Abstract
The removal and cryo-storage of ovarian cortical biopsies is now offered as a fertility preservation option for young women. The only available option to restore fertility using this tissue is by transplantation which may not be a viable option for all patients. The full potential of this tissue to restore fertility could be achieved by the development of in vitro systems that support oocyte development from the most immature stages to maturation. The techniques of in vitro growth (IVG) combined with in vitro maturation (IVM) are being developed in human but comparing different systems has been difficult because of the scarcity of tissue and non-human primates are being used as model systems. There are many challenges to developing a complete culture system that will support human oocyte development and this review outlines the approaches being taken by several groups to support each of the stages of oocyte development using tissue from women and non-human primate models.
Introduction
The development of culture systems with the aim of growing oocytes from the earliest stage of follicle through to maturity for fertilization in vitro (IVF) could have a lasting impact on clinically assisted reproduction, in particular for the growing number of women who are surviving cancer only to face infertility as a result of the gametotoxic effects of cancer therapies (1). Recent success with ovarian tissue transplantation (2–4) has expanded interest in and efforts to cryopreserve and store ovarian tissue for future fertility. Ovarian tissue cryopreservation is the only available option for young patients without partners or who cannot undergo ovarian stimulation for oocyte/embryo cryopreservation and patients for whom the risk for reintroduction of malignant cells precludes transplantation. Thus, the need for follicle culture systems that can efficiently use all classes of ovarian follicles, derived from clinically cryopreserved ovarian tissue, as sources of gametes would maximize reproductive potential for future fertility. However, complete growth in vitro from immature primordial stages with subsequent IVF of oocytes followed by embryo transfer and production of live offspring has, so far, only been achieved in the mouse (5,6). Several groups have focused on culturing later stages of follicle development from rodents and have produced developmentally competent oocytes and viable offspring). The success of these techniques has encouraged the demanding challenge of adapting them for humans and other primates. Whilst these techniques have been used to study the regulation of follicle development, the ultimate aim of follicle culture is to increase the availability of developmentally competent oocytes than would be available from conventional methods, and there is still much to do before follicle culture can be used as a strategy for obtaining competent oocytes. In recent years a great deal of progress has been made in developing culture techniques for humans and non-human primates and in this review we describe the technologies and discuss the prospects as well as the problems of applying them clinically.
Follicular Development
Female reproductive function requires cyclical development and maturation of ovarian follicles on a background of continuous activation from the pool of primordial follicles. Primordial follicles are formed pre-natally and represent a population of germ cells from which recruitment for growth will take place throughout the female’s reproductive life. Follicular growth and development involves a series of complex and precisely regulated events characterised by transition stages that begin with (i) initiation of primordial follicle growth and development to the preantral follicle stage; (ii) the formation of antral follicles where expansion to the pre ovulatory or Graafian follicle is associated with granulosa cell proliferation and antral fluid accumulation within the basement membrane; and (iii) rupture of the Graafian follicle releasing a cumulus-oocyte complex at ovulation in response to the mid-cycle LH surge (Figure 1A).
Figure 1.
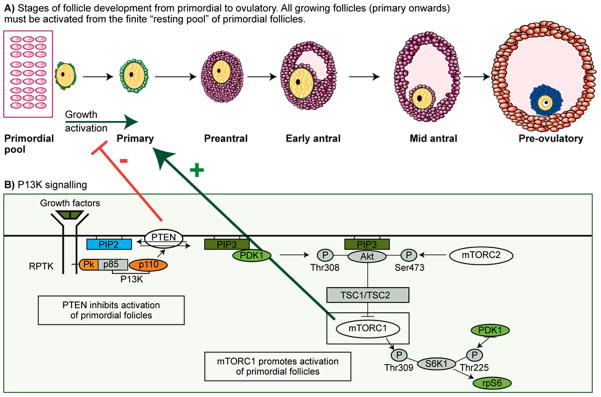
a) Digrammatic representation of follicle growth from the non-proliferating pool of primordial follicles. Primordial follicles are continuously activated into the growing population where they become primary follicles consisting of an oocye arrested at the dictyate stage of prophaseI of meiosis (yellow) surrounded by granulosa cells (green). Primary follicles undergo oocyte growth and granulosa cell proliferation and differentiation (purple) when they form an antral cavity. Antral follicles continue to grow and granulosa cells differentiate into two sub-populations of cells 1) cumulus surrounding the oocyte (blue) and 2) mural lining the wall of the follicle (orange). Exact timings for this developmental sequence to occur in humans are not known but estimations suggest several months, however, it is not known whether the growth profile is continuous or whether there are “resting” phases through follicle development.
b) Simplified version of the PI3K pathway
The factors initiating this process are largely unknown but a body of evidence is emerging to show that the phosphatidylinositol-3’-kinase (PI3K-AKT) signalling pathway is a major regulator of early follicle/oocyte development and that components of this pathway are involved in controlling the rate of activation from the non-growing population of follicles. The phosphatase PTEN converts PIP3 to PIP2, which negatively regulates PI3K activity. Signaling mediated by PI3Ks converge at PDK1. PDK1 phosphorylates Akt and activates it. Akt can phosphorylate and inactivate tuberous sclerosis complex 2 (TSC2, or tuberin), which leads to the activation of mTOR complex (mTORC1). mTORC1 can phosphorylate (activate) S6K1. S6K1 subsequently phosphorylates and activates rpS6, which enhances protein translation that is needed for cell growth. mTORC1 can be inhibited pharmacologically with Rapamycin and stimulated by leucine. The manipulation of this pathway could have important clinical applications in the field of fertility preservation.
As the oocyte grows within the follicle it is held in meiotic arrest at the dictyate stage of Prophase I, but during development within the follicle it must acquire the ability to resume meiosis (meiotic competence) and the ability to support fertilisation and embryonic development (developmental competence). Thus the oocyte is dependent upon the local environment within the follicle for subsequent function as a gamete and the formation and maintenance of connections facilitating bi-directional communication between the oocyte and granulosa cells are key to oocyte development in all species.
