Abstract
The Fc glycosylation of therapeutic antibodies is crucial for their effector functions and their behavior in pharmacokinetics and pharmacodynamics. To monitor the Fc glycosylation in bioprocess development and characterization, high-throughput techniques for glycosylation analysis are needed. Here, we describe the development of a largely automated high-throughput glycosylation profiling method with multiplexing capillary-gel-electrophoresis (CGE) with laser induced fluorescence (LIF) detection using a DNA analyzer. After PNGaseF digestion, the released glycans were labeled with 9-aminopyrene-1,3,6-trisulfonic acid (APTS) in 96-well plates, which was followed by the simultaneous analysis of up to 48 samples. The peak assignment was conducted by HILIC-UPLC-MS/MS of the APTS-labeled glycans combined with peak fractionation and subsequent CGE-LIF analysis of the MS-characterized fractions. Quantitative data evaluation of the various IgG glycans was performed automatically using an in-house developed software solution. The excellent method accuracy and repeatability of the test system was verified by comparison with two UPLC-based methods for glycan analysis. Finally, the practical value of the developed method was demonstrated by analyzing the antibody glycosylation profiles from fermentation broths after small scale protein A purification.
Keywords: monoclonal antibody (mAb), IgG glycosylation, automation, multiplexed capillary electrophoresis, DNA analyzer, HILIC-UPLC, APTS labeling, LC-MS
Introduction
Recombinant monoclonal antibodies (mAbs) are valuable therapeutics for various conditions, including inflammatory diseases and cancer.1,2 The production of these glycoproteins in mammalian cell culture systems results in a remarkable macro- and microheterogeneity. The heterogeneity is often due to post-translational modifications like N-terminal pyroglutamic acid, C-terminal lysine heterogeneity, and glycosylation. Glycosylation is one of the most important post-translational modifications of mAbs because it affects properties critical to their development as therapeutics, e.g., solubility, structural stability, clinical efficacy.3,4
Pronounced heterogeneity of protein glycoforms is often observed. Therapeutic mAbs are normally of the IgG isotype. Each heavy chain has in the Fc part an N-glycan chain linked to asparagine 297.5 Depending on the expression system, various complex-type, hybrid-type and high-mannose type glycans may be found at this site.5,6 The complex-type glycan species often show heterogeneity with regard to the presence of a core fucose and bisecting N-acetylglucosamine. Additionally, they vary in galactose and sialic acid content of the antennae.4-7 IgGs may carry an additional N-glycan in the variable region of the fragment antigen-binding (Fab) portion. It has been shown that the Fab N-glycans are generally highly galactosylated and have a higher degree of sialylation.8,9
Antibody effector functions are greatly influenced by the glycosylation pattern. The different glycan species often represent so-called critical quality attributes (CQAs) and must be carefully monitored.10 Galactosylation can affect the complement-dependent cytotoxicity by improving the binding to C1q.11 The absence of core fucose results in an increase in antibody-dependent cell-mediated cytotoxicity (ADCC).12,13 Sialylation may induce anti-inflammatory effects via Th2 signaling and decrease ADCC via reduced interaction with Fcγ receptors.14,15 mAb glycosylation may also influence pharmacodynamic and pharmacokinetic behavior16-20 and may even be the target of adverse immune reactions against terminal alpha1–3 bound galactose and N-glycolylneuraminic acid.21-24
It is difficult to predict and control glycosylation patterns because N-glycan structures also depend on cultivation conditions, such as temperature, pH, and by-products like lactate.25 Therefore, monitoring IgG glycosylation is essential in the biotechnology industry and glycosylation is an important marker for process robustness in process development, medium development, clone selection, process characterization/validation studies and release analytics.3,5,12
A wide range of analytical methods are available to monitor protein glycosylation targeting intact glycoproteins, glycopeptides (after proteolytic cleavage) or released glycans, including mass spectrometry (MS)-based methods, high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD), and high performance liquid chromatography (HPLC)-based methods relying on fluorescence labeling of the glycans.3,26 These methods have been reviewed by Huhn et al. and Marino et al.27,28
Capillary electrophoresis (CE) techniques have also been used for glycosylation analysis. In this approach, the glycans are generally derivatized with a fluorophore that carries one or more negative charges to allow electrophoretic separation. The tag, in most cases 9-aminopyrene-1,3,6-trisulfonic acid (APTS) is introduced at the reducing terminus of the glycan via reductive amination29-31 and mass spectrometric detection may be applied.32
Callewaert et al. introduced the electrophoretic separation and fluorescence detection of glycans by means of a multicapillary DNA analyzer.33,34 These systems, which are commonly used in molecular biology laboratories, use polyacrylamide-based gels for separation and apply an argon laser to excite the fluorescent tag of the glycans. The suitability of the method for glycosylation analysis was demonstrated by several investigators for a broad range of analytes, including biopharmaceuticals and clinical samples.31,35-41
This approach is particularly amenable for high-throughput analysis of glycans, as DNA analyzers are available with various formats of capillary arrays, allowing the parallel measurement of up to 96 samples.34
We present here the development of a high-throughput capillary-gel-electrophoresis with laser induced fluorescence (CGE-LIF) method for IgG glycosylation analysis with a commercially-available DNA analyzer. For peak identification, APTS-labeled glycans were subjected to two-dimensional separation applying in the first dimension hydrophilic interaction ultra performance liquid chromatography (HILIC-UPLC) with mass spectrometry (MS) detection and in the second dimension CGE-LIF of the MS-characterized HILIC-UPLC peaks. In addition, spiking with commercially-available standards was applied for peak identification. The successful application of the CGE-LIF method in process development studies was demonstrated.
Results
Method development
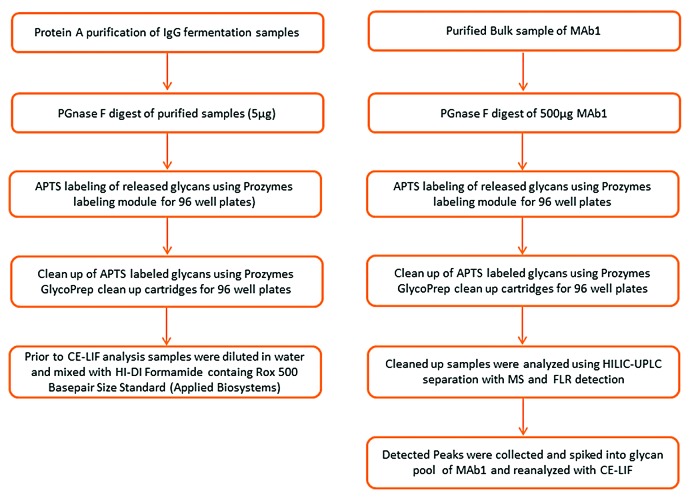
A CGE-LIF method was established for the glycosylation analysis of therapeutic mAbs for two applications: (1) the glycosylation analysis at the drug substance level, and (2) the glycosylation analysis after automated small scale purification of the antibodies with protein A columns directly from fermentation supernatants. The method involves desalting of the IgG samples by ultrafiltration, glycan release by PNGase F, APTS labeling, glycan separation by CGE-LIF using a DNA analyzer, and automated data evaluation (Fig. 1, left).
