Abstract
Phage display, one of today’s fundamental drug discovery technologies, allows identification of a broad range of biological drugs, including peptides, antibodies and other proteins, with the ability to tailor critical characteristics such as potency, specificity and cross-species binding. Further, unlike in vivo technologies, generating phage display-derived antibodies is not restricted by immunological tolerance. Although more than 20 phage display-derived antibody and peptides are currently in late-stage clinical trials or approved, there is little literature addressing the specific challenges and successes in the clinical development of phage-derived drugs. This review uses case studies, from candidate identification through clinical development, to illustrate the utility of phage display as a drug discovery tool, and offers a perspective for future developments of phage display technology.
Keywords: phage display, antibodies, peptides, drug discovery, biologics, clinical trials
Introduction to Phage Display
Historically, novel drugs for the treatment of disease were identified serendipitously or through the isolation of the active components in natural remedies. With the advent of synthetic chemistry and increased understanding of structural biology, more rational drug design approaches were used.1 The modern revolution in molecular biology has added another tool for drug discovery and development. In particular, in vitro recombinant technologies such as phage display have emerged as powerful platforms for the discovery of candidates suitable for drug development.2 Phage display has become one of today’s crucial drug discovery platforms in large part because it allows identification of a broad range of biologics, including peptides, antibodies and other proteins, with the ability to engineer many of the attributes of successful drugs, e.g., potency, specificity, cross-reactivity, stability.
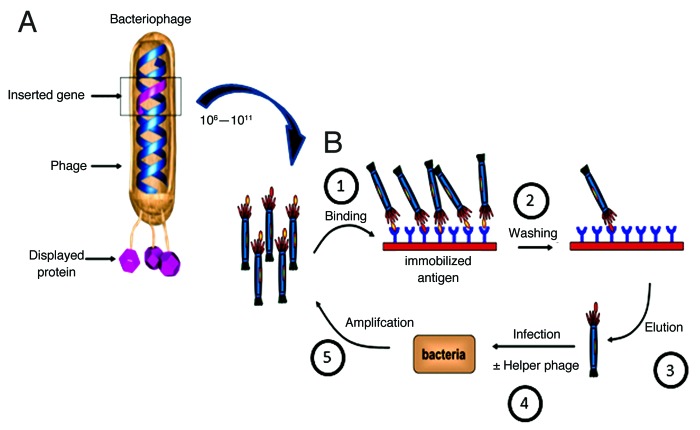
Phage display is a process in which phage DNA is manipulated to produce a fusion of a protein or peptide to one of the phage coat proteins. The most commonly used phage for phage display are members of the Ff family (M13, Fd, f1) and the most commonly used fusion partners are coat proteins of the parental phage, such as protein III of M13.3 For M13, both the N and C termini of the five coat proteins have been used as fusion proteins for library display. When the phage assembles, the fusion protein is incorporated into the phage particle in place of, or in addition to, the naturally occurring coat protein (Fig. 1A).
Figure 1. Phage display and selection. (A) A bacteriophage highlighting the genotype-phenotype coupling that is fundamental to phage display technology. The gene of interest (pink) is cloned into the gene 3 protein (g3p) of phage DNA, which results in the display of the pink protein product (antibody, peptide) on the surface of the phage as a polypeptide fusion. (B) Overview of phage display selection process. (1) A phage library containing 10^6-10^11 clones is incubated with immobilized antigen. (2) Unbound phage are removed by washing. (3) Bound phage are eluted. (4) E.coli are infected with eluted phage with or without helper phage to amplify eluted candidates. (5) Cells are plated onto selective plates and amplified. Process is reiterated 2–3 times resulting in enriched population of antibody/peptide fragments for the antigen of interest. Additional site directed mutagenesis or depletion approaches can be used to further tune desired antibody properties. Adapted and reproduced with permission from Buckler D, Schofield D, Sexton DJ, Lowe D and Vaughan TJ. Selection and screening of antibody phage display libraries. In: Wood CR, ed. Antibody Drug Discovery.©2012 World Scientific Publishing Co.
The power of phage display as a discovery tool stems from two basic features of the system: (1) the linkage of genotype and phenotype, and (2) the ability to build display libraries that range in size from 106 to 1011 distinct drug candidates and select those that bind the target (Fig. 1B). The physical linkage between the displayed protein and the gene that encodes it facilitates characterization of the displayed protein following selection of phage with a desired binding property. Once display of a parent protein has been demonstrated, it is possible to build display libraries of 106–1011 variants from which variants having a desired binding property can be selected. In contrast, other screening methods such as chemical library or cell based screening allow testing of hundreds or thousands of synthetic compounds, or tens of thousands of plated colonies. In fact, 1010 variants in a phage display library is actually a small part of the available theoretical sequence space. That is, the first set of binders to an antigen of interest can be subsequently diversified, retaining the sequence features that initially caused binding. This iterative variegation was used successfully to create high-affinity protease inhibitors of human plasmin, plasma kallikrein and thrombin, and it has become a common strategy in affinity maturation of drug candidates identified by phage display.4,5
Phage display was first described in 1985 and used to display short peptide fragments,6 and the first patent was filed in 1991 (US5223409).7 Since then, phage display has proven to be a reliable method for the generation of peptides with potential therapeutic or diagnostic utility.8 Phage display of single-chain V-domain antibody fragments (scFv) was reported in 1990, along with selective recovery of the phage on the basis of antigen binding.9 It has since become a major discovery platform for the identification of potent, fully human monoclonal antibodies (mAbs).10 More recently described antibody libraries have displayed Fab fragments on the phage protein, which can be readily reformatted to full-length IgG antibodies, usually without loss of binding function.11,12
Sources of diversity for phage display libraries includes immunized and naïve, i.e., non-immunized, animals, as well as synthetic diversity. Use of immunized animals as a source of diversity can be attractive in that the resulting library will be biased toward the target of interest and should contain high-affinity antibodies that have been matured through the natural affinity maturation process. This approach, however, requires construction of new libraries for each new antigen and assumes that an immune response can be generated to the antigen of interest. Alternatively, single large naïve,13 synthetic,14,15 or semi-synthetic libraries (where synthetic diversity is combined with natural diversity)12 have been shown to be capable of producing high affinity antibodies (Kd < 10nM) to a wide range of structurally diverse antigens. The use of such libraries therefore eliminates the need for a specific library for a specific antigen and circumvents issues with immunological tolerance found with mouse immunization approaches.
A critical feature of phage display is the ability to deplete libraries of binders to epitopes that are not of interest. Use of compounds (such as murine antibodies or non-specific enzyme inhibitors) that mask desirable binding sites on the target can be used to deplete libraries and thereby enrich for candidates likely to have desired biological activity (Fig. 2). For example, in the development of an active-site inhibitor of matrix metalloproteinase 14 (MMP-14) the phage display library was first depleted by selecting with MMP-14 bound to its natural, active site inhibitor.16 This depletion step eliminates the majority of antibodies that bind at sites other than the active site. Subsequent selection with active MMP-14 was then used to identify a highly specific candidate antibody that acted as a competitive inhibitor to the target site of interest.3 Selection strategies can also be designed to remove candidates with vulnerability toward challenges such as proteolytic digestion or resistance to denaturation.17 Moreover, the initial protein scaffold itself can be used to steer toward biological activity. For example, Kunitz domains are highly likely to bind the active site of serine proteases and have been used as a successful starting point to create a variety of specific protease inhibitors.18 In addition to Kunitz domains, a variety of other protein scaffolds have been used in phage display such as lipocalins, anticalins, and affibodies.19-21

Figure 2. Depletion strategy for selecting specific bioactive epitopes. In this strategy, a library of candidates is applied to immobilized antigen in the presence of a competitor that binds at the active site of interest. The competitor will prevent the binding of displayed candidates with similar binding characteristics, while allowing undesired candidates to bind. Unbound candidates in solution can be collected and applied to immobilized antigen, this time without the competitor. Such a strategy can facilitate the discovery of therapeutic candidates with exquisite specificity that would not be feasible using in vivo methods.