The development of culture conditions for immature germ cells (both eggs and sperm) is one of the greatest technical challenge to reproductive technology. An understanding of the physiological requirements of the oocyte, granulosa, theca and perhaps even the stromal cells is needed. These requirements are complex and change during growth therefore a major consideration is the starting point of the culture system i.e. which stage of follicle to start with. The majority of follicles within the ovary in all young mammalian females will be at the primordial stage of development and these follicles are continually being utilized during reproductive life (12). We don’t know if this pool represents a homogeneous population but it is thought that at this stage follicles have not yet been exposed to selection processes that lead to follicle degeneration (13,14). Primordial follicles represent the population of germ cells from which recruitment for growth will take place throughout the female’s reproductive life and this process requires a precisely regulated sequence of events to be initiated (Figure 1). Whilst rodents are excellent models for pioneering technologies, intermediate species are needed to test the feasibility for human applications. Follicles of some domestic animals (cows, sheep, and goats) can resemble those of humans in terms of growth rates and size, but the protracted length of folliculogenesis in vivo, estimated in women to be 90 days from the entrance of a preantral follicle into the growing pool to a preovulatory follicles (15), and the long length of the follicular phase of the spontaneous menstrual cycle in nonhuman primates (≈ 2 weeks relative to a few days in domestic animals) more closely reflect that of women. Thus, nonhuman primates are emerging as an important translational model to advance technological developments in follicle culture.
Development of Follicles in vitro
Several approaches have been taken to develop human follicles in vitro using fresh (16,17) and thawed-cryopreserved (17,18 human cortical tissue. It is now clear that if we are to achieve complete development of human oocytes a dynamic multi-step culture system is required to support each of the transitional stages (16, 19–21). The first step is to support the initiation of primordial follicle development and early growth; the second stage is to optimise the growth of follicles from preantral to antral stages with the completion of oocyte growth being achieved during the third stage. The focus should be primarily on oocyte development which may not require the development of large follicular structures but rather the maintenance of differentiated somatic cells in contact with the developing oocyte. A multistep system for in vitro grown (IVG) follicles has been proposed (22,23) to produce competent oocytes from human ovarian cortical tissue. The multi-step approach needs to support the changing requirements of the developing oocyte and its surrounding somatic (granulosa) cells with the main focus being on maintaining oocyte-somatic cell interactions. Several groups have worked on each of the steps required to support human oocyte development in vitro: 1) activation of primordial follicles through culturing ovarian cortex (16,17) 2) isolation and culture of growing preantral follicles to achieve oocyte growth and development (16,24–31). 3) Aspiration and maturation of oocyte cumulus complexes (32,33). The aim of ongoing research in this field has been to combine each of these steps to achieve complete development of human oocytes (16). Progress in achieving this goal and the use of non-human primate models will be reviewed.
Activation of Primordial Follicles
The majority of follicles within ovarian cortical tissue are quiescent primordial, therefore the first consideration of an IVG system should be to optimise initiation of primordial follicles in vitro and support early follicle development. The factors regulating follicle initiation and early growth are still not well defined but the process requires a combination of inhibitory, stimulatory and maintenance factors (34). Studies using knock out mouse models have demonstrated the importance of the phosphatidylinositol-3′-kinase (PI3K-AKT) signalling pathway within the oocyte in regulating follicle activation (Reddy et al., 2008). The phosphatase and tensin homolog deleted on chromosome ten (PTEN) acts as a negative regulator of this pathway and suppresses initiation of follicle development (35). The transcription factor FOXO3a is a downstream effector of this pathway and acts to inhibit follicle recruitment (36). However, primordial follicles of fetal and juvenile macaque ovaries lack FOXO3a expression, suggesting alternative transcription factors may mediate follicle activation in primates (37). Other components of this pathway are dependent on the mammalian target of rapamycin complex 1 (mTORC1), a serine/threonine kinase that regulates cell growth and proliferation in response to growth factors and nutrients and also regulates primordial follicle activation (38). How these pathways regulate human follicle development is unclear, but culture models facilitate the study of these processes. Using pharmacological inhibitors of PTEN in vitro, increased activation of presumably human primordial follicles has been demonstrated following xenotransplantation of ovarian cortical strips into immunodeficient mice (39). A recent study using a human culture model has also demonstrated that treatment with rapamycin (an inhibitor of mTORC) results in decreased activation of primordial follicles but also oocyte loss in growing follicles (40). Figure 1B illustrates components of the PI3K pathway and their influence on follicle activation.
One limitation to determining primordial follicle activation using cultured human ovarian cortex includes difficulty in assessing the follicular cohort within the starting material. In human (41) and nonhuman primate (42) ovaries, the distribution of follicles within the ovarian cortex is heterogeneous. It is important to note that follicular density can vary more than two orders of magnitude in pieces of human cortical tissues from within the same ovary (41). Each cortical piece will have variation in number and stage distribution of follicles, therefore it is difficult to compare numbers of follicles in a piece of control starting tissue to those pieces subjected to different treatments in vitro (43,44). Recently, a procedure using the vital dye, neutral red, was developed for visualizing preantral follicles within ovine cortical pieces and successfully applied to determining follicular density in human cortical tissue (45,46). Whether primordial follicles can develop normally within the human or nonhuman primate ovarian cortex during in vitro culture after exposure to neutral red is not known, but if so, this could prove to be a useful tool for identifying pieces of cortex containing viable primordial follicles for experiments on follicle activation, as well as for screening pieces of tissue prior to ovarian transplantation.
