Abstract
Directed cell migration polarizes the cytoskeleton, allowing the cell to move toward migratory cues. In this issue, Wu et al. (2011) demonstrate that the glycogen synthase kinase 3β (GSK3β) controls microtubule architecture and polarized movement of skin stem cells during wound healing in mammals by regulating the microtubule crosslinking protein ACF7.
Development, inflammation, and wound healing all critically depend upon directed cell migration when effector cells move toward particular extracellular cues. For example, when skin is injured, stem cells travel from the hair follicle to the wound to rebuild the skin epithelium. Directed cell migration requires changes in the cell’s polarity and remodelingof the cytoskeleton (Ridleyet al., 2003), including the regulation of microtubule, actin, and intermediate filaments at the front of the cell (Figure 1). These dynamic structures mediate changes in cellular shape, but they also form a platform for receiving and generating key morphogenic signals.
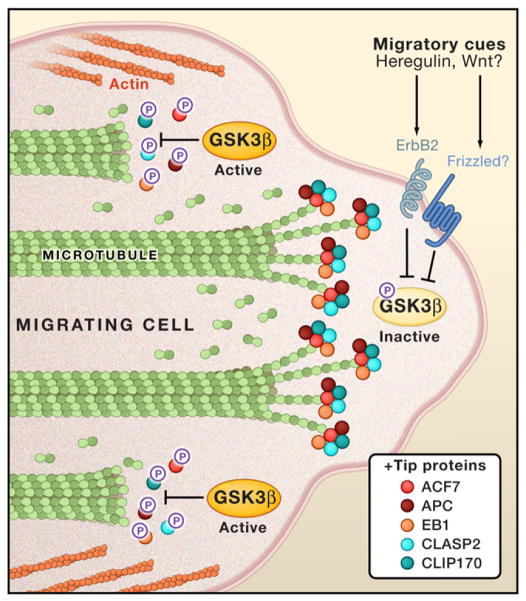
Figure 1. GSK3β Is a Central Regulator of the Cytoskeleton during Cell Migration.
In nonmigrating cells, GSK3β is normally active and phosphorylates proteins that associate with the plus (+) ends of microtubule filaments, called “+tip proteins.” Phosphorylation uncouples +tip proteins from microtubules (Wu et al., 2011), inhibiting growth of the filaments. Migratory cues, such as Heregulin, inactivate GSK3β by phosphorylation (Zaoui et al., 2010), allowing the local extension of microtubules by +tip proteins. This helps to polarize the cytoskeleton and direct cellular movement toward the migratory cues
What cytosolic factors transmit migratory cue information from the cellular membrane to these signaling centers on the cytoskeleton? Although this complex process probably depends on many genetically redundant factors, several kinases and phosphatases are emerging as key players. Initial reports have solidified roles for phosphatidylinositol 3-kinase (PI3K) and the lipid phosphatase PTEN in defining the front and backsides of migrating cells, respectively. Now Wu et al. (2011) find that another workhorse kinase, glycogen synthase kinase 3β (GSK3β), orchestrates polarization and reorganization of microtubules in hair follicle stem cells during wound healing. This study, along with another recent report by Zaoui et al. (2010), demonstrates that GSK3β governs microtubule elongation through its control of the microtubule-actin crosslinking protein ACF7 (actin cross-linking factor-7/microtubule and actin crosslinking factor-1).
GSK3β is a serine-threonine kinase that participates in numerous growth factor and morphogen signaling pathways, including the canonical Wnt pathway. In addition, GSK3β has been implicated in a variety of diseases, such as diabetes, Alzheimer’s disease, bipolar disorder, and cancer (Rayasam et al., 2009). Recent studies have found that GSK3β is a central regulator of cell polarity and cytoskeleton dynamics. The kinase regulates the polymerization of filamentous actin (F-actin) at the front and periphery of the cell by activating the Rho GTPase Rac and ADP-ribosylation factor 6 (Arf6), and by repressing p190a-RhoGAP and APC (adenomatous polyposis coli). Further, GSK3β modulates cell-matrix adhesions by activating paxillin and inhibiting focal adhesion kinase (Sun et al., 2009).
A fascinating link has also recently emerged between GSK3β and proteins that associate with the plus (+) ends of microtubules, called “+tip proteins.” The +tip proteins regulate the growth of the noncapped, (+) ends of microtubule filaments at the front of migrating cells (Figure 1). The +tip proteins also interact with membrane proteins, such as Rho GTPases, to relay extracellular migratory signals to microtubules (Sun et al., 2009). Recent studies indicate that GSK3β phosphorylates and inactivates certain +tip proteins, such as CLASP2, providing a link between GSK3β kinase and microtubule elongation (Kumar et al., 2009).
Another key class of +tip proteins is spectraplakins. The two mammalian spectraplakins, BPAG1/dystonin and ACF7/MACF, are large proteins (>500 kDa) that orient the cytoskeleton by binding and crosslinking actin and microtubule filaments. These spectraplakins also form membrane subdomains and scaffolds for signaling complexes. Mice lacking BPAG1 display skin blistering due to the epidermis splitting from the dermis (Roper et al., 2002). In contrast, loss of ACF7 in the epidermis causes no skin or hair phenotype but delays wound repair. Keratinocytes with mutations in ACF display 80% less movement than wild-type control cells in scratch wound assay in vitro. This phenotype was previously ascribed to defective coordination of microtubule growth along F-actin filaments and stabilization of focal adhesions (Wu et al., 2008).