Another distinct advantage of using in vitro methods, such as phage display, for drug discovery is the ability to select for candidates that bind to both human and non-human targets.3 Having a candidate that binds the mouse, rat or monkey orthologs of a human target simplifies preclinical tests of efficacy and toxicity, and can shorten development timelines. Screening for such candidates is typically conducted by performing selections on a combination of cross-species orthologs, either separately or in succession, and screening for the appropriate reactivity profile.3
Capitalizing on the ability of phage display to tailor critical attributes of therapeutic proteins, a number of drug candidates have been developed using this platform and are now in clinical development. Here, we provide case reviews of identified phage display-derived drugs that are either in Phase 3 development or approved for the treatment of human disease.22-24 Such an analysis is timely, given that the clinical evidence sheds new light on the viability of phage display as a discovery method for identifying therapeutic candidates. Table 1 includes > 40 drugs identified as being derived from phage display and their current stage of clinical development. By examining the discovery and development of the phage display-derived biologics in the most advanced stages of clinical development, our purpose is to connect clinical success to thoughtful design principles implemented at the discovery phase, as well as to provide a commentary on how well phage display is living up to its early promise.
Table 1. Phage display-derived drugs in clinical trials or approved1.
| International non-proprietary name or drug code | Sponsor company | Target | Phage display technology | Development status | Indication(s) | |
|---|---|---|---|---|---|---|
| Raxibacumab (ABthrax) | Discovered by CAT technology and Human Genome Sciences- GlaxoSmithKline (2012) |
Protective antigen (PA) component of anthrax (Bacillus anthracis) | CAT | Approved (2012) |
Prophylaxis and treatment of anthrax | |
| Belimumab(Benlysta) | GlaxoSmithKline | B-lymphocyte stimulator (BLyS) | CAT | Approved (2011) | Autoantibody-positive, systemic lupus | |
| Ecallantide (Kalbitor) | Dyax Corp. | Plasma kallikrein | Dyax | Approved (2009) | Hereditary angioedema | |
| Romiplostim (Nplate) | Amgen | Thrombopoietin receptor (TPOR) | Affymax | Approved (2008) | Immune thrombocytopenic purpura | |
| Ranibizumab (Lucentis) | Genentech | Vascular endothelial growth factor A (VEGF-A) | Genentech | Approved (2006) |
Neovascular (wet) age-related macular degeneration | |
| Adalimumab (Humira) | Abbott Laboratories | Tumor necrosis factor-α (TNF) | CAT | Approved (2002) |
Rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, crohn’s disease, ulcerative colitis, plaque psoriasis | |
| Affinity ligand for Xyntha 2 | Wyeth Pharmaceuticals | Factor VIII | Dyax | used in purification of Factor VIII |
Hemophilia A | |
| Necitumumab (IMC-11F8) | Lilly and Bristol-Myers Squibb (purchased drug program from ImClone) |
Epidermal Growth Factor Receptor (EGFR) |
Dyax | Phase 3 | Cancer (NSCLC) | |
| Ramucirumab (IMC-1121B) | Eli Lilly (collaborator: ImClone) | Vascular endothelial growth factor receptor 2 (VEGFR2) | Dyax | Phase 3 | Cancer (NSCLC, breast, metastatic gastric adenocarcinoma) | |
| Trebananib (AMG 386) | Amgen | Angiopoietin 1 and 2 neutralizing peptibody (Ang1/2) | Dyax | Phase 3 | Cancer (ovarian, peritoneal, fallopian tube) | |
| Ganitumab (AMG 479) | Amgen | Insulin-like growth factor receptor (IGF-1R) | Dyax | Phase 2 | Cancer (pancreatic, colorectal breast, NSCLC) | |
| Cixutumumab (IMC-A12) | ImClone Systems Inc. | Insulin-like growth factor-1 receptor (IGF-1R) | Dyax | Phase 2 | Cancer (NSCLC, metastatic melanoma of the eye, liver) | |
| MM-121 | Merrimack Pharmaceuticals, partner with Sanofi | ErbB3 | Dyax | Phase 2 | Cancer (advanced ovarian, hormone sensitive breast cancer, NSCLC, and HER2 negative neoadjuvant breast cancer) | |
| BIIB 033 | Biogen Idec | Leucine-rich repeat and immunoglobulin-like domain containing, Nogo receptor-interacting protein (LINGO 1) | Dyax | Phase 2 | Acute optic neuritis, Multiple Sclerosis | |
| Mapatumumab | Human Genome Sciences, Inc., a GSK company | TNF-related apoptosis-inducing ligand receptor 4 (TRAIL-4) | CAT | Phase 2 | Cancer (NSCLC, non-Hodgkin lymphoma, liver, cervical) | |
| Tralokinumab (CAT-354) | MedImmune | Interleukin-13 | CAT | Phase 2 | Ulcerative colitis, pulmonary fibrosis, asthma | |
| Mavrilimumab (CAM-3001) | MedImmune | Granulocyte macrophage-colony stimulating factor receptor (GM-CSF) | CAT | Phase 2 | Rheumatoid arthritis | |
| Bertilimumab (iCo) | Immune Pharmaceuticals | Eotaxin-1 (CCL-11) | CAT | Phase 2 | Ulcerative colitis | |
| MOR103 | MorphoSys | GM-CSF | MorphoSys | Phase 2 | Inflammatory diseases (rheumatoid arthritis) | |
| BHQ880 | Novartis | Dickkopf-1 (DKK1) |
MorphoSys | Phase 2 | Multiple myeloma | |
| Carlumab (CNTO 888) | J&J | MCP-1 (CCL-2) | MorphoSys | Phase 2 | Prostate cancer | |
| CNTO 1959 | Janssen | IL-23p19 | MorphoSys | Phase 2 | Plaque-type psoriasis | |
| Gantenerumab | Roche | Beta-amyloid | MorphoSys | Phase 2 (Phase 3 recruiting) | Alzheimer | |
| BYM338 | Novartis | ActRIIB | MorphoSys | Phase 2/3 (recruiting) |
Sporadic inclusion body myositis |
|
| MLDL1278A (BI-204) | Genentech | Oxidized low-density lipoprotein (LDL) | BioInvent | Phase 2 | Stable atherosclerotic vascular disease | |
| Foravirumab (CL-184) | Crucell | Rabies virus glycoprotein | Crucell | Phase 2 | Prophylaxis of rabies | |
| Adecatumumab (MT201) | Amgen | Epithelial cell adhesion molecule EpCAM |
Micromet | Phase 2 | Colorectal liver metastases | |
| Fresolimumab (GC-1008) | Genzyme | TGF-β | CAT | Phase 2 | Primary brain tumors, primary focal segmental glomerulosclerosis | |
| BI-505 | BioInvent | ICAM-1 (CD54) | BioInvent | Phase 2 | Cancer (multiple myeloma) | |
| AMG 780 | Amgen | Angiopoietin | Dyax | Phase 1 | Cancer (advanced solid tumor) | |
| IMC-3C5 | ImClone | Vascular endothelial growth factor receptor-3 (VEGFR-3) |
Dyax | Phase 1 | Cancer (advanced solid tumor) | |
| Anti-MIF | Baxter | Macrophage Migration Inhibitory Factor (MIF) | Dyax | Phase 1 | Cancer (malignant solid tumor) | |
| AMG-745 | Amgen | Myostatin | Dyax | Withdrawn | Muscle loss | |
| Moxetumomab pasudotox (CAT-8015) | MedImmune | CD22 | CAT | Phase 1/2 | Cancer (non-Hodgin lymphoma, Phase 3 for hairy cell leukemia) | |
| CNTO 3157 | Janssen | Toll-Like Receptor 3 (TLR-3) | MorphoSys | Phase 1 | Asthma | |
| MOR202 | MorphoSys | CD38 | MorphoSys | Phase 1/2 | Cancer (multiple myeloma) | |
| BAY 94-9343 | Bayer | Mesothelin | MorphoSys | Phase 1 | Cancer (mesotheliomas, ovarian and pancreatic carcinomas) | |
| OMP-59R5 | OncoMed | Notch 2 and Notch 3 receptors | MorphoSys | Phase 1/2 | Cancer (solid tumors, small cell lung, pancreatic) | |
| Vantictumab OMP-18R5 |
OncoMed | Frizzled 7 receptor | MorphoSys | Phase 1 | Cancer (solid tumors) | |
| MT203 | Takeda | Granulocyte macrophage colony-stimulating factor GM-CSF |
Micromet | Phase 1 | Rheumatoid arthritis | |
| Samalizumab (ALXN6000) | Alexion | CD200 | Alexion | Phase 1/2 | Cancer (B cell chronic lymphocytic leukemia, multiple myeloma) | |
| DX-2930 | Dyax | Plasma kallikrein | Dyax | Phase 1 | Hereditary angioedema | |
1 Data current as of November 1, 2013. Phase and indications verified on ClinicalTrials.gov, company website or in the package insert of approved products.2 The affinity ligand for Xyntha was discovered using phage display. Abbreviations: NSCLC- non-small cell lung cancer
Case Reviews: Approved Phage-Display Derived Drugs or Those in Phase 3 Development
In the cases reviewed below, we highlight properties of phage display-derived candidates (Table 1) that have the potential to affect clinical success. The majority of these candidates originate from a few company-owned libraries: Cambridge Antibody Technology’s (CAT, now MedImmune, subsidiary of AstraZeneca) scFv-fragment library,13 Dyax Corp’s human Fab-fragment libraries,11,12 and MorphoSys’s human combinatorial antibody scFv-fragment (HuCAL®) and Fab-fragment (HuCALGold®) libraries.14,15 The case reviews were developed using information available in both the primary literature and patent information.
