C. E., Gasson J., Iruela-Arispe M. L., Weinmaster G. (2001). Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech. Dev. 108, 161–164 [DOI] [PubMed] [Google Scholar]
- Walls J. R., Coultas L., Rossant J., Henkelman R. M. (2008). Three-dimensional analysis of vascular development in the mouse embryo. PLoS ONE 3, e2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. U., Chen Z. F., Anderson D. J. (1998). Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741–753 [DOI] [PubMed] [Google Scholar]
- Wigle J. T., Oliver G. (1999). Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 [DOI] [PubMed] [Google Scholar]
- Williams C., Kim S. H., Ni T. T., Mitchell L., Ro H., Penn J. S., Baldwin S. H., Solnica-Krezel L., Zhong T. P. (2010). Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev. Biol. 341, 196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L. R., Lin F. J., Lee C. T., DeMayo F. J., Tsai M. J., Tsai S. Y. (2005). Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435, 98–104 [DOI] [PubMed] [Google Scholar]
- Zhong T. P., Childs S., Leu J. P., Fishman M. C. (2001). Gridlock signalling pathway fashions the first embryonic artery. Nature 414, 216–220 [DOI] [PubMed] [Google Scholar]