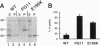Abstract
Prion diseases are a group of fatal neurodegenerative disorders that are unique in being infectious, genetic, and sporadic in origin. Infectious cases are caused by prions, which are composed primarily of PrPSc, a posttranslationally modified isoform of the normal cellular prion protein PrPC. Inherited cases are linked to insertional or point mutations in the host gene encoding PrPC. To investigate the molecular mechanisms underlying inherited prion diseases, we have constructed stably transfected Chinese hamster ovary cells that express mouse PrPs homologous to two human PrPs associated with familial Creutzfeldt-Jakob disease. One mouse PrP molecule carries a Glu-->Lys substitution at codon 199, and the other carries an insertion of six additional octapeptide repeats between codons 51 and 90. We find that both of these mutant PrPs display several biochemical hallmarks of PrPSc when synthesized in cell culture. Unlike wild-type PrP, the mutant proteins are detergent insoluble and are relatively resistant to digestion by proteinase K, yielding an N-terminally truncated core fragment of 27-30 kDa. Pulse-chase labeling experiments demonstrate that these properties are acquired posttranslationally, and are accompanied by increased metabolic stability of the protein. Our results provide the first evidence that a molecule with properties reminiscent of PrPSc can be generated de novo in cultured cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessen R. A., Marsh R. F. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol. 1992 Apr;66(4):2096–2101. doi: 10.1128/jvi.66.4.2096-2101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., Bendheim P. E., Marmorstein A. D., Potempska A. Isolation and structural studies of the intact scrapie agent protein. Arch Biochem Biophys. 1987 Nov 1;258(2):579–590. doi: 10.1016/0003-9861(87)90380-8. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Seligman S. J., Bablanian G., Windsor D., Scala L. J., Kim K. S., Chen C. M., Kascsak R. J., Bendheim P. E. Molecular location of a species-specific epitope on the hamster scrapie agent protein. J Virol. 1991 Jul;65(7):3667–3675. doi: 10.1128/jvi.65.7.3667-3675.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt D. R., Taraboulos A., Prusiner S. B. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem. 1992 Aug 15;267(23):16188–16199. [PubMed] [Google Scholar]