Adalimumab (Humira)
Adalimumab (Humira®; AbbVie Inc., formerly Abbott Laboratories, North Chicago, IL) is a human IgGκ antibody that binds tumor necrosis factor (TNF) and blocks its activation of TNF receptors. The biospecificity of adalimumab demonstrates a realization of the expected advantages of phage display as a drug discovery technology. Additionally, the case of adalimumab demonstrates the contribution of phage display, in conjunction with guided selection methods. Adalimumab is approved for treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn disease, ulcerative colitis and plaque psoriasis.25 According to the boxed warning in the US prescriber information, adalimumab is associated with a risk of serious infections and malignancy.25
Adalimumab was discovered using phage display with a “guided selection” method involving a mouse mAb. Briefly, the heavy chain of a murine antibody able to bind to TNF was combined with a collection of human light chains and selected for binding to hTNF. The selected light chains were then combined with a collection of human heavy chains and further selected for binding to hTNF and to an N-terminal peptide of hTNF.26 The resulting antibody, 2SD4, had an affinity for hTNF of ~15 nM. Additional sequence modification to remove non-wild type framework mutations and to affinity mature 2SD4 resulted in D2E7 or adalimumab.27 The affinity of adalimumab for human TNF is reported in the patents as ~300 pM, and it neutralizes hTNF-mediated cell toxicity of L929 cells with an IC50 of ~200 pM.27 Adalimumab was tested in nine species, including four different non-human primate species, dogs, and marmosets. The affinity of adalimumab for murine TNF is ~1000 fold weaker than for human; preclinical work was done in transgenic mice, mice injected with human TNF, or cynomolgus monkeys.28
Belimumab (Benlysta)
Belimumab (Benlysta®; GlaxoSmithKline, London, UK) is a human IgG1λ that was discovered using antibody phage display through collaboration between Cambridge Antibody Technology and Human Genome Sciences (now GlaxoSmithKline). Belimumab was approved in 2011 for the treatment of systemic lupus erythematosus (SLE).
Belimumab binds the soluble form of human B-lymphocyte stimulator (BLyS), preventing its interaction with any of its three receptors on B lymphocytes: (1) transmembrane activator and calcium-modulating cyclophilin ligand (CAML)-interactor (TACI); (2) B cell maturation antigen (BCMA); and (3) B cell activating factor belonging to TNF family-receptor (BAFF-R)/BLyS receptor-3 (BR3).29 The biological function of BLyS is to promote B-lymphocyte survival and differentiation. As a result, the clinical development of belimumab has focused on reducing B cell subsets responsible for autoantibodies, while normalizing immunoglobulin and complement levels, which are especially important in autoimmune disease such as SLE.29
In contrast to adalimumab, which used relatively small libraries of Fab fragments displayed on phage as described above, belimumab was identified from a large (~1011 member) library of single chain antibodies. Lead candidates identified from the primary selection were further optimized by heavy chain CDR3 mutagenesis to improve potency, resulting in belimumab which has been reported to bind human BLyS with high affinity (Kd = 274 pM).30 This affinity is sufficient to block the binding of BLyS with its receptors by in vitro assays.31 The potency of belimumab in blocking receptor interactions demonstrates that this antibody recognizes a critical BLyS epitope necessary for receptor binding. An advantage of in vitro selection approaches, such as phage display, is that antibody generation can be focused toward bioactive epitopes, for example receptor binding sites. This capability can accelerate discovery and provide a means of identifying antibodies against sought-after epitopes.
Belimumab binds BLyS from cynomolgus monkeys with approximately equal affinity (Kd = 264 pM) as human BlyS. Belimumab also binds mouse BLyS, albeit with lower affinity (Kd = 9.98 nM).30 This result is notable because it can be challenging to obtain biologics, such as antibodies, that cross-react with its target antigen from pre-clinically relevant species. For example, mAbs generated in mice against a human antigen are not expected to cross-react with the mouse ortholog. In vitro based discovery methods, such as phage display, are not restricted by the immunological tolerance induced using in vivo methods. Indeed, cross-reactivity with preclinical orthologs may be advantageous and can be selected using phage display due to the lack of suppression of antibodies against shared epitopes. This is performed by engineering cross-reactivity directly into the selection process by alternating between the human and rodent antigens. Although, this is only feasible if the target epitope exists on the antigen from both species.
Results from Phase 3 clinical trials demonstrated the effects of belimumab on Blys-dependent B cell subtypes.32-36 Patients with serologically and clinically active SLE who were treated with belimumab showed significant decreases in the numbers of naive and activated B cells, and significant decreases in plasma cells. Memory B cells and T cell populations did not decrease. Post-hoc analyses of clinical trial data showed that SLE disease activity and flare risk was more effectively reduced in patients with greater serological activity, i.e., patients who had low C3/C4 levels at baseline and who were anti-dsDNA positive at baseline.36,37
Belimumab has been noted as having a remarkable safety profile, based on similar adverse event rates in belimumab- and placebo-treated patients in clinical trial programs and no additional safety signals demonstrated in long-term observational studies.29,38
Ecallantide (Kalbitor)
Ecallantide (Kalbitor ®; Dyax Corp., Burlington, MA) is a 60 amino acid peptide inhibitor of plasma kallikrein approved for the treatment of acute attacks of hereditary angioedema (HAE), a rare autosomal dominant disease characterized by a deficiency of C1 esterase inhibitor (C1-INH).39 C1-INH is a critical endogenous inhibitor of plasma kallikrein.40 In HAE patients, reduced levels of total C1-INH (in the case of type I HAE) or functional C1-INH (in the case of type II HAE) lead to increased levels of active plasma kallikrein. During an HAE attack, uncontrolled plasma kallikrein activation leads to the excessive production of bradykinin, resulting in increased vascular permeability, edema, inflammation and pain.41,42 Ecallantide is a potent (Ki = 25 pM), selective, and reversible inhibitor of plasma kallikrein that effectively inhibits the active plasma kallikrein generated during an HAE attack.43 According to the boxed warning in the US prescriber information, ecallantide is associated with a risk of anaphylaxis.44
Ecallantide was discovered using a phage display library constructed using the first Kunitz domain of human lipoprotein-associated coagulation inhibitor (LACI-D1) as a scaffold.45 LACI-D1 is also known as tissue factor pathway inhibitor (TFPI). In the development of ecallantide, phage libraries were prepared and screened in an iterative manner as an alternative to building large libraries (i.e., libraries with a phage clonal diversity > 108). First, the region of LACI-D1 identified as making crucial interactions with its natural target (the primary loop) were varied and this library was selected for binding to plasma kallikrein. Next, DNA was prepared from the resulting binding phage and a varied sequence introduced into a second region within the Kunitz domain. This second region contains residues that interact with the primary loop, which may serve to stabilize the primary loop, and also directly contact the target protease. This new library was then selected for binding to plasma kallikrein. Sequence information from the resulting isolates was then used to make a more focused third library, which had limited variability in those residues that were strongly selected and varied positions that were not varied in the initial library. Screening of this third library resulted in an isolate that, when expressed as free protein, was able to bind to plasma kallikrein with high specificity and potency (Ki = 25 pM).
