Abstract
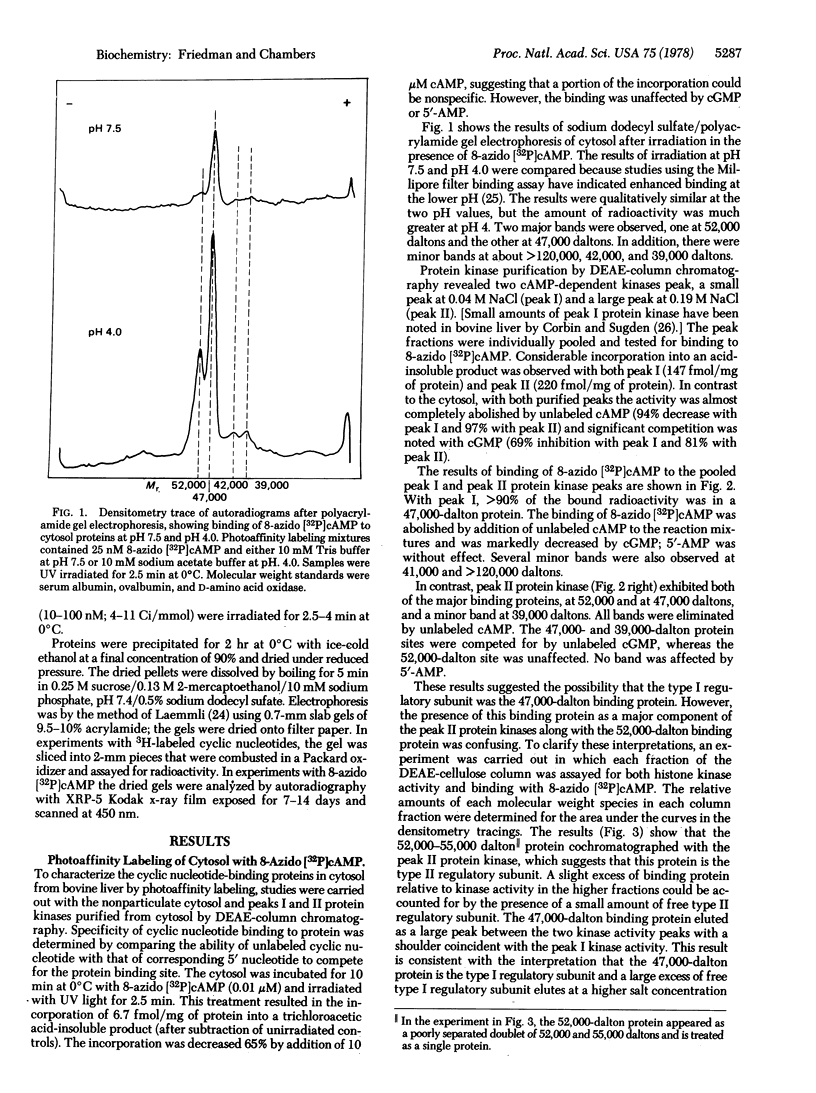
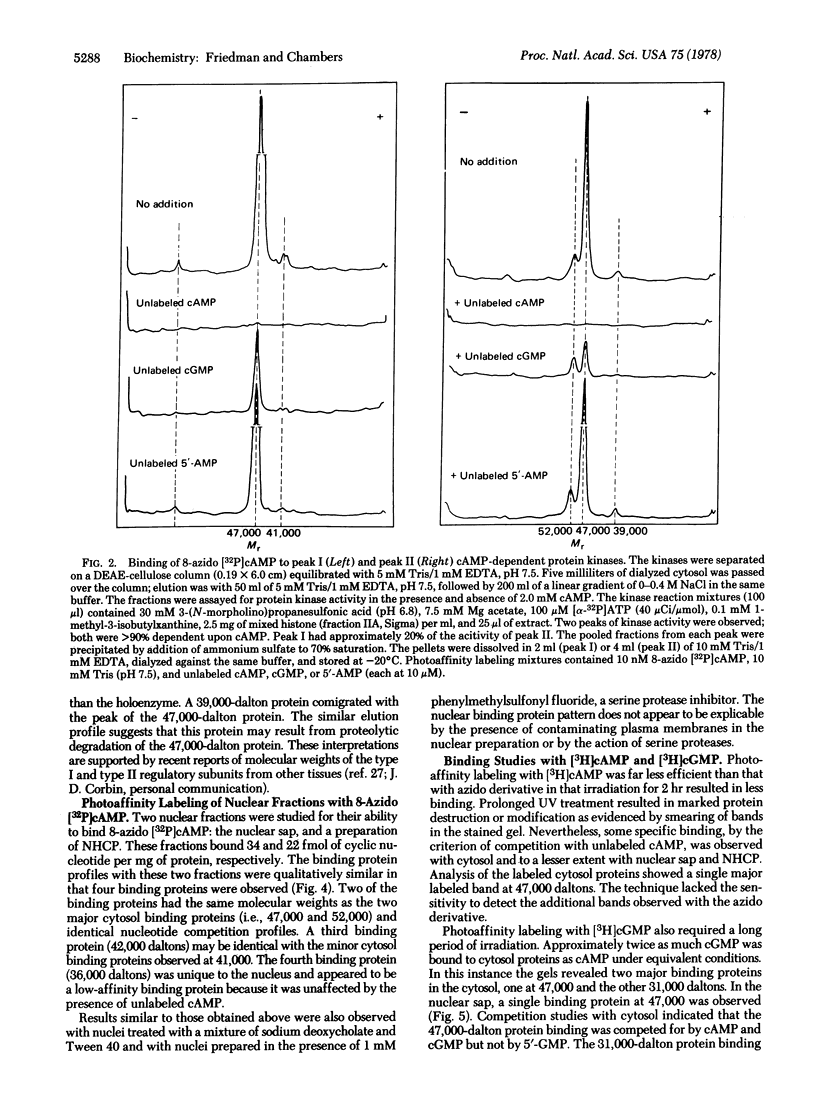
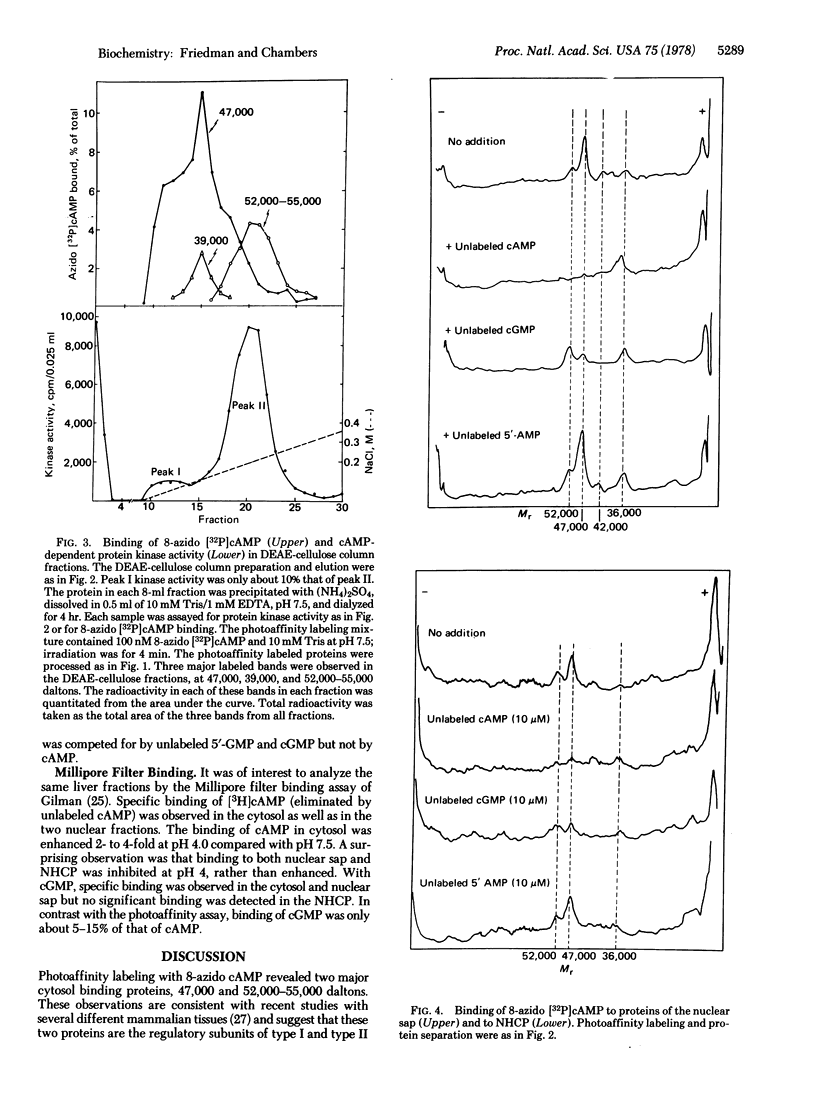
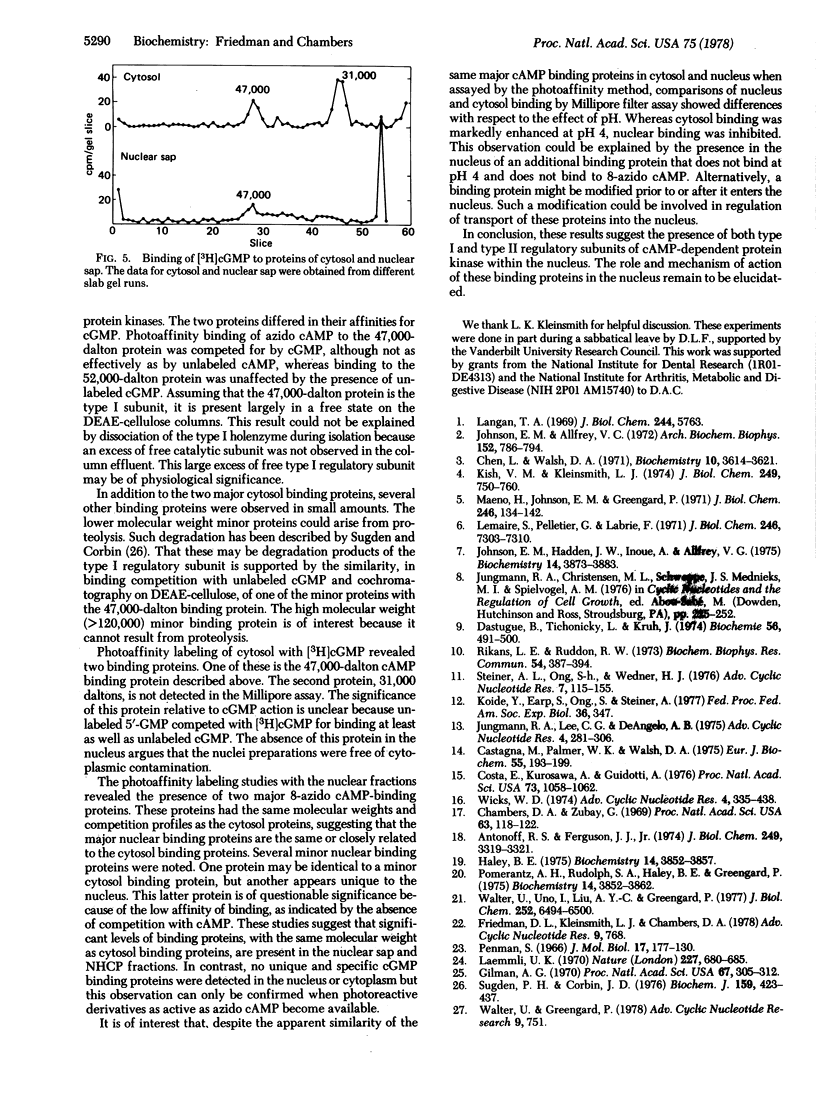
A photoaffinity labeling method was used to characterize and compare cyclic nucleotide-binding proteins of bovine liver cytosol with binding proteins of the nucleus. After photoaffinity labeling of cytosol with 8-azido cyclic [32P]AMP, autoradiographs of sodium dodecyl sulfate polyacrylamide gel electrophoresis revealed two major labeled proteins of 47,000 and 52,000-55,000 daltons. DEAE-cellulose column-derived fractions suggested that the larger protein was the regulatory subunit of peak II cyclic AMP-dependent protein kinase and the smaller protein was the regulatory subunit of peak I kinase. The smaller protein was largely present as the free regulatory subunit. The two binding proteins differed in their ability to bind cyclic GMP. Binding to both proteins was abolished by excess unlabeled cyclic AMP but not by 5′-AMP. Photoaffinity labeling of a 0.14 M salt extract of nuclei and a nonhistone chromosomal protein preparation revealed two major binding proteins with the same molecular weight and competition profiles as those of the cytosol. Detergent-washed nuclei gave similar results. Several minor binding proteins were observed in both cytosol and nucleus. One protein (36,000 daltons) was unique to the nucleus and had low affinity for 8-azido cyclic AMP. Photoaffinity labeling with cyclic [3H]GMP revealed a cytosol protein, absent from the nucleus, of 31,000 daltons and the ligand was competed for by both cyclic GMP and 5′-GMP. These studies suggest that the major specific cyclic AMP-binding proteins of bovine liver are the type I and type II regulatory subunits of cyclic AMP-dependent protein kinase and are present in both nucleus and cytoplasm.