Human primordial follicles can be activated to grow and develop within mechanically loosened cortical pieces. Multilaminar preantral (secondary) stage follicles can be detected in this system after 6 days of culture (16). This system differs from those described in other studies (17,26) as the culture medium is serum free and no supporting matrix is present. The vital step in this process lies in the preparation of tissue which involves the removal of most of the underlying stromal tissue and any growing follicles (Figure 2). When these small fragments of human ovarian cortex are cultured there is a significant shift of follicles from the quiescent to the growing pool over short culture periods of 6 – 10 days (16), an observation repeated in cattle where extensive primordial activation has been reported within 2 days in vitro (22,47,48) indicating that activation results from a release from intra ovarian factors that act to inhibit the initiation of follicle growth (49) (Figure 2). It is clear that tissue shape and stromal density are important factors that contribute to the regulation of follicle growth initiation in-vitro, as solid cubes or strips (1mm thick) of human cortical tissue show lesser growth initiation (17) and a high proportion of atretic follicles (50) than cortex cultured as flattened “sheets,” where much of the underlying stroma is removed (16). Likewise, relatively thin pieces of macaque cortical tissue (0.5 × 1 × 1 mm) exhibit atretic oocytes and follicles within contracted nonviable tissue by 7 days of culture (Ting, personal communication). The physical environment of the follicles within the cortical tissue affects their response to stimulatory and inhibitory factors and therefore influences their ability to grow (49).
Figure 2. Two-Step Culture System To Obtain Growing Human Follicles.
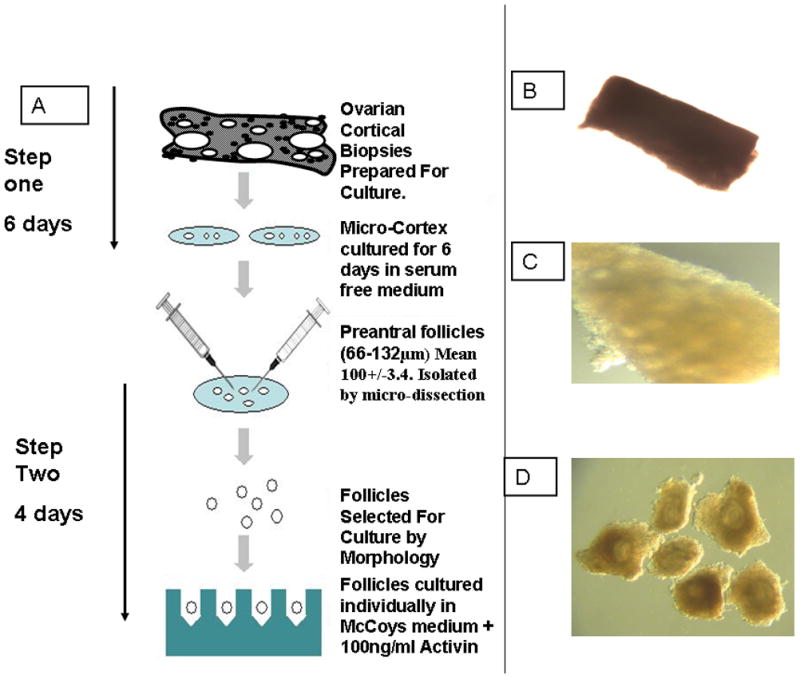
A: An outline of the preparation of human ovarian cortical biopsies to initiate development of primordial follicles (Step 1) and after 6 days in serum free medium dissect out multi laminar growing follicles and grow these individually (16).
Photomicrograph of freshly prepared micro-cortex before culture (B) and after 6 days of culture (C) followed by dissection to isolate multilaminar follicles with theca cells attached (D). Isolated follicles can be cultured individually as outlined in A and antral formation occurs during a further 4 day culture period.
Primordial follicles isolated enzymatically from nonhuman primate (42) and human (50–52) ovarian tissue also spontaneously grow in vitro. Remarkably, both human and macaque primordial follicles initiate their growth at similar sizes (≈ 30–40 μm) after isolation achieve similarand grow to identical diameters (50–58 μm) after one week in culture. In both species, concomitant with the small increase in diameter, granulosa cells surrounding the oocyte apparently proliferate in a subset of these follicles and can be morphologically identified after fixation for hematoxylin and eosin staining (42) or transmission electron microscopy with toliduine blue staining (52) as depicted in Figure 3. Human primordial follicles were reported to reach secondary stages based on the presence of more than one layer of granulosa cells (52), despite their diameters being much less than that typical for secondary follicles (150- 225 μm) isolated from human (29) and macaque (27,28) ovarian cortex. The discrepancy may be attributed to the lack of appreciable oocyte growth as reported for macaque primordial follicles (42) that is believed to occur in primates concomitant with the rapid proliferation of granulosa cells associated with the secondary stage (15). Thus, the multilayer “secondary” morphology may actually represent an earlier developmental stage when the oocyte is just initiating its growth. In these studies, groups of primate primordial follicles were cultured within alginate matrices wherein inhibitory interactions may be more localized, however recent studies in mice suggest group culture of primordial follicles in alginate confers an advantage to subsequent growth via paracrine mechanisms (53). Until primate primordial follicle culture is extended to intervals longer than one week, it is not known whether currently reported culture conditions can upport further growth of both follicles and oocytes beyond ‘early’ secondary stages.
Figure 3.

Left panel depicts representative histological images of isolated rhesus macaque primordial follicles after encapsulation in alignate at Day 0 (A and B), cultured for 6 days without encapsulation (C and D), or cultured for 6 days while encapsulated in 0.5% (E and F) or 2% (G and H) alginate. Scale bar = 50 μm; asterisks denote oocytes. From reference (42) Right panel represents morphology and histology of isolated human primordial follicles encapsulated in alginate before in vitro culture (A), after 7 days in vitro (B), a cryopreserved follicle after 7 days in vitro (C), and a follicle isolated from cryopreserved-thawed ovarian cortex after 7 days in culture (D). Images are at 40X magnification. Semi-thin sections of a cryopreserved isolated follicle (E, 1000X) and a follicle isolated from frozen-thawed ovarian cortex (F, 400X) after 7 days of culture encapsulated in alginate. From reference (53).