The development of ecallantide demonstrates the use of phage display in the discovery of approved therapeutics based on novel protein scaffolds, in contrast to its more extensive use in the production of therapeutic antibodies. This example also demonstrates that phage display can be used to engineer altered specificity into a protein scaffold.18 In this case, while wild type LACI-DI is primarily an inhibitor of coagulation factor VIIa, ecallantide does not inhibit FVIIa at the highest concentration tested (10 µM).46
Romiplostim (Nplate)
Romiplostim (Nplate®; Amgen) is a peptibody, i.e., a peptide-Fc fusion, that binds the thrombopoietin receptor (TPOR) and acts as a highly potent functional mimetic of thrombopoietin (TPO). It was the first peptibody approved by the US Food and Drug Administration (FDA). Romiplostim is indicated for the treatment of immune thrombocytopenic purpura (ITP) an autoimmune disorder characterized by accelerated platelet destruction and decreased platelet production.
The peptide component of romiplostim was originally discovered using recombinant peptide libraries displayed as fusions to the pVIII coat protein of phage or to the E. coli lac repressor protein (peptides-on-plasmids).47 Six pVIII phagemid libraries and three peptides-on-plasmid libraries were screened for three to four rounds against immobilized TPOR. Two families of TPOR peptide ligands, unrelated in their sequences, were identified that specifically bound TPOR. Chemically synthesized peptides most closely resembling the consensus sequence of each family were then created and tested for competition with TPO for receptor binding.
Variants from one of the TPOR peptide ligand families were subsequently screened under affinity-selective conditions and yielded a 14-amino acid peptide, AF12505, with an IC50 = 2 nM and an EC50 = 400 nM.47 Based upon the observation that covalently linked dimeric forms of erythropoietin mimetic peptides have increased potency, a similar strategy was undertaken with AF12505. The strategy proved effective, as the dimeric form of the peptide had an IC50 of 0.5 nM and an EC50 of 100 pM, more than 4000 times as potent as the monomer from which it was derived. The dimer both stimulated the in vitro proliferation and maturation of megakaryocytes, the cells giving rise to platelets, and potently stimulated platelet formation in mice.47 Romiplostim, a 60 Da peptibody, was ultimately produced by covalently linking two tandem dimers to the C-terminus of human IgG1 (Fc fragment).48
Preclinical studies in mouse, rat and cynomolgus monkey demonstrated that both subcutaneously and intravenously administered romiplostim dose-dependently increased platelet levels in all species.48,49 Romiplostim was ultimately approved based upon the results of two double-blind placebo controlled Phase 3 studies, performed in both splenectomised and non-splenectomised patients with ITP (splenectomy is a standard procedure in ITP patients to reduce platelet destruction).50 In these trials, the overall platelet response rate was 88% in non-splenectomised and 79% in splenectomised patients, compared with 14% in placebo-treated patients.50 The drug was well tolerated and many patients were able to reduce or discontinue other ITP medications. An open-label extension study in ITP patients found the incidence of anti-drug antibodies to be low, and they did not cross react with TPO.51 The lack of cross-reactivity to TPO is not surprising as romiplostim has no sequence homology to TPO. However, the observation is important because first generation recombinant TPO products were abandoned due to development of neutralizing antibodies that cross-reacted to endogenous TPO, leading to thrombocytopenia and a dependence on platelet transfusion.52
Raxibacumab
Raxibacumab (GlaxoSmithKline, London, UK) is a human monoclonal IgG1λ antibody approved for the treatment and prevention of inhaled anthrax. Of particular note, it is the mAb approved using the FDA’s Animal Efficacy rule. The Animal Efficacy rule allows efficacy findings from adequate and well-controlled animal studies to support FDA approval when it is not feasible or ethical to conduct trials in humans, as is the case with inhaled anthrax.53 Importantly, the case of raxibacumab highlights the substantial utility of in vitro technologies, in this case phage display, in the development of therapeutics for antigens that prove too lethal in vivo.
Raxibacumab binds the Bacillus anthracis protective antigen (PA) to prevent the lethal factor (LF) and edema factor (EF) from engaging the anthrax toxin receptor (ATR) or capillary morphogenesis protein 2 (CMG2) on mammalian cell surfaces, which is responsible for virus entry into cells.54 That the mechanism of action of raxibacumab does not compete with currently approved antibiotics for treating anthrax infection is a distinct advantage.
Raxibacumab was discovered using phage display by Human Genome Sciences under a license from Cambridge Antibody Technology.55 Monomeric PA is produced by Bacillus anthracis as an 83 kDa protein that is cleaved by a furin-like protease to a 63 kDa form that assembles as a heptamer on the cell surface and binds LF and EF leading to their endocytosis.56 Raxibacumab has been shown to bind the 63 kDa form of PA with a binding constant (Kd) of 2.78 nM, as measured by Biacore surface plasmon resonance, and to exhibit a potency (IC50) of 503 pM in an in vitro assay that measures the inhibition of PA binding to its recombinant soluble anthrax toxin receptor (TEM8). Raxibacumab was shown to significantly increase 28 d survival in rabbits and monkeys.57
Ranibizumab (Lucentis)
Ranibizumab (Lucentis®; Genentech) is an antigen-binding fragment (Fab) that binds and neutralizes the activity of vascular endothelial growth factor A (VEGF-A). Ranibizumab is approved for the treatment of wet age-related macular edema and is an interesting case study for how phage display can be used for antibody humanization. VEGF-A plays a critical role in angiogenesis, a process critical for normal growth and development, as well as wound healing.58 Angiogenesis is also involved in the pathology of disease such as tumor progression and ocular neovascularization, which occurs in diabetic retinopathy and age-related macular edema.59,60 As such, VEGF-A is a candidate drug target where angiogenesis contributes to the pathology of disease.
A mouse mAb to VEGF-A, A4.6.1, was found to effectively neutralize VEGF-A activity in vitro and in a variety of mouse tumor models. This mouse antibody was humanized by grafting the mouse CDRs onto a human antibody backbone (VLκ1, VHIII), followed by site-directed mutagenesis.61 The resulting antibody, bevacizumab (Avastin), is an IgG1 approved for treatment of a variety of cancer types.
Ranibizumab was also created using A4.6.1 as a starting point and phage display was used in candidate optimization.62 In the first round of optimization, the CDRs of A4.6.1 were grafted onto a human antibody backbone. The resulting antibody had a binding activity ~4000-fold weaker than a chimera consisting of the VL and VH regions from A4.6.1 and human constant regions.63 A library was then constructed in which select framework residues in VL and VH regions were varied, with the resulting library displayed on phage and selected for binding to immobilized VEGF. Analysis of the selected clones resulted in the identification of an isolate that demonstrated a 125-fold improvement in affinity over the parental clone.63 From additional sequence and structural analysis, an additional light chain residue was identified for mutagenesis that resulted in an additional 6-fold improvement in affinity.63
A clone from the first round was used for alanine scanning of the CDRs and inspection of a crystal structure of the Fab form of bevacizumab, Fab-12, identified residues within the three heavy chain CDRs to be varied.64 Using an iterative approach, three individual CDR display libraries were prepared and selected for binding to VEGF-A.64 In a second step, the selected residues in the CDRs were combined to produce a high affinity variant. This isolate had a Kd of ~0.1 nM, a ~10-fold improvement over the chimera, 100-fold improvement over Fab-12, and 40,000-fold improvement over the initial CDR grafted construct.