Keywords: 8-azido cyclic AMP, cyclic GMP, nuclear binding proteins
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonoff R. S., Ferguson J. J., Jr Photoaffinity labeling with cyclic nucleotides. J Biol Chem. 1974 May 25;249(10):3319–3321. [PubMed] [Google Scholar]
- Castagna M., Palmer W. K., Walsh D. A. Nuclear protein-kinase activity in perfused rat liver stimulated with dibutyryl-adenosine cyclic 3':5'-monophosphate. Eur J Biochem. 1975 Jun 16;55(1):193–199. doi: 10.1111/j.1432-1033.1975.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Chambers D. A., Zubay G. The stimulatory effect of cyclic adenosine 3'5'-monophosphate on DNA-directed synthesis of beta-galactosidase in a cell-free system. Proc Natl Acad Sci U S A. 1969 May;63(1):118–122. doi: 10.1073/pnas.63.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. J., Walsh D. A. Multiple forms of hepatic adenosine 3':5'-monophosphate dependent protein kinase. Biochemistry. 1971 Sep 14;10(19):3614–3621. doi: 10.1021/bi00795a020. [DOI] [PubMed] [Google Scholar]
- Costa E., Kurosawa A., Guidotti A. Activation and nuclear translocation of protein kinase during transsynaptic induction of tyrosine 3-monooxygenase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1058–1062. doi: 10.1073/pnas.73.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B. E. Photoaffinity labeling of adenosine 3',5'-cyclic monophosphate binding sites of human red cell membranes. Biochemistry. 1975 Aug 26;14(17):3852–3857. doi: 10.1021/bi00688a018. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Allfrey V. G. Differential effects of cyclic adenosine-3',5'-monophosphate on phosphorylation of rat liver nuclear acidic proteins. Arch Biochem Biophys. 1972 Oct;152(2):786–794. doi: 10.1016/0003-9861(72)90274-3. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Hadden J. W., Inoue A., Allfrey V. G. DNA binding by cyclic adenosine 3',5'-monophosphate dependent protein kinase from calf thymus nuclei. Biochemistry. 1975 Aug 26;14(17):3873–3884. doi: 10.1021/bi00688a022. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Lee S., DeAngelo A. B. Translocation of cytoplasmic protein kinase and cyclic adenosine monophosphate-binding protein to intracellular acceptor sites. Adv Cyclic Nucleotide Res. 1975;5:281–306. [PubMed] [Google Scholar]
- Kish V. M., Kleinsmith L. J. Nuclear protein kinases. Evidence for their heterogeneity, tissue specificity, substrate specificities, and differential responses to cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1974 Feb 10;249(3):750–760. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Action of adenosine 3',5'-monophosphate-dependent histone kinase in vivo. J Biol Chem. 1969 Oct 25;244(20):5763–5765. [PubMed] [Google Scholar]
- Lemaire S., Pelletier G., Labrie F. Adenosine 3',5'-monophosphate-dependent protein kinase from bovine anterior pituitary gland. II. Subcellular distribution. J Biol Chem. 1971 Dec 10;246(23):7303–7310. [PubMed] [Google Scholar]
- Maeno H., Johnson E. M., Greengard P. Subcellular distribution of adenosine 3',5'-monophosphate-dependent protein kinase in rat brain. J Biol Chem. 1971 Jan 10;246(1):134–142. [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Pomerantz A. H., Rudolph S. A., Haley B. E., Greengard P. Photoaffinity labeling of a protein kinase from bovine brain with 8-azidoadenosine 3',5'-monophosphate. Biochemistry. 1975 Aug 26;14(17):3858–3862. doi: 10.1021/bi00688a019. [DOI] [PubMed] [Google Scholar]
- Rikans L. E., Ruddon R. W. Role of 3',5'-cyclic AMP in the control of nuclear protein kinase activity. Biochem Biophys Res Commun. 1973 Sep 5;54(1):387–394. doi: 10.1016/0006-291x(73)90934-0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Ong S. H., Wedner H. J. Cyclic nucleotide immunocytochemistry. Adv Cyclic Nucleotide Res. 1976;7:115–155. [PubMed] [Google Scholar]
- Sugden P. H., Corbin J. D. Adenosine 3':5'-cyclic monophosphate-binding proteins in bovine and rat tissues. Biochem J. 1976 Nov;159(2):423–437. doi: 10.1042/bj1590423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter U., Uno I., Liu A. Y., Greengard P. Identification, characterization, and quantitative measurement of cyclic AMP receptor proteins in cytosol of various tissues using a photoaffinity ligand. J Biol Chem. 1977 Sep 25;252(18):6494–6500. [PubMed] [Google Scholar]