- Brown P., Gibbs C. J., Jr, Rodgers-Johnson P., Asher D. M., Sulima M. P., Bacote A., Goldfarb L. G., Gajdusek D. C. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994 May;35(5):513–529. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- Butler D. A., Scott M. R., Bockman J. M., Borchelt D. R., Taraboulos A., Hsiao K. K., Kingsbury D. T., Prusiner S. B. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol. 1988 May;62(5):1558–1564. doi: 10.1128/jvi.62.5.1558-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. A., Scott M. R., Bockman J. M., Borchelt D. R., Taraboulos A., Hsiao K. K., Kingsbury D. T., Prusiner S. B. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol. 1988 May;62(5):1558–1564. doi: 10.1128/jvi.62.5.1558-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büeler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993 Jul 2;73(7):1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Caughey B. W., Dong A., Bhat K. S., Ernst D., Hayes S. F., Caughey W. S. Secondary structure analysis of the scrapie-associated protein PrP 27-30 in water by infrared spectroscopy. Biochemistry. 1991 Aug 6;30(31):7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J., Ernst D., Race R. E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991 Dec;65(12):6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991 Sep 25;266(27):18217–18223. [PubMed] [Google Scholar]
- Cohen F. E., Pan K. M., Huang Z., Baldwin M., Fletterick R. J., Prusiner S. B. Structural clues to prion replication. Science. 1994 Apr 22;264(5158):530–531. doi: 10.1126/science.7909169. [DOI] [PubMed] [Google Scholar]
- Collinge J., Brown J., Hardy J., Mullan M., Rossor M. N., Baker H., Crow T. J., Lofthouse R., Poulter M., Ridley R. Inherited prion disease with 144 base pair gene insertion. 2. Clinical and pathological features. Brain. 1992 Jun;115(Pt 3):687–710. doi: 10.1093/brain/115.3.687. [DOI] [PubMed] [Google Scholar]
- Collinge J., Palmer M. S., Campbell T., Sidle K. C., Carroll D., Harding A. Inherited prion disease (PrP lysine 200) in Britain: two case reports. BMJ. 1993 Jan 30;306(6873):301–302. doi: 10.1136/bmj.306.6873.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb L. G., Brown P., Mitrovà E., Cervenáková L., Goldin L., Korczyn A. D., Chapman J., Gálvez S., Cartier L., Rubenstein R. Creutzfeldt-Jacob disease associated with the PRNP codon 200Lys mutation: an analysis of 45 families. Eur J Epidemiol. 1991 Sep;7(5):477–486. doi: 10.1007/BF00143125. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Huber M. T., van Dijken P., Shyng S. L., Chait B. T., Wang R. Processing of a cellular prion protein: identification of N- and C-terminal cleavage sites. Biochemistry. 1993 Feb 2;32(4):1009–1016. doi: 10.1021/bi00055a003. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Meiner Z., Kahana E., Cass C., Kahana I., Avrahami D., Scarlato G., Abramsky O., Prusiner S. B., Gabizon R. Mutation of the prion protein in Libyan Jews with Creutzfeldt-Jakob disease. N Engl J Med. 1991 Apr 18;324(16):1091–1097. doi: 10.1056/NEJM199104183241604. [DOI] [PubMed] [Google Scholar]
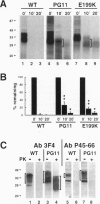
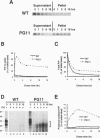
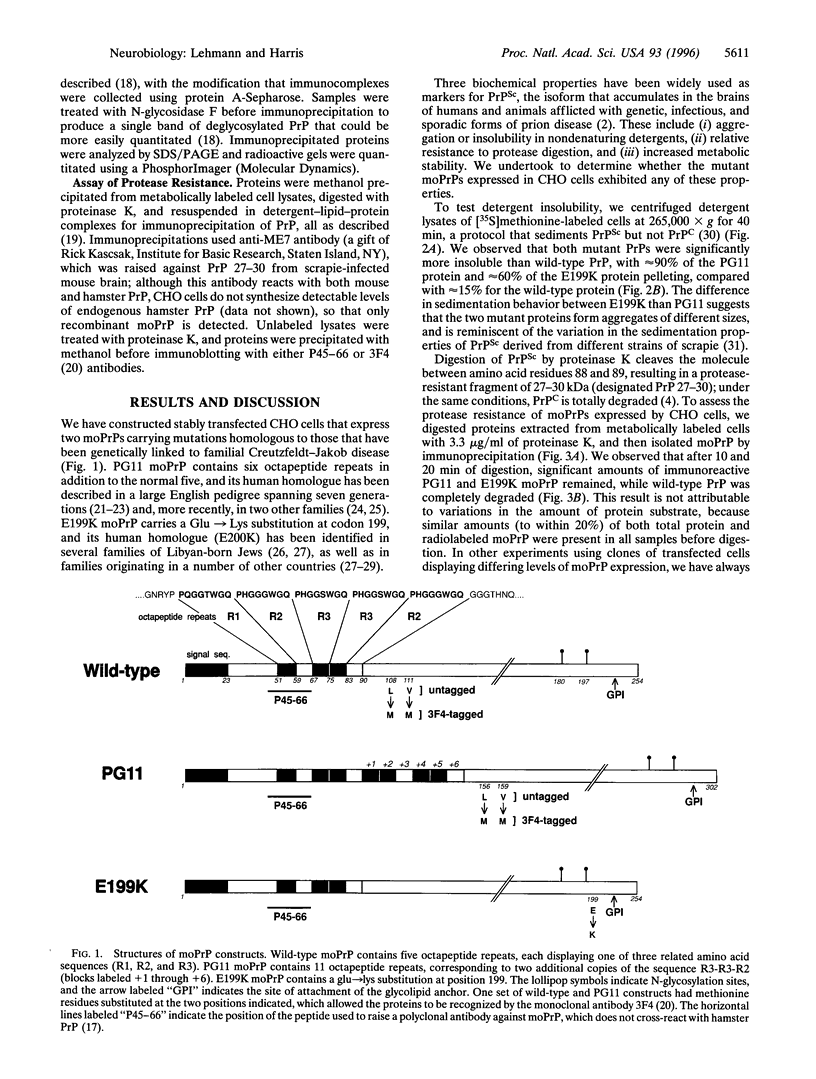
- Inoue I., Kitamoto T., Doh-ura K., Shii H., Goto I., Tateishi J. Japanese family with Creutzfeldt-Jakob disease with codon 200 point mutation of the prion protein gene. Neurology. 1994 Feb;44(2):299–301. doi: 10.1212/wnl.44.2.299. [DOI] [PubMed] [Google Scholar]
- Kocisko D. A., Priola S. A., Raymond G. J., Chesebro B., Lansbury P. T., Jr, Caughey B. Species specificity in the cell-free conversion of prion protein to protease-resistant forms: a model for the scrapie species barrier. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3923–3927. doi: 10.1073/pnas.92.9.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S., Harris D. A. A mutant prion protein displays an aberrant membrane association when expressed in cultured cells. J Biol Chem. 1995 Oct 13;270(41):24589–24597. doi: 10.1074/jbc.270.41.24589. [DOI] [PubMed] [Google Scholar]
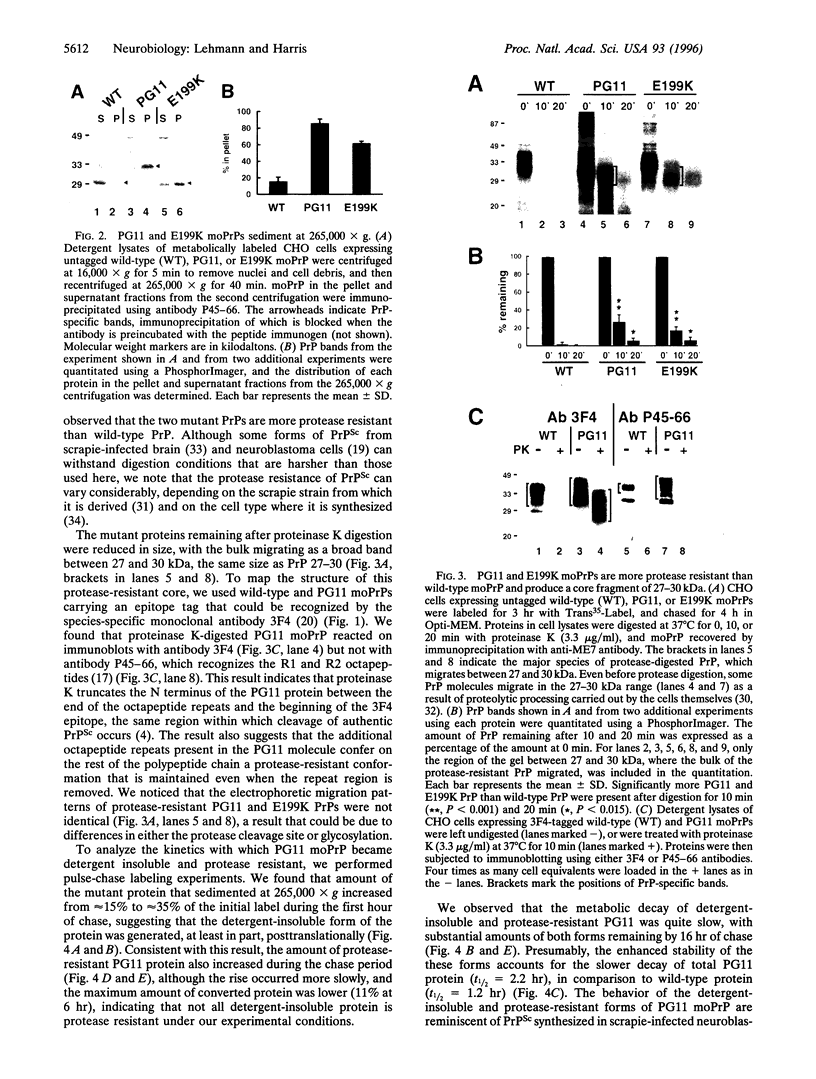
- Lehmann S., Harris D. A. Mutant and infectious prion proteins display common biochemical properties in cultured cells. J Biol Chem. 1996 Jan 19;271(3):1633–1637. doi: 10.1074/jbc.271.3.1633. [DOI] [PubMed] [Google Scholar]
- Meiner Z., Halimi M., Polakiewicz R. D., Prusiner S. B., Gabizon R. Presence of prion protein in peripheral tissues of Libyan Jews with Creutzfeldt-Jakob disease. Neurology. 1992 Jul;42(7):1355–1360. doi: 10.1212/wnl.42.7.1355. [DOI] [PubMed] [Google Scholar]
- Nicholl D., Windl O., de Silva R., Sawcer S., Dempster M., Ironside J. W., Estibeiro J. P., Yuill G. M., Lathe R., Will R. G. Inherited Creutzfeldt-Jakob disease in a British family associated with a novel 144 base pair insertion of the prion protein gene. J Neurol Neurosurg Psychiatry. 1995 Jan;58(1):65–69. doi: 10.1136/jnnp.58.