The choice of the Fab rather than a full-length IgG was supported by preclinical studies. At the time of development of ranibizumab, it was thought that a full-length IgG would not adequately penetrate all layers of the retina. This belief was supported by observations that trastuzumab intravitreal injections could not penetrate the limiting inner membrane of the retina in rhesus monkeys.65 In contrast, intravitreal injection of ranibizumab was shown to penetrate all layers of the rabbit retina.66 Pharmacokinetics also supported use of the Fab format for ranibizumab. Ranibizumab distributes rapidly to the retina of cynomolgus monkeys with a tmax of 6 h and was subsequently found to be cleared from the ocular compartment with a half-life of ~3 d.66 Following intravitreal injection, ranibizumab is found in the serum at maximum concentration >1000-fold lower than that of the concentration found in the vitreous, with peak concentration reached at ~6 h and a terminal half-life that parallels that of clearance from the ocular compartment.66 Notably, the serum half-life of ranibizumab following IV injection is ~14 h.66 The relatively low serum concentrations of ranibizumab and relatively rapid clearance from this compartment, when compared with an IgG, have been proposed to be important in minimizing impact on normal healthy processes that involve VEGF.62
The preclinical studies suggested that a once monthly dose of 300–500 μg would be sufficient to effectively neutralize VEGF in the eye of patients.66 In a Phase 1 study in patients with age-related macular edema, the maximum tolerated dose was found to be 500 μg.62 In a subsequent multiple-dose escalating Phase 2 study, patients were shown to tolerate doses up to 2000 μg with only mild, transient ocular inflammation observed.62 In the pivotal Phase 3 trials, ANCHOR and MARINA, ranibizumab was dosed once a month for 2 y with the rate of loss or gain of visual acuity as a primary end point.67 In both trials, ~95% of patients treated with ranibizumab lost fewer than 15 letters from visual acuity compared with ~60% of patients that were sham injected. Approximately 30–40% of treated patients gained 15 letters or more in visual acuity, compared with 5% of patients that were sham injected.67 On the basis of this data, ranibizumab was approved in the US in June 2006 for the treatment of age-related macular edema.68
Trebananib (AMG-386)
Trebananib (Amgen) is a peptibody that binds to angiopoietin-1 (ang-1) and angiopoietin-2 (ang-2) and blocks their interaction with the Tie-2 receptor. Trebananib is currently being evaluated clinically as a treatment for a variety of tumor types.
While VEGF inhibitors have demonstrated success in reducing angiogenesis, and therefore affect the growth of tumors, in developing trebananib it was thought that targeting the signaling pathways leading to angiogenesis could result in improved anti-tumor efficacy.69 One such pathway is the angiopoetin-Tie-2 signaling pathway. Tie-2 is a receptor tyrosine kinase expressed on vascular endothelial cells which, when activated by the angiopoietins (ang-1, ang-2, ang-3, and ang-4), leads to increased cell survival, proliferation, and motility.69 Current evidence suggests that ang-2 plays a dominant role in mediating angiogenesis by enhancing vasculature plasticity and allowing vascular remodeling.
Trebananib was identified by interrogating both linear and constrained loop peptide libraries displayed on the surface of phage with biotinylated ang-2 immobilized on streptavidin-coated beads.69 Identified peptides were then subjected to further affinity maturation using newly created libraries that maintained a selected motif while varying other residues. Both a linear and constrained loop peptide were identified and further characterized by comparison to an Ang-2 specific human antibody, also identified using phage display. The affinities of the peptides were in the range of those more commonly associated with antibodies, ~50 pM and 23 pM for the linear and constrained loop peptides, respectively.69 The specificity was equally impressive. The linear peptide bound human, mouse and rat ang-2 with affinities of 54 pM, 71 pM and 160 pM, but did not cross react with ang-1, ang-3, ang-4 or VEGF.69 As might have been expected, the constrained loop peptide’s affinities for ang-2 (23 pM, 21 pM, and 49 pM for human, mouse and rat, respectively) were higher than those of the linear peptide.69 The homology between ang-2 and ang-1 is ~60%, but cross-reactivity with ang-1 was observed for the constrained loop, albeit a much lower affinity (900 pM for human and 640 pM for mouse ang-1).69
The peptibody was created by fusing the identified peptides to the carboxy-terminus of a human IgG1 Fc.69 When evaluated in preclinical tumor xenograft models, there was no substantial difference in efficacy between the two peptides, although the constrained loop peptide had a half-life of 96 h, approximately twice that of the linear peptide. The peptides were typically dosed intravenously twice weekly, resulting in tumor stasis at doses of < 0.6 mg/kg.69 The mechanism of tumor growth inhibition was through inhibition of endothelial cell proliferation rather than inhibition of growth of the tumor cells, which supported the proposed anti-angiogenic mechanism of action.69
An open label Phase 1 study with trebananib was conducted in patients with advanced solid tumors that were refractory to standard treatment.70 Trebananib was found to be generally well tolerated with most toxicities being mild to moderate in severity. The half-life of the molecule was found to be 3–6 d, with weekly doses of <3 mg/kg resulting in trough levels of drug above the serum concentrations found to be effective in preclinical models.70
Trebananib has been evaluated in Phase 2 studies in combination with three different standard of care regimens: 5-fluorouracil, irinotecan and leuocvorin (FOLFIRI); sorafenib; and cisplatin and capecitabine.71,72 In each case, the progression-free survival rate was found to be similar to the standard of care plus placebo. In the case of combination with FOLFIRI, while the progression-free survival rate was similar to control, there was a trend for improvement in overall response rate. Trebananib is currently being evaluated in three Phase 3 studies in women with recurrent partially platinum sensitive or resistant epithelial ovarian, primary peritoneal or fallopian tube cancer (clinicaltrials.gov numbers NCT01281254, NCT01204749, and NCT01493505).
Necitumumab
Necitumumab (IMC-11F8) was discovered via phage display by ImClone Systems (subsidiary of Eli Lilly, Indianapolis, IN) that binds epidermal growth factor receptor (EGF-R) and blocks binding of EGF. EGF signaling is thought to play an important role in the progression of several kinds of cancer by mediating the proliferation of EGFR-dependent tumor cells. The antibody library used for the discovery of necitumumab was constructed using PCR of VH and VL from normal donors.11 Necitumumab was discovered using a selection against this naïve library without resorting to affinity maturation. US Patent 7,598,350 reports that the Fabs of necitumumab and cetuximab (an approved chimeric mouse/human antibody also targeting EGF-R) have affinity for EGF-R of 1.8 nM and 0.5 nM, respectively.73 The IgG form of necitumumab has an affinity of 0.32 nM, which is greater than the affinity of EGF for EGF-R at 0.60 nM.74 In addition to its high affinity for EGF-R, necitumumab may pose a low risk of hypersensitivity reaction because it is a fully human mAb.
Preclinical studies indicated that the antitumor activity of necitumumab was comparable or superior to cetuximab, and several clinical trials in which EGF is targeted have been or are being conducted for malignant solid tumors, non-small cell lung cancer, squamous non-small cell lung cancer, metastatic colorectal cancer, and advanced solid tumors.75 Two of the trials are in Phase 3 and six are Phase 2. One Phase 3 trial (NCT00982111, necitumumab plus pemetrexed and cisplatin) was stopped because of excessive thromboembolytic events in the experimental arm.76 There are as yet no publications on the mechanism of these thromboembolytic events. Additional Phase 3 data of the use of necitumumab in combination with gemcitabine-cisplatin for the treatment of in Stage IV non-small cell lung carcinoma are expected in late 2013.