Once follicle growth has been initiated within cortical tissue, human follicles can develop to multilaminar stages. Large multilaminar follicles do not survive well within the cortical environment and it appears to be inhibitory to further growth resulting in a loss of follicle integrity and oocyte survival (16,26). Therefore in order to support further development, it appears that multilaminar follicles need to be released from the cortical stromal environment and cultured individually to limit the effect of follicle interactions (16,22), and/or the limitations of oxidative damage and nutrient diffusion into the tissue in vitro (Figure 2).
Early Follicle Development In Vitro
Because of the limited amount of human tissue available for clinical use and research, as well as declining follicle numbers in specimens obtained from older vs. younger patients, optimizing the yield of good quality follicles is important. Isolation of preantral follicles from cortical tissue post culture can be achieved by mechanical dissection, enzymatic isolation or a combination of both. Whilst enzymatic isolation using collagenase and DNase, to remove preantral follicles from stromal tissue results in more follicles than by mechanical dissection (54,55) this method can cause damage which leads to poor survival of follicles. Growing follicles need theca layers to retain their structure and to survive the second stage of IVG and collagenase treatment may compromise these layers (56). Some of this damage may be avoided by using new purified enzyme preparations including liberase (42,43,52,58).
Careful mechanical isolation using fine needles has the advantage of preserving follicular integrity by maintaining the basal lamina and thecal layers of the follicle, but the yield is low and the procedure slow due to the dense fibrous cortical tissue in human ovaries where follicles are embedded in the tough fibrous cortex and relatively inaccessible. In contrast, hundreds of secondary follicles can be isolated from the similarly dense and fibrous ovarian cortex in a pair of rhesus macaque ovaries (27,28) and this is also age-dependent since tissue from older primates yields fewer follicles (27).
When culturing large mammalian follicles the use of v-shaped micro-well plates has allowed maintenance of three dimensional follicular architecture in vitro while promoting growth and differentiation in bovine (59–61) and human follicles (16) with antral formation occurring within 10 days. In addition to v-shaped micro-well culture plates, follicle encapsulation in alginate hydrogels has been used to support secondary human (29), rhesus monkey (27,28,30) and baboon (31) follicle growth in vitro. Alginate encapsulation is believed to mimic the extracellular matrix in vivo in terms of its ability to facilitate nutrient, oxygen, hormone and growth factor exchange between the follicle and the culture medium. Furthermore, its flexibility can accommodate cell proliferation, but its rigidity prevents dissociation of the 3-dimensional follicular unit. The rigidity of the alginate capsule affects follicle development as inhibition of growth and reduced steroidogenesis have been reported in murine follicles embedded in 1% alginate gels (62) whereas fully grown human (29) and macaque (27,28,30) oocytes have been produced using 0.5% and 0.25% gels, respectively. Indeed, a more rigid environment of 2% alginate supported macaque primordial follicle growth in vitro (42).
The progression of human follicles following isolation from the cortex is remarkable. In the presence of FSH, enzymatically isolated secondary human follicles can differentiate, become steroidogenically active and complete oocyte growth in 30 days (29; Figure 4), quiescent follicles activated to grow within cultured fragments of cortex and mechanically isolated as secondary follicles become steroidogenic and undergo differentiation after a 10 day in vitro period with and without activin (16). These observations confirm that local ovarian factors inhibit follicle development in vivo, however, the question remains as to whether the growth rate observed in vitro is accelerated or whether it represents growth without the brakes that are required in vivo to regulate follicle development within the context of the reproductive cycle. Likewise, recent advancements in understanding primate folliculogenesis have been modelled in nonhuman primates wherein preantral follicle growth in vitro is initiated at the secondary stage. By this stage, early follicles have undergone activation, and selection through two steps of folliculogenesis in vivo. Macaque secondary follicles (125–225 μm diameter) isolated mechanically and encapsulated in alginate can survive (≈ 50% of the total isolated) and routinely achieve growth to the small antral stage (1 mm diameter) in 4–5 weeks under defined culture conditions (27,28; Figure 4). Follicle survival and growth in vitro is a function of stage of the menstrual cycle, ovarian age, oxygen concentration during culture and the inclusion of fetuin in the media (27,28,30). A semidegradable matrix containing fibrin, alginate, and Matrigel also facilitated baboon follicle expansion and antrum formation in vitro (31; Figure 4).
Figure 4.
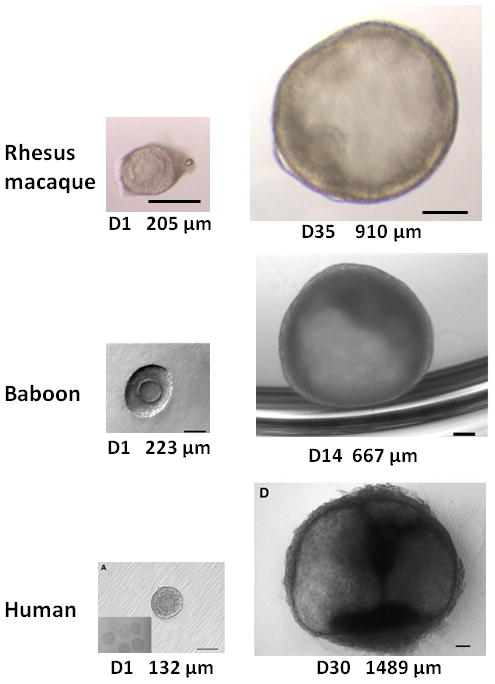
Representative images illustrating in vitro growth of nonhuman primate and human preantral follicles enclosed in a 3D matrix to the small antral stage. The day of culture and the follicular diameter is noted under each picture. Top panel represents growth of a rhesus macaque secondary follicle (left) to a small antral follicle (right); scale bars = 250 μm; from M. Zelinski. Middle panel depicts growth of a baboon multilayer follicle (left; scale bar = 100 μm) to a small antral follicle (scale bar = 50 μm); from reference (31). Bottom panel shows a human secondary follicle that grew to a small antral follicle (scale bars = 100 μm); from reference (29).