Figure 1. Schematic overview of experimental workflow (left) and workflow for identification of glycan structures (right).
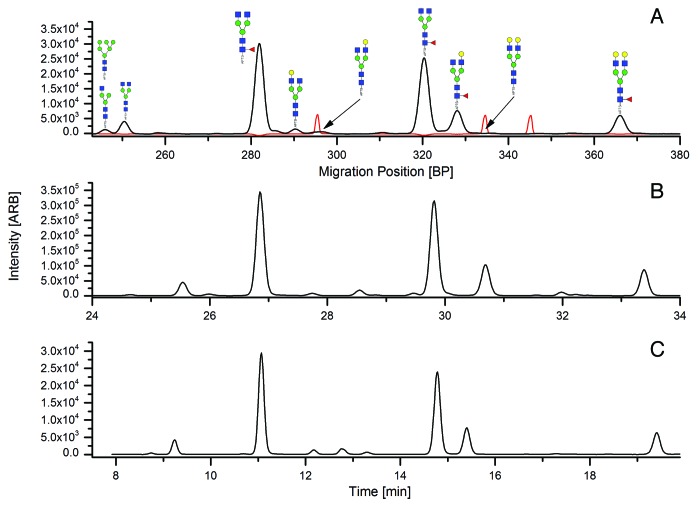
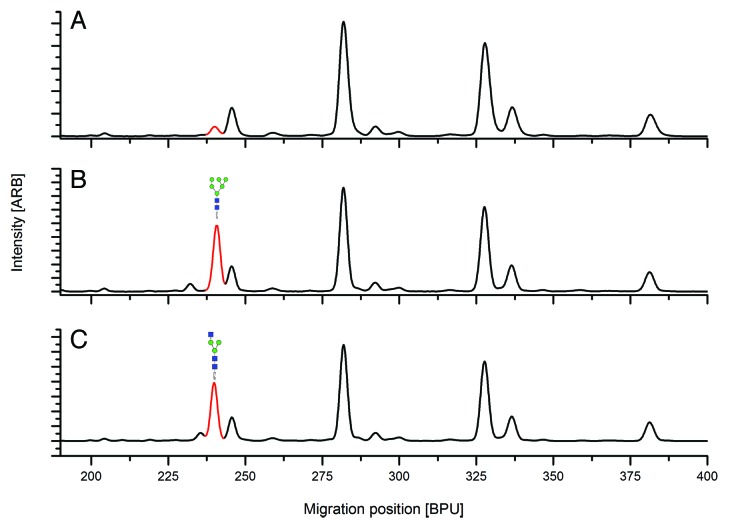
A typical CGE-LIF profile for the APTS-labeled glycans of MAb1, the formulated drug substance of a marketed therapeutic antibody, including the basepair standard as internal reference for migration times, is shown in Figure 2A. For comparison, the corresponding HILIC-UPLC profiles obtained after APTS labeling and 2-AB labeling are shown in Figure 2B and 2C, respectively. The profiles show high similarity, with smaller glycans eluting first in both CGE-LIF and HILIC-UPLC.
Figure 2. CGE-LIF electopherogram (in black) with basepairs used for calibration (in red) (A); HILIC-UPLC of APTS-labeled glycans used for fractionation of peaks and for quantitation (B); HILIC-UPLC of AB-labeled glycans used for comparison (C).
For the CGE-LIF separation, migration positions of glycan peaks were expressed in basepair units relying on a fluorescently-labeled DNA ladder that was detected by excitation at 587 nm and emission at 607 nm, not interfering with the detection of APTS-labeled glycans that occurs at 473 nm excitation and 520 nm emission.
CGE-LIF peak assignment on a DNA-analyzer is not feasible via direct coupling to a mass spectrometer, nor is the collection of eluting APTS-labeled glycans possible on such a system. Therefore, we relied on two approaches for peak assignment in CGE-LIF (Fig. 1, right): (1) HILIC-UPLC with online mass spectrometric analysis, fractionation and subsequent CGE-LIF analysis of the collected APTS-labeled glycans that were assigned on the basis of the mass spectrometric data; and (2) CGE-LIF peak assignment via spiking experiments with commercially-available glycan standards that were APTS-labeled.
CGE-LIF peak assignment via spiking with APTS-labeled glycans after HILIC-UPLC fractionation
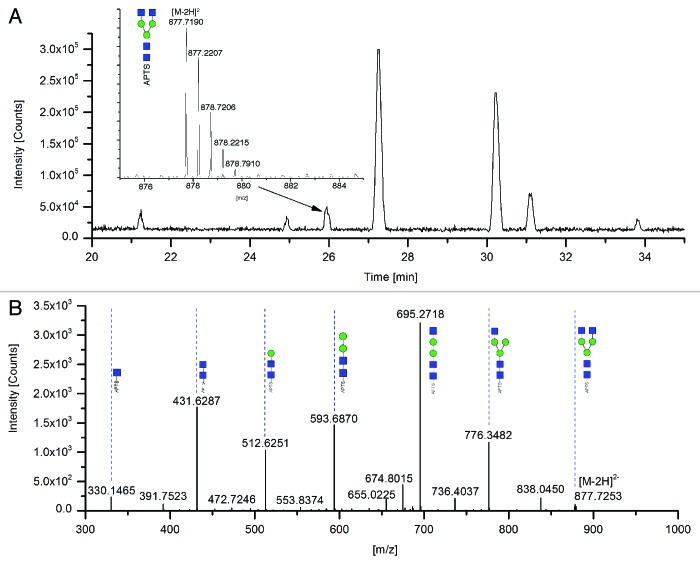
Enzymatically released glycans of MAb1 were APTS-labeled and separated by HILIC-UPLC. For quantitation, peaks were detected with a fluorescence detector and the outlet of the column was online coupled to an ESI mass spectrometer. The fluorescence chromatogram is shown in Figure 2B. The corresponding total ion current (TIC) chromatogram is shown in Figure 3A. The identification of the HILIC-UPLC peaks was accomplished by comparing the experimentally detected molecular masses of the APTS-labeled glycans with their theoretical masses (Table 1). Because we used an Orbitrap mass spectrometer with a high mass accuracy, all abundant experimental masses could be unambiguously assigned to the corresponding glycan structure. As an example, the mass spectrum of the double-charged species of G0-F (H3N4) is shown in Figure 3A. Moreover, all detectable glycan structures were confirmed by MS/MS experiments as shown, for example, by the MS/MS spectrum of G0-F (H3N4) (Fig. 3B). The fragmentation was conducted in the negative ion mode. Predominantely [M-2H]2- ions of the y-type were observed. This might be due to the negative charge of the APTS label. Only minor signals corresponding to ring fragments could be detected, and the loss of hexoses and n-acetyl-hexose could be detected, and hence the structure unambiguously assigned.
Figure 3. HILIC-UPLC-MS of APTS-labeled N-glycan pool from MAb1. (A) Total ion chromatogram (TIC). The peak indicated by an arrow corresponds to G0-F (H5N2) with retention time of 25.9 min. Inset: zoomed view of the [M-2H]2- ions. (B) MS-MS spectrum in negative ion mode, detected masses are [M-2H]2- ions.