Ramucirumab
Ramucirumab (IMC 1121B) (Eli Lilly) is a human mAb that binds and blocks the kinase insert domain containing region (KDR, VEGF-R2), a receptor for VEGF. Inappropriate stimulation of KDR is thought to be involved in several forms of cancer due to its demonstrated role in angiogenesis, and blocking the pathway is an area of considerable interest. Ramucirumab blocks KDR activation by acting as a receptor antagonist and preventing the binding of VEGF.
The development of ramucirumab began with the use of a non-immunized phage antibody library of human Fabs obtained by PCR amplification of human VHs and VLs.11 The extracellular domains of KDR (fused to alkaline phosphatase) were used as the target antigen and Fabs D2C6, D2H2, D1H4, and D1F7 were selected.77 D2C6, D2H2, D1H4, and D1F7 were then expressed as soluble Fabs and tested for their ability to block binding between KDR-AP and VEGF. The affinities of these Fabs for KDR are 1.97, 3.93, 2.76, and 45.2 nM, respectively.77 Fabs D2C6, D2H2, D1H4 are specific for human KDR, while D1F7 shows some binding to the murine ortholog (Flk-1). Often, cross-reactivity to animal orthologs is seen as an advantage, but in this case attention was focused on the higher-affinity Fabs that are specific to human KDR and share a single heavy chain. A new library was constructed by combining the shared heavy chain with the full light chain diversity of the library and Fabs SA1, SA3, SB10, SB5, SC7, SD2, SD5, SF2, SF7, and 1121 were selected.77 The affinities of the Fab 1121 (IMC 1121) and the IgG1 version (ramucirumab) for KDR are 110 pM and 50 pM, respectively.77
In preclinical development, ramucirumab demonstrated significant antitumor activity in a range of animal tumor models both as a single agent and in combination with other therapeutics.78 As one example using the HL60 mouse leukemia model, mice received intraperitoneal injection of various doses of ramucirumab twice weekly and were observed for signs of toxicity and recorded for time of survival. Untreated mice died within 17 d, while mice treated with ramucirumab survived 63 ± 12 d, with no overt toxicities observed over the course of the experiment.79
Following promising preclinical results and nonclinical toxicology studies, an open label Phase 1 study was conducted in patients with advanced solid malignancies treated once weekly with escalating doses of ramucirumab. Ramucirumab was generally well-tolerated as a weekly 1 h infusion and associated with both decrements in tumor perfusion and vascularity, as well as partial response or stable disease in 73% of patients.78 Ramucirumab has since been entered into 32 clinical trials, 6 of which are Phase 3 studies. These studies involve the use of ramucirumab as a first-line or second-line therapy, or as a combination with conventional chemotherapeutics, for hepatocellular carcinoma, gastric cancer, breast cancer, non-small-cell lung cancer, and colorectal cancer.80 Recently, a marketing application for the use of ramucirumab as a monotherapy in second-line gastric cancer was submitted to the FDA.
Recombinant anti-hemophilic factor (Xyntha®) purification peptide
Xyntha (Pfizer) is a recombinant Factor VIII (FVIII) approved in 2008 for the treatment of hemophilia. This protein is expressed in Chinese hamster ovary cells and purified using a process that involves an affinity chromatography step based on a FVIII-specific peptide that was identified using phage display.81 The FVIII-specific affinity peptide was obtained using a peptide phage display library that contained varied amino acids constrained in a loop by a disulfide. The peptides were synthesized and attached to a solid support in order to assess their ability to capture recombinant FVIII from typical expression systems and elute under mild conditions.81 Using the selected peptide (Tn 8.2) that bound with a dissociation constant of ~0.72 µM as determined by isothermal calorimetry, an affinity chromatographic purification procedure was developed for the production of recombinant FVIII under GMP conditions.82 Xyntha does not tolerate changes of pH, salt or temperature, and as such is eluted with 50% ethylene glycol.
Replacing the antibody affinity column purification step with a peptide affinity column in the Xyntha manufacturing process eliminated the use of human- or animal-derived proteins. This was an important consideration in the development of recombinant FVIII due to the negative perception that surrounds the use of human plasma derived proteins for hemophilia products. The case of Xyntha also highlights the flexible use of phage display not only as a discovery technology for the production of human therapeutics, but as a platform for product development in the area of purification and manufacturing technology.
Summary
The number of phage display-derived candidates currently in clinical development demonstrates the value of phage display as an established and reliable drug discovery platform. As shown in Table 1, over 40 biological drugs or drug candidates were discovered using phage display. Five are approved drugs (4 antibodies and 1 peptide), and an additional 3 antibody-based candidates (trebananib, ramucirumab and necitumumab) are in Phase 3 development for the treatment of various cancers. Of these, completion of Phase 3 studies (either primary or estimated study completion) of both ramucirumab (NCT01170663, NCT00917384, NCT00703326) and necitumumab (NCT00981058, NCT00982111) are estimated for 2013.
Most of the phage display candidates in Table 1 are antibodies or antibody derivatives, with ecallantide (Kalbitor®), Xyntha purification peptide, and the peptibody trebananib (AMG-386) being the exceptions. While peptide-based therapeutics are clinically promising due to their small size and established manufacturing processes, their short half-life initially limited their potential as therapeutic strategies. Advances in protein engineering such as peptide cyclization, use of fusion proteins (e.g., the Fc fragment), unnatural amino acids and conjugation to polyethylene glycol and polysialic acids have mitigated some of these limitations.83 As a consequence, there has been a recent re-emergence of peptide-based therapeutics development.8 With the power and versatility of phage display, it is likely that phage display will continue to provide candidate peptides for therapeutic or diagnostic applications.84,85
Anecdotal evidence has been used to support the hypothesis that a higher percentage of mAbs selected from synthetic or semi-synthetic phage libraries have lower solubility profiles compared with murine hybridoma antibodies.86 However, this phenomenon, if true, is easily overcome by employing a variety of techniques. Screening library outputs using a combination of cross interaction chromatography and dynamic light scattering have proven effective as early solubility screening techniques.87 Furthermore, protein engineering approaches using targeted mutagenesis,88,89 structure based engineering efforts,90 and process modifications88 have also been employed successfully to increase the solubility profile of mAbs.
The ability of phage display to tailor desired drug characteristics through the use of strategies such as depletion, guided selection and engineered cross-species reactivity, as identified in the examples above, suggests the platform will continue to provide useful therapeutic candidates for clinical development. In addition, advances in the automation of the selection process continue to enhance throughput such that therapeutic candidates can be obtained on the order of weeks. Indeed, perhaps the biggest limitation in the discovery of novel therapeutics is not the ability to produce specific and efficacious biopharmaceuticals, but the identification of appropriate disease targets.
Disclosure of Potential Conflicts of Interest
Nixon AE, Sexton DJ, and Ladner RC are all full-time employees of Dyax Corp., Burlington MA.
Glossary
Abbreviations:
- Abs
antibody
- Ang
angiopoietin
- ATR
anthrax toxin receptor
- BLyS
B-lymphocyte stimulator
- C1-INH
C1 inhibitor
- CDR
complementarity determining region
- EGF
epidermal growth factor
- FVIII
factor VIII HAE, hereditary angioedema
- IgG
immunoglobulin-G
- KDR
kinase inserting domain
- LACI-D1
lipoprotein-associated coagulation inhibitor domain 1
- PA
protective antigen
- scFv
single-chain variable fragment
- SLE
systemic lupus erythematosus
- TFPI
tissue factor pathway inhibitor
- TNF
tumor necrosis factor
- VEGF-A
vascular endothelial growth factor A
- VH
variable heavy chain
- VL
variable light chain
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/27240
References
- 1.Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960–4. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury AR, Sidhu S, Dübel S, McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol. 2011;29:245–54. doi: 10.1038/nbt.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckler DR, Schofield D, Sexton DJ, Lowe D, Vaughan TJ. Selection and Screening of Antibody Phage Display Libraries. In: Wood CR, ed. Antibody Drug Discovery. London: Imperial College Press, 2012. [Google Scholar]
- 4.Markland W, Ley AC, Ladner RC. Iterative optimization of high-affinity protease inhibitors using phage display. 2. Plasma kallikrein and thrombin. Biochemistry. 1996;35:8058–67. doi: 10.1021/bi952629y. [DOI] [PubMed] [Google Scholar]
- 5.Markland W, Ley AC, Lee SW, Ladner RC. Iterative optimization of high-affinity proteases inhibitors using phage display. 1. Plasmin. Biochemistry. 1996;35:8045–57. doi: 10.1021/bi9526286. [DOI] [PubMed] [Google Scholar]
- 6.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 7.Guterman S, Kent RB, Ladner R, Ley A, Markland W, Roberts BL. Direct evolution of novel binding proteins. US Patent 5223409A. Protein Engineering Corp., Mar 1, 1991.