Notably, surviving secondary follicles from macaques were heterogeneous with respect to their growth rates in vitro when encapsulated in alginate as single follicles; a consistent feature of secondary follicles in this system in every animal studied to date. Three distinct cohorts were observed based on their diameters at week 5 (27,28). The cohort that does not significantly change in diameter (< 250 μm) is termed “no-grow” follicles. “Slow-grow” follicles represent another cohort that doubles their diameters (250 μm–500 μm). The group that increases their diameters by a minimum of three-fold (> 500 μm, in some instances over 1 mm) are designated “fast-grow” follicles. All slow- and fast-grow follicles exhibit an antral cavity within 3–4 weeks of culture. Differences in follicle growth rate of single human secondary follicles encapsulated in alginate were less obvious, however it was noted that the majority of follicles developed antral cavities, while others remained at the multilayer stage through 4 weeks of culture (29). Thus, the population of secondary follicles in the primate ovary at early follicular phase of the cycle is heterogeneous in their capacity to grow in vitro. Growth rates may differ due to follicle response to FSH or other hormones (27,28), or to produce and respond to other local factors that regulate follicular growth. The ability of human (29) and macaque (27,28) follicles to produce steroid hormones is also a function of follicle growth. Only growing follicles synthesized increasing levels of steroids beginning at antrum formation, with levels of estradiol, androstenedione and progesterone greater in fast- than slow-grow follicles. Vascular endothelial growth factor (VEGF) production also parallels steroidogenesis in growing follicles (27), and may reflect a requirement for vascularization by the small antral stage follicle to achieve further development in vivo with additional substrates and release of hormones. In addition to an angiogenic action, VEGF may protect antral follicles from apoptosis and atresia. Interestingly, anti-Mullerian hormone (AMH) production by growing follicles exhibited a transient elevation at weeks 3–4 of culture; levels were greater in fast- relative to slow-grow follicles (28). These data suggest that AMH may be a biomarker for early preantral follicle growth, in addition to an important local regulator of folliculogenesis in primates. Direct actions of AMH on primate preantral follicles await demonstration.
Xu and colleagues (63) recently developed a serum-free, alginate-encapsulated culture system for isolated primary follicles from rhesus macaques. A number of unique features were noted relative to secondary follicle culture in that primary follicles a) required 13 weeks to achieve the small antral stage in vitro; b) displayed only two growth patterns in vitro, no grow and fast grow; c) grew to secondary follicles within 4 weeks at which time they produced AMH; and d) did not produce steroids or VEGF production until they began to rise with the intiation of antrum formation at 9 weeks in vitro. Thus, macaque primary follicles encapsulated in alginate grow very slowly and appear to be quiescent for a month with respect to production of somatic cell-derived hormones and factors until granulosa cells proliferate with the beginning of secondary follicle formation in vitro. For the first time, a mature oocyte was produced by an isolated macaque primary follicle in 3D culture, but only developed to a zygote after insemination in vitro. Since primary follicles are more abundant within the ovarian cortex than secondary follicles, they can provide an additional cohort of growing preantral follicles for potential production of mature gametes in vitro.
A question arises as to just how large primate antral follicles need to progress to produce a competent oocyte. The typical diameter of a preovulatory follicle in the macaque is 5–6 mm, whereas it is much larger, 15–20 mm, in women. This poses a technical challenge for growing preantral follicles to preovulatory diameters in vitro. Recent data provides evidence that perhaps achieving the preovulatory size is not necessary, and that preantral follicles can be grown to much smaller antral sizes compatible with oocyte growth, maturation and competence. In macaques, small antral follicles can be derived from the medulla of ovaries during early follicular phase. This pool of follicles (0.5 – 2mm diameter) represents the cohort from which the single, dominant preovulatory follicle will be selected. Developmental competence of macaque oocytes to the blastocyst stage was possible when oocytes matured in vitro were derived from 1–2 mm small antral follicles (64). Definitive confirmation of oocyte developmental competence from follicles smaller than preovulatory diameters was recently provided by Guzman et al (65) who reported live births from in vitro-matured human oocytes retrieved from antral follicles ≤6 mm.
Table 1 summarizes the growth characteristics and oocyte outcomes of growing primate follicles encapsulated in varying alginate matrices. Collectively, these studies represent the most recent advances in 3D primate follicle culture and demonstrate the principle that all classes of growing, preantral follicles can contribute to the reproductive potential of women. Furthermore, these studies provide a glimpse into the follicle selection process in that cohorts of primordial, primary and secondary follicles isolated from the ovarian cortex are not homogeneous with respect to their survival, growth rates and endocrine/paracrine functions in vitro. By using a 3D supportive matrix, primary and secondary follicles maintained bidirectional communication between somatic cells and the germ cell creating an environment conducive for oocyte growth and steroid production. Clearly, current culture systems can support somatic cell functions of the preantral follicular unit. The next crucial step is to demonstrate whether the oocytes produced in these systems are capable of in vitro maturation and to determine whether the growth pattern in vitro is deleterious to oocyte function, epigenetic changes and health.
Table 1.