Table 1. Structures, Summary of identification results.
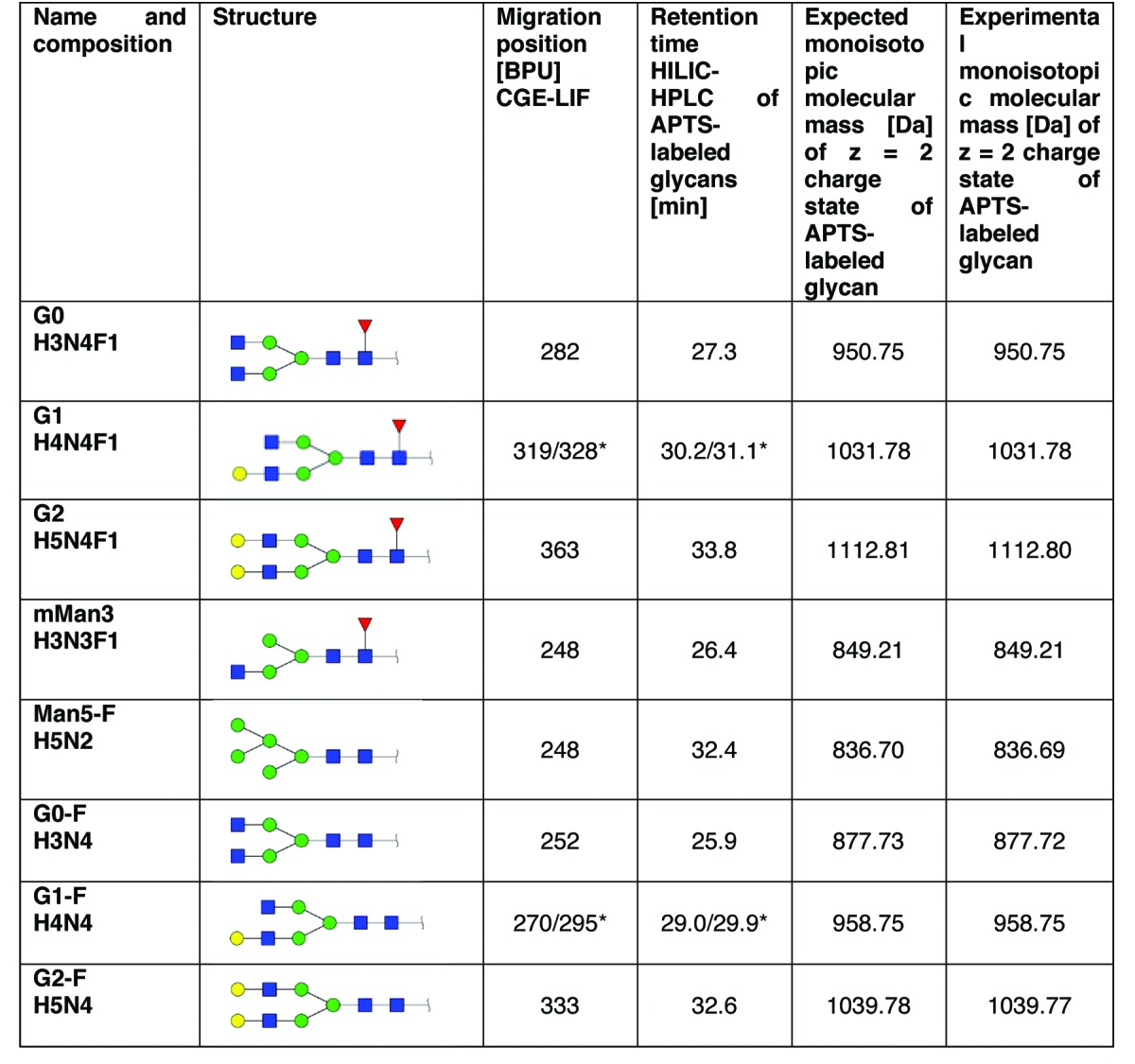
Because G1 and G1-F exist in two different isomers that can be separated with both methods, two values are given.
The two isomeric structures of G1 (H4N4F1) corresponding to the man α 1,3- and the α 1,6-arm could be separated with HILIC-UPLC and CGE-LIF, but could not be unambiguously assigned with MS/MS. The same holds true for the two isomeric structures of G1-F (H4N4); however, literature suggests that the earlier eluting peaks correspond to the α 1,6-arm and the later eluting peak to the α 1,3 arm. The relative amount of the peaks is consistent with that reported for IgG1 glycan structures.42 Because the effect of the two isomeric structures is unclear, we report the sum of G1 (H4N4F) and G1-F (H4N4), respectively.
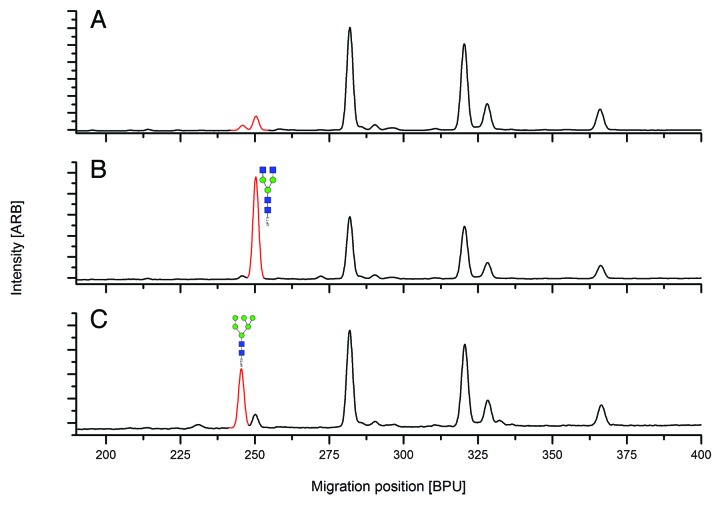
From another HILIC-UPLC run, fractions of the APTS-labeled glycans of MAb1were collected and individual fractions corresponding in each case to a peak in HILIC-UPLC were spiked into the APTS-labeled glycans of MAb1 prior to CGE-LIF analysis. Figure 4A shows the CGE-LIF electropherogram of APTS-labeled glycans of MAb1, Figure 4B and 4C show the CGE-LIF results of APT- labeled glycans of MAb1 spiked with the collected peak assigned by mass spectrometric analysis as G0-F (H3N4) or Man5-F (H5N2), respectively. The first spiking experiment identified G0-F (H3N4) as the minor peak eluting at 252 base pair units (Fig. 4B). In the second experiment Man5-F (H5N2) was spiked into the APTS-labeled glycans of MAb1 showing co-elution with the peak at 248 base pair units. The same holds true when mMan3 (H3N3F1) is spiked into the APTS-labeled glycans of MAb1. It could thus be shown that G0-F (H3N4) could be unambiguously assigned in the CGE-LIF glycan pattern, whereas Man5-F (H5N2) and mMan3 (H3N3F1) co-elute. With the exception of these co-eluting glycan structures, all abundant peaks of the CGE-LIF electropherogram could be assigned by applying this strategy (summarized in Table 1).