- 8.Brown KC. Peptidic tumor targeting agents: the road from phage display peptide selections to clinical applications. Curr Pharm Des. 2010;16:1040–54. doi: 10.2174/138161210790963788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–4. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 10.Bradbury AR, Marks JD. Antibodies from phage antibody libraries. J Immunol Methods. 2004;290:29–49. doi: 10.1016/j.jim.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, de Bruïne AP, Arends JW, Hoogenboom HR. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218–30. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 12.Hoet RM, Cohen EH, Kent RB, Rookey K, Schoonbroodt S, Hogan S, Rem L, Frans N, Daukandt M, Pieters H, et al. Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat Biotechnol. 2005;23:344–8. doi: 10.1038/nbt1067. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan TJ, Williams AJ, Pritchard K, Osbourn JK, Pope AR, Earnshaw JC, McCafferty J, Hodits RA, Wilton J, Johnson KS. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol. 1996;14:309–14. doi: 10.1038/nbt0396-309. [DOI] [PubMed] [Google Scholar]
- 14.Knappik A, Ge L, Honegger A, Pack P, Fischer M, Wellnhofer G, Hoess A, Wölle J, Plückthun A, Virnekäs B. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296:57–86. doi: 10.1006/jmbi.1999.3444. [DOI] [PubMed] [Google Scholar]
- 15.Rothe C, Urlinger S, Löhning C, Prassler J, Stark Y, Jäger U, Hubner B, Bardroff M, Pradel I, Boss M, et al. The human combinatorial antibody library HuCAL GOLD combines diversification of all six CDRs according to the natural immune system with a novel display method for efficient selection of high-affinity antibodies. J Mol Biol. 2008;376:1182–200. doi: 10.1016/j.jmb.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, Chang E, Tao Q, Vanhove M, Lejeune A, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69:1517–26. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 17.Brockmann EC, Cooper M, Strömsten N, Vehniäinen M, Saviranta P. Selecting for antibody scFv fragments with improved stability using phage display with denaturation under reducing conditions. J Immunol Methods. 2005;296:159–70. doi: 10.1016/j.jim.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Zani ML, Moreau T. Phage display as a powerful tool to engineer protease inhibitors. Biochimie. 2010;92:1689–704. doi: 10.1016/j.biochi.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Beste G, Schmidt FS, Stibora T, Skerra A. Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold. Proc Natl Acad Sci U S A. 1999;96:1898–903. doi: 10.1073/pnas.96.5.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binz HK, Amstutz P, Plückthun A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat Biotechnol. 2005;23:1257–68. doi: 10.1038/nbt1127. [DOI] [PubMed] [Google Scholar]
- 21.Gebauer M, Skerra A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr Opin Chem Biol. 2009;13:245–55. doi: 10.1016/j.cbpa.2009.04.627. [DOI] [PubMed] [Google Scholar]
- 22.Reichert JM. Which are the antibodies to watch in 2012? MAbs. 2012;4:1–3. doi: 10.4161/mabs.4.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichert JM. Which are the antibodies to watch in 2013? MAbs. 2013;5:1–4. doi: 10.4161/mabs.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–57. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 25.HUMIRA® [package insert]. Abbott Park, Il: Abbott Laboratories, 2012.
- 26.Jespers LS, Roberts A, Mahler SM, Winter G, Hoogenboom HR. Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen. Biotechnology (N Y) 1994;12:899–903. doi: 10.1038/nbt0994-899. [DOI] [PubMed] [Google Scholar]
- 27.Salfeld JG, Allen DJ, Hoogenboom HRJM, Kaymakcalan Z, Labkovsky B, Mankovich JA, et al. Human antibodies that bind human TNFα. US Patent 6090382. US BASF Aktiengesellschaft, Germany, Feb 9, 1996.
- 28.Pharmacology Review(s): Adalimumab (BLA 125057/0). 2002.
- 29.Stohl W. Biologic differences between various inhibitors of the BLyS/BAFF pathway: should we expect differences between belimumab and other inhibitors in development? Curr Rheumatol Rep. 2012;14:303–9. doi: 10.1007/s11926-012-0254-6. [DOI] [PubMed] [Google Scholar]
- 30.Pharmacology Review(s): Belimumab (BLA 125370). 2011.
- 31.Baker KP, Edwards BM, Main SH, Choi GH, Wager RE, Halpern WG, Lappin PB, Riccobene T, Abramian D, Sekut L, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253–65. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- 32.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, et al. BLISS-76 Study Group A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–30. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, Mackay M, Aranow C, Diamond B, Davidson A. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62:201–10. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manzi S, Sánchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, Ginzler EM, D’Cruz DP, Doria A, Cooper S, et al. BLISS-52 and BLISS-76 Study Groups Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis. 2012;71:1833–8. doi: 10.1136/annrheumdis-2011-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, et al. BLISS-52 Study Group Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 36.Stohl W, Hiepe F, Latinis KM, Thomas M, Scheinberg MA, Clarke A, Aranow C, Wellborne FR, Abud-Mendoza C, Hough DR, et al. BLISS-52 Study Group. BLISS-76 Study Group Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64:2328–37. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, Zhong ZJ, Freimuth W. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. 2012;71:1343–9. doi: 10.1136/annrheumdis-2011-200937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merrill JT, Ginzler EM, Wallace DJ, McKay JD, Lisse JR, Aranow C, Wellborne FR, Burnette M, Condemi J, Zhong ZJ, et al. LBSL02/99 Study Group Long-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64:3364–73. doi: 10.1002/art.34564. [DOI] [PubMed] [Google Scholar]
- 39.Stolz LE, Horn PT. Ecallantide: a plasma kallikrein inhibitor for the treatment of acute attacks of hereditary angioedema. Drugs Today (Barc) 2010;46:547–55. doi: 10.1358/dot.2010.46.8.1507205. [DOI] [PubMed] [Google Scholar]
- 40.Joseph K, Tuscano TB, Kaplan AP. Studies of the mechanisms of bradykinin generation in hereditary angioedema plasma. Ann Allergy Asthma Immunol. 2008;101:279–86. doi: 10.1016/S1081-1206(10)60493-0. [DOI] [PubMed] [Google Scholar]
- 41.Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio-oedema. Lancet. 1998;351:1693–7. doi: 10.1016/S0140-6736(97)09137-X. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman N, Page JD, Pixley RA, Schein R, Schmaier AH, Colman RW. Alpha 2-macroglobulin-kallikrein complexes detect contact system activation in hereditary angioedema and human sepsis. Blood. 1991;77:2660–7. [PubMed] [Google Scholar]
- 43.Bernstein JA, Qazi M. Ecallantide: its pharmacology, pharmacokinetics, clinical efficacy and tolerability. Expert Rev Clin Immunol. 2010;6:29–39. doi: 10.1586/eci.09.60. [DOI] [PubMed] [Google Scholar]
- 44.KALBITOR® (ecallantide) [package insert]. In: Corp. D, ed. Burlington, MA: Dyax Corp., 2012.