Growth characteristics and oocyte outcome of nonhuman primate and human growing follicles in 3-dimensional culture.
| Species
|
Ref
|
3D matrix
|
Initial follicle stage (diameter, μm)
|
Days to Antrum formation
|
Days to Antral stages (>500 um)
|
IFMb or IVMc
|
Oocyte nuclear stages reported at recoveryd
|
Fertilization and embryo development
|
|---|---|---|---|---|---|---|---|---|
| Rhesus | 27,28 | alginate | Secondary (125–225) | 21 | 35 – 40 | IFM | GV, MII, degenerating | ICSIe, early cleavage |
| Rhesus | 63 | alginate ± fibrin | Primary (80–120) | 63 | 91 | IFM | GV, MII, degenerating | ICSI, zygote |
| Baboon | 31 | FAMa | Multilayer (250) | 10 | ≥14 | IVM | GV, IVM to MI and MII | Not inseminated |
| Human | 29 | alginate ± Matrigel | Secondary (125–225) | 15 | ≥35 | IFM | GV | Not inseminated |
FAM = semidegradable fibrin, aliginate, Matrigel matrix
IFM = in follicle maturation; rhesus: 1000 IU r-hCG in media, oocytes harvested and inseminated 34 hr post-hCG; human: hCG was not administered
IVM = in vitro maturation; cumulus-oocyte complex removed from antral follicle, FSH/LH/hCG/EGF in media, oocytes denuded and inseminated 42–46 hr later
GV= germinal vesicle-intact oocyte; MI = metaphase I oocyte; MII = metaphase II oocyte
ICSI = intracytoplasmic sperm injection
Development of fully grown oocytes
The ultimate aim of a follicle culture system is to obtain developmentally competent oocytes therefore, these oocytes need to be matured in vitro. In vitro maturation (IVM) of oocytes already exists as a separate strategy and is utilised routinely in human assisted reproductive technology processes with varying degrees of success (66). As discussed earlier achieving and sustaining oocyte growth is the major objective of any complete in vitro development system as this is a size specific indicator of the oocyte’s ability to resume meiosis (67,68). The system must also be capable of supporting nuclear maturation and cytoplasmic differentiation of oocytes in vitro (69).
It is widely accepted that while 40–80% of immature human oocytes can successfully complete in vitro maturation and fertilization giving rise to live births, the rate of maturation of immature oocytes is still well below that of oocytes harvested from stimulated ovaries, indicating that the protocols are sub-optimal or many of the harvested oocytes are intrinsically unable to undergo maturation (66). In vitro grown oocytes may require a further period of growth within the cumulus complex before maturation (22). One way to achieve this final growth phase of the oocyte is removal of this oocyte-cumulus cell unit from the antral follicle. Maintaining oocyte somatic cell interactions and cytoskeleton stability is important at this stage and in bovine follicles it has been demonstrated that the correct balance of activin and FSH in vitro affects these processes (222).
Oocyte growth within human complexes has been demonstrated in vitro (33) and live births have been reported using bovine oocyte-granulosa cell complexes which were aspirated from immature follicles and grown for 14 days until the oocyte was large enough to be matured in vitro (70). Compact COCs isolated from IVG baboon antral follicles underwent in vitro maturation to yield oocytes that reinitiated meiosis to the metaphase II stage, with a normal appearing spindle structure (31). Fertilization was not attempted in this study. These results give encouragement that a similar system could be applied to human cumulus-oocyte complexes aspirated from IVG follicles in order to achieve oocyte diameters suitable to undergo IVM.
Alternatively, in vitro developed follicles that reach the antral stage can also be treated with recombinant human chorionic gonadotropin (hCG) in the media to examine reinitiation of meiotic maturation of the oocyte within the follicle (27,28,63). Healthy and degenerating oocytes are obtained from small antral stage (0.5–1mm diameter) IVG macaque follicles. The majority of healthy oocytes (~ 100 μm diameter) remain at the germinal vesicle stage. However, metaphase II stage oocytes (> 110 μm) within an expanded cumulus matrix have been retrieved from hCG-treated antral follicles following growth from both primary and secondary follicles under chemically-defined conditions in macaques. Fertilization can occur following insemination by intracytoplasmic sperm injection, but thus far only the resulting zygotes from secondary follicle culture cleave, and embryonic development is arrested within 3 days in vitro. Thus, the few oocytes obtained from IVG primary and secondary follicles using the alginate encapsulated culture system in macaques can achieve nuclear maturation and fertilization, but do not complete cytoplasmic maturation necessary for embryonic development.
Evidence of normal offspring derived from IVG of primordial (5,6) and secondary follicles both with (11) and without (7,71) alginate in the mouse provides an impetus for considering the feasibility of follicle culture to one day achieve births in nonhuman primates, followed by clinical translation to women. A realistic concern remains as to whether culture systems would confer interference at the epigenetic level and perhaps alter imprinting patterns in oocytes and the maintenance of genomic imprinting required for subsequent normal embryonic development. Although only a few investigations have been made to date, correct DNA methylation establishment has been observed in murine and ovine oocytes derived from IVG follicles (72). Whether normal imprinting establishment and maintenance would be sustained in primate oocytes and embryos derived from follicles grown in defined culture conditions is currently unknown.
Since only a small proportion of preantral follicles reaching the antral state in vitro enclose fully-grown oocytes surrounded by cumulus cells, current culture systems favour somatic cell function perhaps at the expense of the oocyte since they fall short of supporting oocyte-granulosa cell communication leading to oocyte competence. Oocyte-derived factors, such as GDF-9 and BMP-15, secreted during their characteristic stages of folliculogenesis would be logical biomarkers of oocyte function to assess during in vitro follicle culture (20. However, reagents for non-invasive assays of oocyte-specific factors to evaluate oocyte function while enclosed in preantral follicles during their growth and development in vitro are not yet available for routine use. Furthermore, if oocytes were healthy and functioning normally within the cultured follicles, there should be no need to add exogenous oocyte-specific factors; however, these experiments have not yet been performed with primate follicles. Thus, manipulation of current follicle culture systems (i.e. media components) to optimize oocyte-somatic cell communication throughout growth to the antral stage in vitro are limited to morphological measurements and hormone production of somatic cells, with invasive removal of cumulus-oocyte complexes at the end of culture to assess oocyte viability and competence.