Figure 4. Spiking of HILIC-UPLC separated APTS-labeled glycans into CGE-LIF glycan profile. (A) CGE-LIF electropherogram of MAb1glycan pool without spiking. (B) CGE-LIF electropherogram of MAb1 glycan pool with spiking of G0-F (H3N4). (C) CGE-LIF electropherogram of MAb1glycan pool with spiking of Man5-F (H5N2).
CGE-LIF peak assignment via spiking with commercially-available glycan standards
To confirm the assignment of the peaks of the CGE-LIF glycan pattern and to corroborate the co-elution of Man5-F (H5N2) and mMan3 (H3N3F1), commercially-available glycan standards were APTS-labeled and spiked into the glycans of MAb1 after APTS-labeling and subsequently analyzed with CGE-LIF.
As an example, the spiking of MAb1 with APTS-labeled G0-F (H3N4), Man5-F (H5N2) and mMan3 (H3N3F1) is shown in comparison to the non-substituted MAb1 sample in Figure 5.
Figure 5. Spiking of APTS-labeled commercially-available reference glycans into CGE-LIF glycan profile. (A) CGE-LIF electropherogram of MAb1 glycan pool without spiking. (B) CGE-LIF electropherogram of MAb1 glycan pool with spiking of Man5-F (H5N2). (C) CGE-LIF electropherogram of MAb1 with spiking of mMan3 (H3N3F1).
These experiments confirm the results achieved via spiking with APTS-labeled glycans after HILIC-UPLC fractionation and demonstrate that the two glycan species Man5-F (H5N2) and mMan3 (H3N3F1) cannot be separated by the applied CGE-LIF system. This must be taken into account if, for example, the amount of afucosylation of the therapeutic antibody has to be determined, as this peak may contain both a core-fucosylated (mMan3) and a non-core-fucosylated glycan species (Man5-F). With HILIC-UPLC analysis of APTS-labeled and 2-AB-labeled glycans of MAb1, we could elucidate that the selected material does contain both mMan3 and Man5-F structures amounting to 0.7% and 1.7% with HILIC-UPLC of APTS-labeled glycans and 0.5% and 2.0% with HILIC-UPLC of 2-AB-labeled glycans, respectively (Table 2). All major glycan peaks could be assigned with the employed strategy (Table 1, raw data not shown).
Table 2. Quantitation, comparison to alternative methods Where applicable standard deviations are given in parentheses. Six replicates were analyzed side by side.
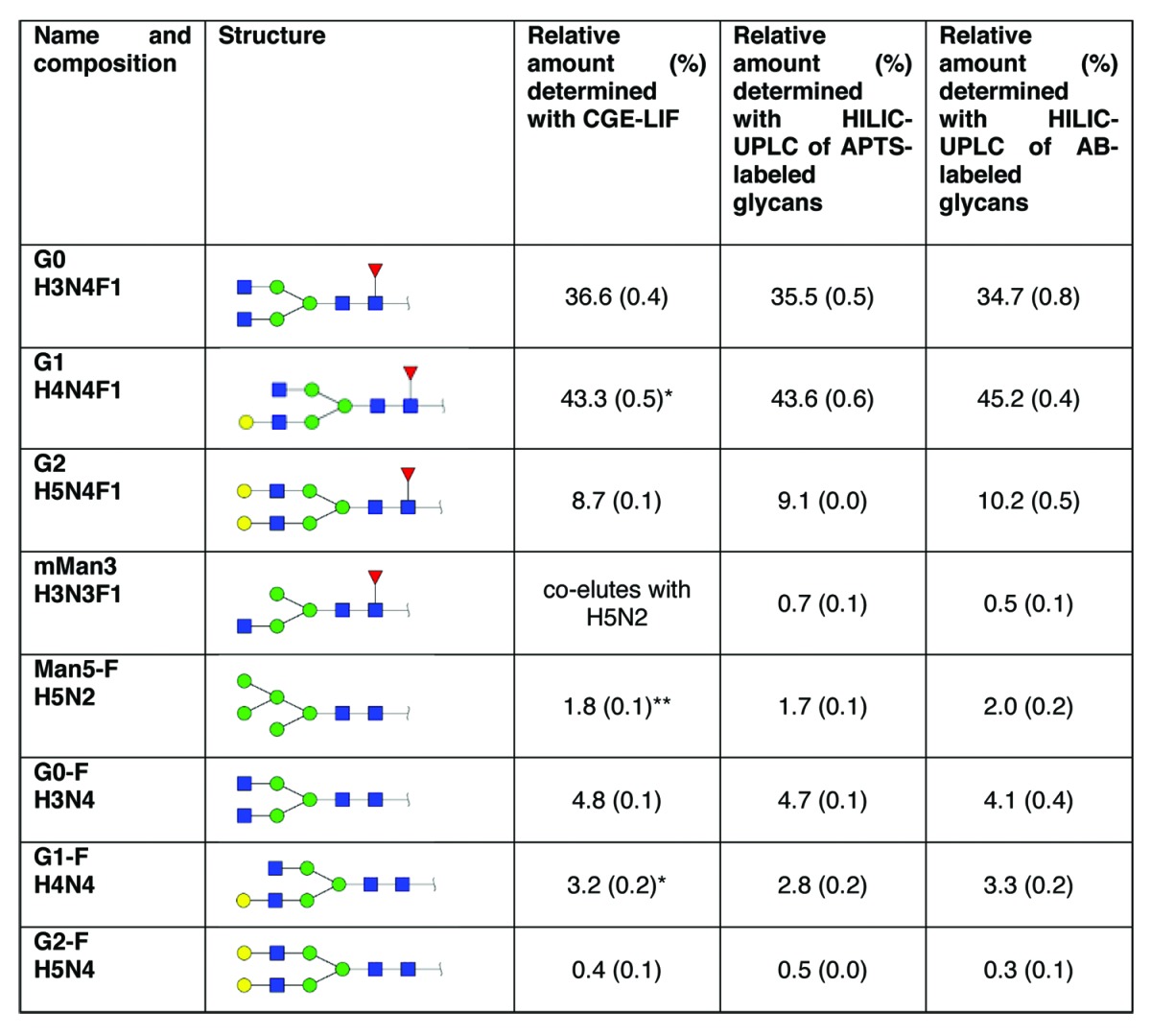
The sum of the relative amounts of the two isomeric structures is given. ** Because mMan3 and Man5-F co-elute, the sum of the relative amounts is given.
In summary, the main species detected were G0 (H3N4F1), G1 (H4N4F1) and G2 (H5N4F1) with a relative abundance of 36.6%, 43.3% and 8.7% and migration positions of 282, 319, and 363 base pair units (Table 1 and 2). Minor components, however, could also be detected at migration positions ranging from 248 to 333 base pair units. These are G0-F (H3N4) with a relative abundance of 4.8%, G1-F (H4N4) with a relative abundance of 3.2% and G2-F (H5N4) with a relative abundance of 0.4%. As previously stated, the glycan species mMan3 (H3N3F1) and Man5-F (H5N2) co-elute at 248 basepairs so they can only be reported as sum, with a relative abundance of 1.8% (see Tables 1 and 2).