Now Wu et al. and Zaoui et al. elegantly link GSK3β activity with the regulation of ACF7-associated microtubules and directed cell migration. Wu and colleagues investigate the wounding phenotype of mice with mutations in the ACF gene and find a significant delay in wound closure. They found that phosphorylation of the carboxyl terminus of ACF7 by GSK3β diminishes ACF7’s binding to microtubules. In addition, using a phospho-specific antibody, the authors show that phospho-ACF7 localizes to the cytoplasm but not to microtubules, confirming that phosphorylation of ACF7 uncouples the +tip protein from microtubules (Figure 1). Next, the authors generate a mutant version of ACF7 that is refractory to phosphorylation by GSK3β, and indeed, this mutant rescues portions of the ACF7 mutant phenotype.
Similarly, Zaoui and colleagues investigate GSK3β’s role in the migration of breast cancer cells responding to the epidermal growth factor (EGF) ligand Heregulin through stimulation of the tyrosine kinase receptor ErbB2. The group had previously shown that Heregulin induces directed cell protrusions by triggering the Memo (mediator of ErbB2 motility) membrane complex at the leading edge. In addition, they found that +tip proteins APC and CLASP2 mediate microtubule formation at cell protrusions during these cells’ migration. In their most recent study, Zaoui and colleagues demonstrate that Memo inactivates GSK3β, and this inhibition, in turn, targets APC and CLASP2 to the membrane. In addition, Heregulin activity localizes ACF7 to the plasma membrane and microtubules, and this localization depends on GSK3β and APC.
Together, the data presented by Wu et al. and Zaoui et al. strongly link GSK3β activity and the phosphorylation status of ACF7 with cell migration and ACF7 association with microtubules. However, the biochemical mechanism of ACF7 regulation by GSK3β remains mysterious. Wu and colleagues report that neither the kinase-refractile nor the phosphomimetic mutants of ACF7 could rescue the polarity and directional movement defect in ACF7 mutant hair follicle stem cells. This surprising result portends that regulation of ACF7 by GSK3β is more complex than simply inhibition by phosphorylation and suggests that subsets of the multiple phosporylation sites on ACF7 may have distinct functions. Indeed, such behavior has been observed for the +tip protein CLASP2 (Kumar et al., 2009). Alternatively, cycling of ACF7 phosphorylation status may be needed for prolonged microtubule elongation. Experiments examining the phosphorylation and function of ACF7 (and other +tip proteins) in single-molecule, dynamic microtubule assays will be necessary to understand precisely how GSK3β operates at the leading edge.
These two studies also generate many additional questions about how migratory cues sculpt the cytoskeleton through key kinases, such as GSK3β. One central question is the nature of the interactions between +tip proteins and how their localization on microtubules regulates actin and microtubule architecture, cell migration, and polarity. The complex of ACF7 and another +tip protein, EB1 (end-binding protein 1), appears necessary and sufficient for microtubule elongation in some cells in culture, but how the other +tip proteins function in different physiological contexts remains unexplored. Mice with mutations in ACF7 develop normal skin morphology, suggesting that other spectraplakins or +tip proteins must control the polarized growth of the hair follicle or developing skin epithelium.
Another open question is how the signaling pathways of PI3K intersect with those of GSK3β in generating cell polarity. It is well-known that PI3K regulates GSK3β (Cross et al., 1995), but it is still unknown whether migratory cues necessarily regulate PI3K, GSK3β, or both kinases simultaneously. Having cues affect different kinases could allow for greater complexity of cell shapes and motility outcomes.
Finally, identifying endogenous migratory cues that control directional migration through GSK3β may lead to the discovery of new therapeutic agents. Heregulin and other EGF ligands are well-known migratory cues in wound healing and development. They appear, at least in breast cancer cells, to be a migratory cue that alters the cytoskeleton by acting through GSK3β (Zaoui et al., 2010).
In addition, Wnt ligands are certainly present in wounded skin, and noncanonical Wnt signaling to the cytoskeleton through Rho kinase plays a well-established role in morphogenic processes, such as convergent extension during gastrulation (i.e., when tissue restructures to extend along a perpendicular axis). However, a clear role for canonical Wnt signaling through GSK3β is currently lacking for wound healing in skin (Ito et al., 2007). Nevertheless, given the central role of GSK3β and +tip proteins in regulating the cytoskeleton during directed cell migration, additional roles in establishing the cytoskeleton’s global positional device system are sure to appear.
References
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kumar P, Lyle KS, Gierke S, Matov A, Danuser G, Wittmann T. J Cell Biol. 2009;184:895–908. doi: 10.1083/jcb.200901042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Br J Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Roper K, Gregory SL, Brown NH. J Cell Sci. 2002;115:4215–4225. doi: 10.1242/jcs.00157. [DOI] [PubMed] [Google Scholar]
- Sun T, Rodriguez M, Kim L. Dev Growth Differ. 2009;51:735–742. doi: 10.1111/j.1440-169X.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Kodama A, Fuchs E. Cell. 2008;135:137–148. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Shen Q, Oristian D, Lu C, Zheng Q, Wang H, Fuchs E. Cell. 2011;144:341–352. doi: 10.1016/j.cell.2010.12.033. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaoui K, Benseddik K, Daou P, Salaun D, Badache A. Proc Natl Acad Sci USA. 2010;107:18517–18522. doi: 10.1073/pnas.1000975107. [DOI] [PMC free article] [PubMed] [Google Scholar]