Conclusions
Thus, major hurdles remain for improving culture systems to promote coordinated development of the COC to achieve oocyte growth, the competence to produce embryos, and attain live offspring as the ultimate clinical endpoint. Current yields of mature oocytes from IVG follicles are low; therefore a concomitant goal is to increase the efficiency and reproducibility of producing mature, competent oocytes. This is no small task in view of the complex systems that regulate oocyte development and ultimate function (73).. A looming challenge remains in evaluating the utility of current culture systems to support the growth and function of cryopreserved-thawed follicles for clinical use in patients who have banked ovarian tissue. Early investigations are yielding promising results. The survival and growth of preantral follicles from frozen-thawed human (51) and vitrified-thawed macaque (44,74,75) ovarian tissue has been recently demonstrated. Continued growth of alginate-encapsulated secondary follicles from vitrified ovarian tissue to the antral stage in macaques is accompanied by steroidogenesis in vitro (44,74,75). Individually cryopreserved macaque preantral follicles (76) and human follicles enclosed in biomatrices prior to cryopreservation (53) can also survive and grow post-thaw in vitro.
Advances in bioengineering novel matrices that support follicle survival, growth, somatic cell differentiation and germ cell maturation as historically and elegantly demonstrated in the mouse model is critical for further progress in human follicle culture. For example, biodegradable matrices with interpenetrating networks (IPN) of fibrin (77) can be engineered to link proteins within the matrix allowing local and sustained delivery of growth factors. Indeed, a novel VEGF-containing IPN matrix supported folliculogenesis and oocyte maturation resulting in live offspring when transplanted to the ovarian bursa in mice (78). These new matrices will allow studies of individual or combinations of growth factors to improve folliculogenesis in vitro that supports the coordinated development of somatic and germ cells necessary for embryonic competence and production of live offspring. Emerging design of hydrogels containing cross-linked peptides that degrade in response to proteases secreted by the follicle can locally allow follicle expansion, while maintaining the integrity of the hydrogel essential for supporting the 3D architecture of the follicle in vitro that is essential for supporting oocyte growth and competence (79). In contrast to alginate which is not degradable, these synthetic hydrogel systems with “tunable” properties allow the follicle to influence its own local environment. These systems will enhance the ability of human follicles to volumetrically expand beyond a 1 mm diameter, currently achieved with the alginate matrix, to diameters (i.e., 5 mm in women) that represent those capable of producing competent oocyte and live births in vivo.
Technologies for human follicle culture today are comparable to the early beginnings of human in vitro fertilization. Consistent and careful research in human and nonhuman primate models will focus on optimizing culture conditions that favour efficient production of meiotically and developmentally competent oocytes from fresh or cryopreserved ovarian tissue that can be performed in a clinical infertility setting (21). While aiming toward the lofty goal of fertility preservation, the amazing recent advancements in the culture of individual or groups of primate follicles in vitro is also providing insights into the basic biology of primate folliculogenesis that have never before been possible. Whether these systems ever reach clinical application or not, they are still invaluable for providing insight into a system that has previously been unattainable.
Acknowledgments
Supported by The Medical Research Council (EET), The Oncofertility Consortium NIH UL1 RR024926 (R01-HD058293, R01-HD058294, HD058295, PL1-EB008542), U54-HD18185 (Eunice Kennedy Shriver Specialized Cooperative Centers Program in Reproduction and Infertility Research), ONPRC 8P51OD011092.
Footnotes
EET and MBZ. Have nothing to disclose.
References
- 1.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43:437–50. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 3.Andersen CY, Kristensen SG, Greve T, Schmidt KT. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 2012:8595–608. doi: 10.2217/fon.12.47. [DOI] [PubMed] [Google Scholar]
- 4.Silber SJ, Barbey N. Scientific molecular basis for treatment of reproductive failure in the human: an insight into the future. Biochim Biophys Acta. 2012;1822:1981–96. doi: 10.1016/j.bbadis.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–6. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 7.Cortvrindt R, Smitz J, Van Steirteghem AC. A morphological and functional study of the effect of slow freezing followed by complete in-vitro maturation of primary mouse ovarian follicles. Hum Reprod. 1996;11:2648–55. doi: 10.1093/oxfordjournals.humrep.a019187. [DOI] [PubMed] [Google Scholar]
- 8.Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–76. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- 9.Roy SK, Greenwald GS. Hormonal requirements for the growth and differentiation of hamster preantral follicles in long-term culture. J Reprod Fertil. 1989;87:103–14. doi: 10.1530/jrf.0.0870103. [DOI] [PubMed] [Google Scholar]
- 10.Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod. 1994;9:527–32. doi: 10.1093/oxfordjournals.humrep.a138539. [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81:433–42. doi: 10.1530/jrf.0.0810433. [DOI] [PubMed] [Google Scholar]
- 13.Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136:703–15. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- 14.Thomas FH, Walters KA, Telfer EE. How to make a good oocyte: An update on in vitro models to study follicle regulation. Hum Reprod Update. 2003;9:1–15. doi: 10.1093/humupd/dmg042. [DOI] [PubMed] [Google Scholar]
- 15.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–55. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 16.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two step serum free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–8. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 17.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–36. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 18.Picton HM, Gosden RG. In vitro growth of human primordial follicles from frozen-banked ovarian tissue. Mol Cell Endocrinol. 2000;166:27–35. doi: 10.1016/s0303-7207(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 19.Telfer EE, McLaughlin M. In vitro growth (IVG) systems for human oocytes: from primordial to maturation. In: Kim S, Donnez J, editors. In Principles and Practice in Fertility Preservation. 2011a. [Google Scholar]
- 20.Telfer EE, McLaughlin M. Human follicle activation and development in vitro. Semin Reprod Med. 2011b;29:15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 21.Smitz J, Dolmans M-M, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 2010;139:971–8. doi: 10.1530/REP-10-0025. [DOI] [PubMed] [Google Scholar]