Repeatability of the CGE-LIF method
To test the repeatability of the developed CGE-LIF method, eight individual samples of MAb1 were deglycosylated and the glycans were labeled separately with APTS and subsequently analyzed with CGE-LIF by the DNA analyzer. The analyses were repeated twice on different days. As can be seen in Figure 6, the repeatability (given in the standard deviation of the individual bars) and the intermediate precision (given in the difference between the individual bars in different color for each glycan species) was excellent. The relative standard deviation for the major 4 peaks was found to be below 0.5% throughout. In conclusion, our glycan analysis method is very robust and precise.
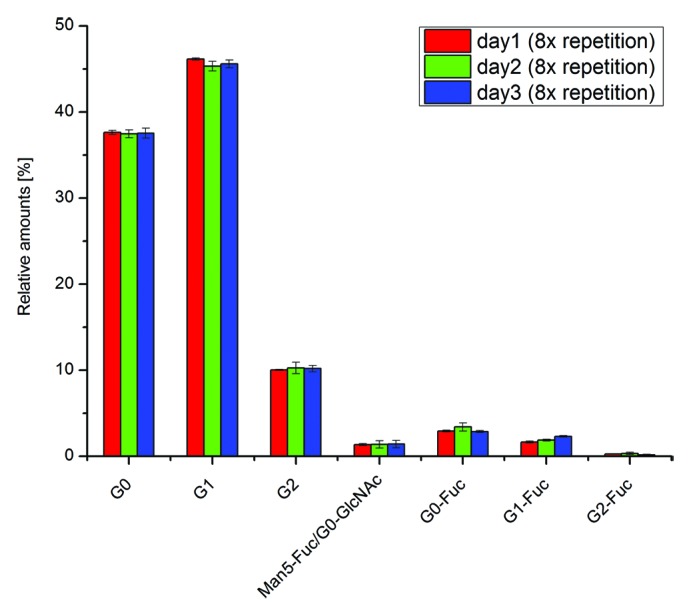
Figure 6. Repeatability of the IgG glycosylation analysis by CGE-LIF. IgG samples were analyzed 8-fold on three different days. Released glycans were analyzed by CGE-LIF after APTS-labeling, peaks were picked and assigned, and peak areas were normalized. Relative peak areas are given, and standard deviations are indicated.
Sensitivity of the CGE-LIF method
All glycans expected to be present in the Fc part of an antibody expressed in Chinese hamster ovary (CHO) cells could be detected with a sensitivity that is comparable to the sensitivity achieved with chromatographic methods. The absolute sensitivity of the CGE-LIF method, however, is much higher. We start with 5 µg IgG (~33 pmol). Because a 1:20 dilution step is included in the sample preparation, it should also be possible to analyze 0.25 µg IgG corresponding to 1.6 pmol. The limit of detection is surely far below as we could also detect and quantitate peaks with a relative abundance of 0.4%.
Comparison to other methods for glycosylation analysis
The developed method was used to analyze IgG glycosylation of MAb1. For comparison, two other methods for glycosylation analysis were applied. In the first approach, glycans of MAb1 were cleaved with PNGase F, APTS-labeled, separated with a HILIC-UPLC and analyzed with a fluorescence detector.
The second method, HILIC-UPLC of 2-AB labeled glycans, is the “gold standard” established in our laboratory. Briefly, glycans were released with PNGase F, labeled with 2-aminobenzamide, cleaned up with a Nanosep centrifugal device and separated with HILIC-UPLC with fluorescence detection.
For better comparison of all methods, six replicates were analyzed and the standard deviations were calculated.
Besides the main glycoforms that are expected for a therapeutic antibody produced in CHO cells, which are H3N4F1 (G0), H4N4F1 (G1) and H5N4F1 (G2) for all applied methods, the same minor glycan species could be detected and quantified as well, with standard deviations between 0.1% and 0.8%, which signify a high precision and repeatability (Table 2). Because mMan3 (H3N3F1) and Man5-F (H5N2) cannot be separated by CGE-LIF, the sum value is given under Man5-F. The relative amounts of MAb1 N-glycans as determined by the three methods were very similar, although different labels and separation techniques were employed.
In conclusion, our newly developed method gives essentially the same results as our gold standard method that we have validated for glycosylation analysis of biotherapeutics.
Application for process development
One of the typical applications for the developed test method is analysis of the glycosylation profile of a fermentation time-course. In this case, samples were taken each day from the beginning of the fermentation to day 10, purified by protein A chromatography, and analyzed with CGE-LIF after glycan release and labeling. Glycan analysis was performed in a single experiment after finalization of the fermentation.
The time-course for six different glycoforms from day 1 to day 10 is shown in Figure 7. As expected for a fermentation of therapeutic antibodies in CHO cells, the relative amount of G0 glycan (H3N4F1) increases with time and, accordingly, G1 glycan (H4N4F1) decreases with time. For the other glycans, the difference with fermentation time is less pronounced.25
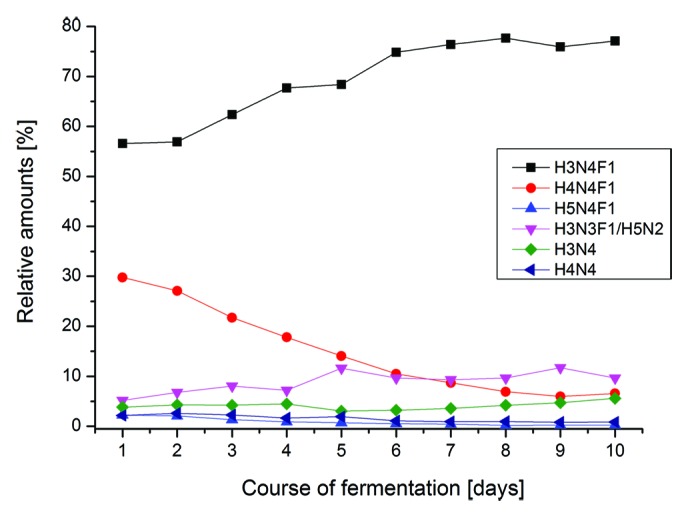
Figure 7. Glycosylation time-course of a fermentation experiment
Discussion
The biological relevance of glycosylation and state-of-the-art analytical glycan profiling methods have been reviewed,43-45 and high-throughput technologies for glycan analysis have been recently reported.46-48 We developed a high-throughput method for the glycosylation analysis of recombinantly expressed IgGs by capillary gel electrophoresis using a DNA analyzer. The high-throughput analysis is achieved by a high degree of automation and by multiplexing that allows up to 96 samples to be analyzed concurrently. We used a commercially-available labeling kit from Prozyme that was originally developed for conventional capillary electrophoresis applications, which helps to minimize the hands-on time in the laboratory. The theoretical sample throughput is enormous. Using the 96-capillary setting, and allowing for the 45 min run-time for one CGE-LIF analysis, up to 3000 samples can be analyzed in a day.