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Kitamoto T., Tateishi J., Mitsuhashi T., Iwabuchi K., Haga C., Oguni E., Kato Y., Tominaga I., Yanai K. Prion disease with 144 base pair insertion in a Japanese family line. Acta Neuropathol. 1995;90(1):80–86. doi: 10.1007/BF00294463. [DOI] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Owen F., Poulter M., Shah T., Collinge J., Lofthouse R., Baker H., Ridley R., McVey J., Crow T. J. An in-frame insertion in the prion protein gene in familial Creutzfeldt-Jakob disease. Brain Res Mol Brain Res. 1990 Apr;7(3):273–276. doi: 10.1016/0169-328x(90)90038-f. [DOI] [PubMed] [Google Scholar]
- Pan K. M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R. J., Cohen F. E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter M., Baker H. F., Frith C. D., Leach M., Lofthouse R., Ridley R. M., Shah T., Owen F., Collinge J., Brown J. Inherited prion disease with 144 base pair gene insertion. 1. Genealogical and molecular studies. Brain. 1992 Jun;115(Pt 3):675–685. doi: 10.1093/brain/115.3.675. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., DeArmond S. J. Prion diseases and neurodegeneration. Annu Rev Neurosci. 1994;17:311–339. doi: 10.1146/annurev.ne.17.030194.001523. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Hsiao K. K. Human prion diseases. Ann Neurol. 1994 Apr;35(4):385–395. doi: 10.1002/ana.410350404. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Race R. E., Caughey B., Graham K., Ernst D., Chesebro B. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J Virol. 1988 Aug;62(8):2845–2849. doi: 10.1128/jvi.62.8.2845-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M., Yehiely F., Scott M., Prusiner S. B. Conversion of truncated and elongated prion proteins into the scrapie isoform in cultured cells. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3182–3186. doi: 10.1073/pnas.90.8.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar J., Roller P. P., Gajdusek D. C., Gibbs C. J., Jr Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem. 1993 Sep 25;268(27):20276–20284. [PubMed] [Google Scholar]
- Taraboulos A., Raeber A. J., Borchelt D. R., Serban D., Prusiner S. B. Synthesis and trafficking of prion proteins in cultured cells. Mol Biol Cell. 1992 Aug;3(8):851–863. doi: 10.1091/mbc.3.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboulos A., Serban D., Prusiner S. B. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J Cell Biol. 1990 Jun;110(6):2117–2132. doi: 10.1083/jcb.110.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]