- 45.Markland W, Ley AC, Ladner RC. Iterative optimization of high-affinity protease inhibitors using phage display. 2. Plasma kallikrein and thrombin. Biochemistry. 1996;35:8058–67. doi: 10.1021/bi952629y. [DOI] [PubMed] [Google Scholar]
- 46.Williams A, Baird LG. DX-88 and HAE: a developmental perspective. Transfus Apher Sci. 2003;29:255–8. doi: 10.1016/S1473-0502(03)00170-8. [DOI] [PubMed] [Google Scholar]
- 47.Cwirla SE, Balasubramanian P, Duffin DJ, Wagstrom CR, Gates CM, Singer SC, Davis AM, Tansik RL, Mattheakis LC, Boytos CM, et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science. 1997;276:1696–9. doi: 10.1126/science.276.5319.1696. [DOI] [PubMed] [Google Scholar]
- 48.Molineux G, Newland A. Development of romiplostim for the treatment of patients with chronic immune thrombocytopenia: from bench to bedside. Br J Haematol. 2010;150:9–20. doi: 10.1111/j.1365-2141.2010.08140.x. [DOI] [PubMed] [Google Scholar]
- 49.Shimamoto G, Gegg C, Boone T, Quéva C. Peptibodies: A flexible alternative format to antibodies. MAbs. 2012;4:586–91. doi: 10.4161/mabs.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 51.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161–71. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
- 52.Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser JS, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355:1672–81. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
- 53.Burns DL. Licensure of vaccines using the Animal Rule. Curr Opin Virol. 2012;2:353–6. doi: 10.1016/j.coviro.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Raxibacumab MS. MAbs. 2009;1:531–8. doi: 10.4161/mabs.1.6.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang JX, Bishop-Hurley SL, Cooper MA. Development of anti-infectives using phage display: biological agents against bacteria, viruses, and parasites. Antimicrob Agents Chemother. 2012;56:4569–82. doi: 10.1128/AAC.00567-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Froude JW, 2nd, Thullier P, Pelat T. Antibodies against anthrax: mechanisms of action and clinical applications. Toxins (Basel) 2011;3:1433–52. doi: 10.3390/toxins3111433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, et al. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009;361:135–44. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 58.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care. 2012;25:349–70. doi: 10.1097/01.ASW.0000418541.31366.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106–14. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 60.Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–27. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 61.Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 62.Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp Pharmacol 2008:131-50. [DOI] [PubMed] [Google Scholar]
- 63.Baca M, Presta LG, O’Connor SJ, Wells JA. Antibody humanization using monovalent phage display. J Biol Chem. 1997;272:10678–84. doi: 10.1074/jbc.272.16.10678. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P, de Vos AM, Lowman HB. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865–81. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 65.Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, Meng YG, Fei DT, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–44. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 66.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 67.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 68.LUCENTIS® Label. San Francisco, CA: Genentech, 2006. [Google Scholar]
- 69.Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–16. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Herbst RS, Hong D, Chap L, Kurzrock R, Jackson E, Silverman JM, Rasmussen E, Sun YN, Zhong D, Hwang YC, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–65. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 71.Eatock MM, Tebbutt NC, Bampton CL, Strickland AH, Valladares-Ayerbes M, Swieboda-Sadlej A, Van Cutsem E, Nanayakkara N, Sun YN, Zhong ZD, et al. Phase II randomized, double-blind, placebo-controlled study of AMG 386 (trebananib) in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann Oncol. 2013;24:710–8. doi: 10.1093/annonc/mds502. [DOI] [PubMed] [Google Scholar]
- 72.Peeters M, Strickland AH, Lichinitser M, Suresh AV, Manikhas G, Shapiro J, Rogowski W, Huang X, Wu B, Warner D, et al. A randomised, double-blind, placebo-controlled phase 2 study of trebananib (AMG 386) in combination with FOLFIRI in patients with previously treated metastatic colorectal carcinoma. Br J Cancer. 2013;108:503–11. doi: 10.1038/bjc.2012.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu M, Zhu Z. Human anti-epidermal growth factor receptor antibody. US Patent 7598350.: ImClone LLC, March 21, 2005.
- 74.Sanders JM, Wampole ME, Thakur ML, Wickstrom E. Molecular determinants of epidermal growth factor binding: a molecular dynamics study. PLoS One. 2013;8:e54136. doi: 10.1371/journal.pone.0054136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dantas-Barbosa C, de Macedo Brigido M, Maranhao AQ. Antibody phage display libraries: contributions to oncology. Int J Mol Sci. 2012;13:5420–40. doi: 10.3390/ijms13055420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dienstmann R, Felip E. Necitumumab in the treatment of advanced non-small cell lung cancer: translation from preclinical to clinical development. Expert Opin Biol Ther. 2011;11:1223–31. doi: 10.1517/14712598.2011.595709. [DOI] [PubMed] [Google Scholar]
- 77.Zhu Z. Human antibodies specific to KDR and uses therof. US Patent 7498414 B2. US: Imclone Systems Incorporated, March 4, 2003.
- 78.Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O’Bryant C, Chow LQ, Serkova NJ, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–7. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu Z, Hattori K, Zhang H, Jimenez X, Ludwig DL, Dias S, Kussie P, Koo H, Kim HJ, Lu D, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia. 2003;17:604–11. doi: 10.1038/sj.leu.2402831. [DOI] [PubMed] [Google Scholar]
- 80.Vacchelli E, Eggermont A, Galon J, Sautès-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Monoclonal antibodies in cancer therapy. Oncoimmunology. 2013;2:e22789. doi: 10.4161/onci.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelley BD, Booth J, Tannatt M, Wub QL, Ladner R, Yuc J, Potter D, Ley A. Isolation of a peptide ligand for affinity purification of factor VIII using phage display. J Chromatogr A. 2004;1038:121–30. doi: 10.1016/j.chroma.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 82.Kelley B, Jankowski M, Booth J. An improved manufacturing process for Xyntha/ReFacto AF. Haemophilia. 2010;16:717–25. doi: 10.1111/j.1365-2516.2009.02160.x. [DOI] [PubMed] [Google Scholar]
- 83.Krumpe LR, Mori T. Potential of phage-displayed peptide library technology to identify functional targeting peptides. Expert Opin Drug Discov. 2007;2:525. doi: 10.1517/17460441.2.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finlay WJ, Bloom L, Cunningham O. Phage display: a powerful technology for the generation of high specificity affinity reagents from alternative immune sources. Methods Mol Biol. 2011;681:87–101. doi: 10.1007/978-1-60761-913-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pande J, Szewczyk MM, Grover AK. Phage display: concept, innovations, applications and future. Biotechnol Adv. 2010;28:849–58. doi: 10.1016/j.biotechadv.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 86.Spencer S, Bethea D, Raju TS, Giles-Komar J, Feng Y. Solubility evaluation of murine hybridoma antibodies. MAbs. 2012;4:319–25. doi: 10.4161/mabs.19869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobs SA, Wu SJ, Feng Y, Bethea D, O’Neil KT. Cross-interaction chromatography: a rapid method to identify highly soluble monoclonal antibody candidates. Pharm Res. 2010;27:65–71. doi: 10.1007/s11095-009-0007-z. [DOI] [PubMed] [Google Scholar]
- 88.Conley GP, Viswanathan M, Hou Y, Rank DL, Lindberg AP, Cramer SM, Ladner RC, Nixon AE, Chen J. Evaluation of protein engineering and process optimization approaches to enhance antibody drug manufacturability. Biotechnol Bioeng. 2011;108:2634–44. doi: 10.1002/bit.23220. [DOI] [PubMed] [Google Scholar]
- 89.Pepinsky RB, Silvian L, Berkowitz SA, Farrington G, Lugovskoy A, Walus L, Eldredge J, Capili A, Mi S, Graff C, et al. Improving the solubility of anti-LINGO-1 monoclonal antibody Li33 by isotype switching and targeted mutagenesis. Protein Sci. 2010;19:954–66. doi: 10.1002/pro.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu SJ, Luo J, O’Neil KT, Kang J, Lacy ER, Canziani G, Baker A, Huang M, Tang QM, Raju TS, et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel. 2010;23:643–51. doi: 10.1093/protein/gzq037. [DOI] [PubMed] [Google Scholar]