Because the DNA analyzer cannot be directly coupled to a mass spectrometer, alternative methods for peak characterization must be employed. We used a pre-fractionation approach with concomitant MS/MS characterization of APTS-labeled glycans. As an alternative method, commercially-available standards were used in spiking experiments. The two presented strategies for the characterization of the glycans should be applicable for the glycan analysis of mAbs from various expression systems, as well as other therapeutic glycoproteins. Glycan structural assignment has to be conducted only once for an individual antibody because the characterized peaks can be linked directly to basepair units in the developed software, and hence the peaks can be unambiguously assigned in each run.
The CGE-LIF DNA analyzer system has a separation power comparable to HILIC-UPLC after APTS-labeling and 2-AB labeling, respectively. The entire glycan repertoire of MAb1, which is typical for a therapeutic antibody expressed in CHO cells, could be separated with the exception of mMan3 (H3N3F1) and Man5-F (H5N2), which were found to co-elute. If an exact level of afucosylation must be calculated, this has to be taken into account as mMan3 (H3N3F1) is fucosylated and Man5-F (H5N2) is not.
The detected relative amounts of MAb1 glycan species were highly comparable to the results we obtained with our “gold standard” method HILIC-UPLC following 2-AB-labeling or APTS-labeling. The method proved to be very robust. We used 4 different batches of capillaries and at least 5 different batches of running buffer. As our laboratories are temperature-controlled, we do not know to which extent the separation is influenced by temperature changes.
With the experiments described here, we could show that the CGE-LIF method has an excellent separation power, a very good repeatability and intermediate precision, and possesses a high robustness. The standard deviations were very close to those reported previously by Schwarzer et al. The use of DNA fragments for normalization was reported by other groups and proved to be very useful in our hands.40 The comparison to other methods gives us also a very high confidence that we determine ratios of glycan species without a vast bias. Over the course of two years, we analyzed thousands of samples and the CGE-LIF with a DNA analyzer is now used routinely for samples for process development. We are currently developing adapted CGE-LIF methods for the analysis of glycoengineered antibodies, Fab glycosylated antibodies and other glycoproteins like EPO.
The CGE-LIF method can be used with fermentation broths. In this case, small scale purification with protein A material must also be employed, but this step can be automated. We presented the glycosylation data of a fermentation time-course used for process development, process characterization and process validation studies. The change of the glycosylation status of an IgG in the course of a fermentation could be shown. Likewise, the method should be capable of detecting glycosylation changes associated with process changes.
In conclusion, CGE-LIF analysis with a DNA analyzer can generate data that help to keep a production process within the desired design space or assess that a comparable drug substance is being produced after process changes (comparability), but could be used for release analytics due to its robustness. Because the method is rather simple and does not rely on mass spectrometry, we expect that it can also be run in non-specialized laboratories or in an at-line analytics setting near the fermentation process. The method is fast, robust, and cost-efficient, and has great potential for use in all laboratories involved in glycomics.
Materials and Methods
MAb1, a marketed antibody, was produced in a CHO cell line and purified by the Downstream Processing Group at Roche GmbH. rProtein A-Sepharose™ fast flow product was obtained from GE Healthcare. N-Glycosidase and 10 × phosphate buffered saline (PBS) were obtained from Roche Diagnostics. Water was purchased from J.T.Baker. Acetonitrile was bought from Fluka. Glycan standards were purchased from Prozyme. The sample preparation method was designed in 96-well plates allowing full automation. For automation of pipetting steps we used a Hamilton Microlab Star Robot.
Small scale purification with Protein A columns
One ml of fermentation supernatant was filtered in AcroPrep™ Advance 96-Well Filter Plates 1.2 µm Supor from Pall and applied to a Atoll column filled with 50 µl MabSelect SuRe (GE Healthcare). MAb1 was eluted with 2.5 mM HCl pH2.6. The protein content was assessed with a UV spectrometer and 5 µg MAb1 were automatically transferred to a AcroPrep™ Advance 96-Well Filter Plates 30K Omega from Pall by means of a TECAN Freedom Evo robot. Water was added to give a final volume of 300 µl. The plates were centrifuged three times after addition of 300 µl water for five min with 1500 g.
Sample preparation bulk samples
Samples from small purification with Protein A columns or bulk samples with an IgG content of about 5 µg were transferred to AcroPrep™ Advance 96-Well Filter Plates 30K Omega from Pall and water was added to give a final volume of 300 µl. The plates were centrifuged three times after addition of 300 µl water for five minutes with 1500 g.
Deglycosylation of IgGs
Samples were reconstituted in 50 µl of water containing 1 µl of PGNaseF (250 U of enzyme were dissolved in 250 µl H2O). Filter plates were sealed and the samples were directly incubated on the filter plates at 37 °C overnight. The released glycans were separated from IgG via the filter plates by centrifugation for 5 min at 1500 g into 96-well receiver plates (Prozyme). Samples were dried by vacuum centrifugation.
Labeling and purification of glycans
Labeling was performed with the APTS-labeling module for 96-well plates (Prozyme, #GP96NG-APTS), consisting of reductant solution, APTS solution and APTS catalyst solution. For 96 samples, typically 104 µl of reductant, 260 µl of APTS catalyst and 104 µl of APTS solution were mixed. Dried glycans were reconstituted in 4.5 µl of the prepared APTS-labeling master mix. 96-well receiver plates were then sealed and the labeling was performed under light protection for 4 h at 50 °C. Clean up after labeling reaction was done with GlykoPrep clean up (CU) cartridges (Prozyme). Twenty milliliters of 5 × APTS sample loading buffer (Prozyme) was filled up to 100 ml with acetonitrile. Samples were then diluted in 200 µl APTS sample loading buffer with thorough mixing and subsequently loaded onto the CU cartridges using 3 min centrifugal force at 300 g followed by 1 min at 1000 g. Then CU cartridges were washed 2 times with 200 µl of APTS sample loading buffer by centrifugation for 3 min at 300 g to remove excess dye and labeling side products. Samples were then eluted with 2 times 50 µl of water by centrifugation for 3 min at 1000 g.
Sample preparation for CGE-LIF
For 96 samples, 1250 µl Hi-Di Formamide (Applied Biosystems product code 4311320) was mixed vigorously with 3.5 µl of Basepair Size Standard (Applied Biosystems 500 Rox Size Standard product code 401734). Cleaned up samples were diluted 1:20 with water. Prior to analysis, 2 µl of diluted samples were then mixed with 10µl HI-DI Formamide Basepair Size Standard mixture.
CGE-LIF measurement of APTS-labeled glycans
For the analysis, we used a 48 capillary array of 50 cm length (Applied Biosystem filled with Pop-7™ Polymer (Applied Biosystems). Injection was performed with an injection voltage of 3 kV for 15 s. Run time was set to 1800 s and run time voltage was set at 15 kV.
HILIC-UPLC of 2-AB labeled glycans
Glycans were released with PNGaseF, dried in a vacuum centrifuge, labeled with 2-aminobenzamide cleaned up with a NanoSep centrifugal device (Pall Life Sciences) and separated by HILIC-HPLC with fluorescence detection.49,50
HILIC-UPLC-MS/MS of APTS-labeled glycans
APTS-labeled glycans were prepared as described before, but we used 500 µg instead of 5 µg of Mab1 for HILIC-UPLC-MS/MS experiments. HILIC-UPLC analysis were perfomed using ACQUITY UPLC BEH Glycan column from Waters (2.1 × 150 mm, 1.7 µm) with constant flow of 500 µl/min using gradient elution from 25% to 40% of Eluent A in 38.5 min. Eluent A consisted of 50 mM ammonium formate at pH 4.4 in water and Eluent B was acetonitrile (J.T. Baker MS grade). Prior to analysis 25 µl of labeled glycans in water were diluted in 75 µl acetonitrile (J.T Baker MS grade). 35 µl of the diluted glycans were injected. The UPLC system was equipped with a fluorescence detector (APTS detection extinction 473 nm; emission 520 nm) and directly coupled to an Orbitrap Velos mass spectrometer (Thermo scientific). Full MS and MS/MS of APTS-labeled glycans were acquired online in one experiment. The MS was operated in the negative ion mode. Source settings for MS were chosen as followes; capillary voltage was set to 5.0 kV; capillary temperature at 275 °C, sheeth gas flow at 42.0 (arb) and auxiliary gas flow at 5.0 (arb). For full MS we used analyzer FTMS (Fourier transform mass spectrometry) mode, scan type full, resolution at 30000 and data acquisition type profile. MS/MS experiments were conducted in the Ion Trap mode, collision energy was set fix at 35 mV and data acquisition type centroid. For fraction collection, fractions were collected manually.
CGE-LIF data analysis
The data analysis was performed by an in-house developed Matlab application. The software normalized the migration time on the internal base pair standard by a regression function of 2nd polynomial order. To eliminate minor deviations and to increase the comparability of different data sets, the peak corresponding to G0 (H3N4F1) was shifted to an bpu-value of 281.9.31 For the assignment of the remaining peaks, a database that contained the bpu-values and the accepted deviation of the expected glycans was used. Finally, the area of the assigned peaks was determined and the relative area of the glycans calculated.
Disclosure of Potential Conflicts of interest
No potential conflict of interest was disclosed.
Acknowledgments
We thank Jana Gassner, Bernd Maier and Stephanie Esslinger for excellent technical assistance. M Wuhrer was supported by the European Union's Seventh Framework Program (FP7-Health-F5–2011) under grant agreement n°278535 (HighGlycan).
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/26712
References
- 1.Beck A, Wurch T, Corvaïa N. Therapeutic antibodies and derivatives: from the bench to the clinic. Curr Pharm Biotechnol. 2008;9:421–2. doi: 10.2174/138920108786786420. [DOI] [PubMed] [Google Scholar]
- 2.Dimitrov DS, Marks JD. Therapeutic antibodies: current state and future trends--is a paradigm change coming soon? Methods Mol Biol. 2009;525:1–27, xiii. doi: 10.1007/978-1-59745-554-1_1. [xiii.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck A, Wagner-Rousset E, Bussat MC, Lokteff M, Klinguer-Hamour C, Haeuw JF, Goetsch L, Wurch T, Van Dorsselaer A, Corvaïa N. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr Pharm Biotechnol. 2008;9:482–501. doi: 10.2174/138920108786786411. [DOI] [PubMed] [Google Scholar]
- 4.Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21:11–6. doi: 10.1021/bp040016j. [DOI] [PubMed] [Google Scholar]
- 5.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–34. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 6.Durocher Y, Butler M. Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol. 2009;20:700–7. doi: 10.1016/j.copbio.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–7. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Biolsi S, Bales KR, Kuchibhotla U. Impact of variable domain glycosylation on antibody clearance: an LC/MS characterization. Anal Biochem. 2006;349:197–207. doi: 10.1016/j.ab.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Lim A, Reed-Bogan A, Harmon BJ. Glycosylation profiling of a therapeutic recombinant monoclonal antibody with two N-linked glycosylation sites using liquid chromatography coupled to a hybrid quadrupole time-of-flight mass spectrometer. Anal Biochem. 2008;375:163–72. doi: 10.1016/j.ab.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 10.del Val IJ, Kontoravdi C, Nagy JM. Towards the implementation of quality by design to the production of therapeutic monoclonal antibodies with desired glycosylation patterns. Biotechnol Prog. 2010;26:1505–27. doi: 10.1002/btpr.470. [DOI] [PubMed] [Google Scholar]
- 11.Boyd PN, Lines AC, Patel AK. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol Immunol. 1995;32:1311–8. doi: 10.1016/0161-5890(95)00118-2. [DOI] [PubMed] [Google Scholar]
- 12.Higgins E. Carbohydrate analysis throughout the development of a protein therapeutic. Glycoconj J. 2010;27:211–25. doi: 10.1007/s10719-009-9261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 14.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–3. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–3. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 16.Alessandri L, Ouellette D, Acquah A, Rieser M, Leblond D, Saltarelli M, Radziejewski C, Fujimori T, Correia I. Increased serum clearance of oligomannose species present on a human IgG1 molecule. MAbs. 2012;4:509–20. doi: 10.4161/mabs.20450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correia IR. Stability of IgG isotypes in serum. MAbs. 2010;2:221–32. doi: 10.4161/mabs.2.3.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, Flynn GC. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology. 2011;21:949–59. doi: 10.1093/glycob/cwr027. [DOI] [PubMed] [Google Scholar]
- 19.Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, Kuni-Kamochi R, Nakano R, Yano K, Kakita S, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17:104–18. doi: 10.1093/glycob/cwl057. [DOI] [PubMed] [Google Scholar]
- 20.Wright A, Sato Y, Okada T, Chang K, Endo T, Morrison S. In vivo trafficking and catabolism of IgG1 antibodies with Fc associated carbohydrates of differing structure. Glycobiology. 2000;10:1347–55. doi: 10.1093/glycob/10.12.1347. [DOI] [PubMed] [Google Scholar]
- 21.Bosques CJ, Collins BE, Meador JW, 3rd, Sarvaiya H, Murphy JL, Dellorusso G, Bulik DA, Hsu IH, Washburn N, Sipsey SF, et al. Chinese hamster ovary cells can produce galactose-α-1,3-galactose antigens on proteins. Nat Biotechnol. 2010;28:1153–6. doi: 10.1038/nbt1110-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, Chen X, Varki A. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18:818–30. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–81. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 25.Hossler P, Khattak SF, Li ZJ. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology. 2009;19:936–49. doi: 10.1093/glycob/cwp079. [DOI] [PubMed] [Google Scholar]
- 26.Reusch D, Haberger M, Selman MH, Bulau P, Deelder AM, Wuhrer M, Engler N. High-throughput work flow for IgG Fc-glycosylation analysis of biotechnological samples. Anal Biochem. 2013;432:82–9. doi: 10.1016/j.ab.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics. 2009;9:882–913. doi: 10.1002/pmic.200800715. [DOI] [PubMed] [Google Scholar]
- 28.Mariño K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6:713–23. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 29.Guttman A, Chen FT, Evangelista RA, Cooke N. High-resolution capillary gel electrophoresis of reducing oligosaccharides labeled with 1-aminopyrene-3,6,8-trisulfonate. Anal Biochem. 1996;233:234–42. doi: 10.1006/abio.1996.0034. [DOI] [PubMed] [Google Scholar]
- 30.Guttman A, Pritchett T. Capillary gel electrophoresis separation of high-mannose type oligosaccharides derivatized by 1-aminopyrene-3,6,8-trisulfonic acid. Electrophoresis. 1995;16:1906–11. doi: 10.1002/elps.11501601314. [DOI] [PubMed] [Google Scholar]
- 31.Ruhaak LR, Hennig R, Huhn C, Borowiak M, Dolhain RJ, Deelder AM, Rapp E, Wuhrer M. Optimized workflow for preparation of APTS-labeled N-glycans allowing high-throughput analysis of human plasma glycomes using 48-channel multiplexed CGE-LIF. J Proteome Res. 2010;9:6655–64. doi: 10.1021/pr100802f. [DOI] [PubMed] [Google Scholar]
- 32.Gennaro LA, Delaney J, Vouros P, Harvey DJ, Domon B. Capillary electrophoresis/electrospray ion trap mass spectrometry for the analysis of negatively charged derivatized and underivatized glycans. Rapid Commun Mass Spectrom. 2002;16:192–200. doi: 10.1002/rcm.564. [DOI] [PubMed] [Google Scholar]
- 33.Callewaert N, Geysens S, Molemans F, Contreras R. Ultrasensitive profiling and sequencing of N-linked oligosaccharides using standard DNA-sequencing equipment. Glycobiology. 2001;11:275–81. doi: 10.1093/glycob/11.4.275. [DOI] [PubMed] [Google Scholar]
- 34.Laroy W, Contreras R, Callewaert N. Glycome mapping on DNA sequencing equipment. Nat Protoc. 2006;1:397–405. doi: 10.1038/nprot.2006.60. [DOI] [PubMed] [Google Scholar]
- 35.Callewaert N, Contreras R, Mitnik-Gankin L, Carey L, Matsudaira P, Ehrlich D. Total serum protein N-glycome profiling on a capillary electrophoresis-microfluidics platform. Electrophoresis. 2004;25:3128–31. doi: 10.1002/elps.200406020. [DOI] [PubMed] [Google Scholar]
- 36.Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, Contreras R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429–34. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 37.Kottler R, Mank M, Hennig R, Müller-Werner B, Stahl B, Reichl U, Rapp E. Development of a high-throughput glycoanalysis method for the characterization of oligosaccharides in human milk utilizing multiplexed capillary gel electrophoresis with laser-induced fluorescence detection. Electrophoresis. 2013;34:2323–36. doi: 10.1002/elps.201300016. [DOI] [PubMed] [Google Scholar]
- 38.Lee KJ, Jung JH, Lee JM, So Y, Kwon O, Callewaert N, Kang HA, Ko K, Oh DB. High-throughput quantitative analysis of plant N-glycan using a DNA sequencer. Biochem Biophys Res Commun. 2009;380:223–9. doi: 10.1016/j.bbrc.2009.01.070. [DOI] [PubMed] [Google Scholar]
- 39.Murray S, McKenzie M, Butler R, Baldwin S, Sutton K, Batey I, Timmerman-Vaughan GM. Quantitative, small-scale, fluorophore-assisted carbohydrate electrophoresis implemented on a capillary electrophoresis-based DNA sequence analyzer. Anal Biochem. 2011;413:104–13. doi: 10.1016/j.ab.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Schwarzer J, Rapp E, Reichl U. N-glycan analysis by CGE-LIF: profiling influenza A virus hemagglutinin N-glycosylation during vaccine production. Electrophoresis. 2008;29:4203–14. doi: 10.1002/elps.200800042. [DOI] [PubMed] [Google Scholar]
- 41.Bunz SC, Rapp E, Neusüß C. Capillary Electrophoresis / Mass Spectrometry of APTS-labeled Glycans for the Identification of Unknown Glycan Species in Capillary Electrophoresis/ Laser Induced Fluorescence Systems. Anal Chem. 2013 doi: 10.1021/ac401930j. [DOI] [PubMed] [Google Scholar]
- 42.Royle L, Dwek RA, Rudd PM. Determining the structure of oligosaccharides N- and O-linked to glycoproteins. Curr Protoc Protein Sci. 2006;Chapter 12:6. doi: 10.1002/0471140864.ps1206s43. [DOI] [PubMed] [Google Scholar]
- 43.Beck A, Diemer H, Ayoub D, Debaene F, Wagner-Rousset E, Carapito C, et al. Analytical characterization of biosimilar antibodies and Fc-fusion proteins. Trac-Trend Anal Chem. 2013;48:81–95. doi: 10.1016/j.trac.2013.02.014. [DOI] [Google Scholar]
- 44.Beck A, Wagner-Rousset E, Ayoub D, Van Dorsselaer A, Sanglier-Cianférani S. Characterization of therapeutic antibodies and related products. Anal Chem. 2013;85:715–36. doi: 10.1021/ac3032355. [DOI] [PubMed] [Google Scholar]
- 45.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys. 2012;526:159–66. doi: 10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Behne A, Muth T, Borowiak M, Reichl U, Rapp E. glyXalign: High-throughput migration time alignment preprocessing of electrophoretic data retrieved via multiplexed capillary gel electrophoresis with laser-induced fluorescence detection-based glycoprofiling. Electrophoresis. 2013;34:2311–5. doi: 10.1002/elps.201200696. [DOI] [PubMed] [Google Scholar]
- 47.Burnina I, Hoyt E, Lynaugh H, Li H, Gong B. A cost-effective plate-based sample preparation for antibody N-glycan analysis. J Chromatogr A. 2013;1307:201–6. doi: 10.1016/j.chroma.2013.07.104. [DOI] [PubMed] [Google Scholar]
- 48.Stöckmann H, Adamczyk B, Hayes J, Rudd PM. Automated, High-Throughput IgG-Antibody Glycoprofiling Platform. Anal Chem. 2013;85:8841–9. doi: 10.1021/ac402068r. [DOI] [PubMed] [Google Scholar]
- 49.Lund J, Takahashi N, Nakagawa H, Goodall M, Bentley T, Hindley SA, Tyler R, Jefferis R. Control of IgG/Fc glycosylation: a comparison of oligosaccharides from chimeric human/mouse and mouse subclass immunoglobulin Gs. Mol Immunol. 1993;30:741–8. doi: 10.1016/0161-5890(93)90145-2. [DOI] [PubMed] [Google Scholar]
- 50.Royle L, Campbell MP, Radcliffe CM, White DM, Harvey DJ, Abrahams JL, Kim YG, Henry GW, Shadick NA, Weinblatt ME, et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal Biochem. 2008;376:1–12. doi: 10.1016/j.ab.2007.12.012. [DOI] [PubMed] [Google Scholar]