- 23.Telfer EE, McLaughlin M. Strategies to support human oocyte development in vitro. Int J Dev Biol. 2012;56:901–7. doi: 10.1387/ijdb.130001et. [DOI] [PubMed] [Google Scholar]
- 24.Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59:783–90. [PubMed] [Google Scholar]
- 25.Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril. 1997;68:682–8. doi: 10.1016/s0015-0282(97)00264-1. [DOI] [PubMed] [Google Scholar]
- 26.Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod. 1999;14:2519–24. doi: 10.1093/humrep/14.10.2519. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young and older adult, rhesus monkeys during encapsulated three-dimensional (3D) culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–97. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Lawson MS, Yeoman RR, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011a;26:1061–72. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M, Barrett SL, West-Farrell ER, Kondipalli LA, Kieswetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009a;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009b;81:587–94. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu M, Fazleabas AT, Shikanov A, Jackons E, Barrett SL, Hirshefeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal phase baboon ovary produce mature oocytes. Biol Reprod. 2011b;84:680–97. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alak BM, Coskun S, Friedman CI, Kennard EA, Kim MH, Seifer DB. Activin A stimulates meiotic maturation of human oocytes and modulates granulosa cell steroidogenesis in vitro. Fertil Steril. 1998;70:1126–30. doi: 10.1016/s0015-0282(98)00386-0. [DOI] [PubMed] [Google Scholar]
- 33.Cavilla JL, Kennedy CR, Byskov AG, Hartshorne GM. Human immature oocytes grow during culture for IVM. Hum Reprod. 2008;23:37–45. doi: 10.1093/humrep/dem178. [DOI] [PubMed] [Google Scholar]
- 34.Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2012 doi: 10.1093/humupd/dms043. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–3. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 36.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–18. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 37.Ting AY, Zelinski MB. Distribution of FOXO transcription factors in ovaries of fetal and juvenile rhesus macaques. Biol Reprod. 2012;(Suppl):191. doi: 10.1093/biolre/iox034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle. 2010;9:1673–4. doi: 10.4161/cc.9.9.11626. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010:10710280–4. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaughlin M, Patrizio P, Kayisli U, Luk J, Thomson TC, Anderson RA, Telfer EE, Johnson J. mTOR kinase inhibition results in oocyte loss characterized by empty follicles in human ovarian cortical strips cultured in vitro. Fertil Steril. 2011;96:1154–9. doi: 10.1016/j.fertnstert.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt KL, Byskov AG, Andersen AN, Andersen CY. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–64. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 42.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27:1801–10. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice S, Ojha K, Mason H. Human ovarian biopsies as a viable source of pre-antral follicles. Hum Reprod. 2008;23:600–5. doi: 10.1093/humrep/dem390. [DOI] [PubMed] [Google Scholar]
- 44.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461–72. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chambers EL, Gosden RG, Yap C, Picton HM. In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. Hum Reprod. 2010;25:2559–68. doi: 10.1093/humrep/deq192. [DOI] [PubMed] [Google Scholar]
- 46.Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum Reprod. 2011;26:157–66. doi: 10.1093/humrep/deq318. [DOI] [PubMed] [Google Scholar]
- 47.Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod. 1996;55:942–8. doi: 10.1095/biolreprod55.5.942. [DOI] [PubMed] [Google Scholar]
- 48.Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12:1993–2001. doi: 10.1093/humrep/12.9.1993. [DOI] [PubMed] [Google Scholar]
- 49.McLaughlin EA. McIver SCAwakening the oocyte: controlling primordial follicle development. Reproduction. 2009;37:1–11. doi: 10.1530/REP-08-0118. [DOI] [PubMed] [Google Scholar]
- 50.Sanfilippo S, Canis M, Romero S, Sion B, Déchelotte P, Pouly JL, Janny L, Smitz J, Brugnon F. Qualityand functionality of human ovarian tissue after cryopreservation using an original slow freezing procedure. J Assist Reprod Genet. 2013;30:25–34. doi: 10.1007/s10815-012-9917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amorim CA, Van Langendonckt A, David A, Dolmans M-M, Donnez J. Survival of human pre-antral follicles after cryopreservation of human tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–9. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- 52.Vanacker J, Camboni A, Dath C, et al. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil Steril. 2011;96:379–83. doi: 10.1016/j.fertnstert.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 53.VanAcker J, Luyckx V, Amorim C, Dolmans M-M, Van Langendonckt A, Donnex J, Camboni A. Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil Steril. 2013 doi: 10.1016/j.fertnstert.2012.12.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145:19–32. doi: 10.1530/REP-12-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Telfer EE. The development of methods for isolation and culture of preantral follicles from bovine and porcine ovaries. Theriogenology. 1996;45:101–10. [Google Scholar]
- 56.Park KS, Lee TH, Park YK, Song HB, Chun SS. Effects of isolating methods (mechanical or enzymatical) on structure of pre-antral follicles in mouse. J Assist Reprod Genet. 2005;22:355–9. doi: 10.1007/s10815-005-6796-z. [DOI] [PubMed] [Google Scholar]
- 57.Telfer EE, Binnie JP, McCaffery FH, Campbell B. In vitro development of oocytes from porcine and bovine primary follicles. Mol Cell Endocrinol. 2000;163:117–23. doi: 10.1016/s0303-7207(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 58.Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Van Langendonckt A, Nottola SA, Donnez J. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod. 2006;21:413–20. doi: 10.1093/humrep/dei320. [DOI] [PubMed] [Google Scholar]
- 59.Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62:1322–8. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- 60.Thomas FH, Armstrong DG, Campbell BK, Telfer EE. Effects of insulin-like growth factor-1 bioavailability on bovine preantral follicular development in vitro. Reproduction. 2007;133:1121–8. doi: 10.1530/REP-06-0382. [DOI] [PubMed] [Google Scholar]
- 61.Walters KA, Binnie JP, Campbell BK, Armstrong DG, Telfer EE. The effects of IGF-I on bovine follicle development and IGFBP-2 expression are dose and stage dependent. Reproduction. 2006;13:515–23. doi: 10.1530/rep.1.00682. [DOI] [PubMed] [Google Scholar]
- 62.Heise M, Koepsel R, Russell AJ, McGee EA. Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reprod Biol Endocrinol. 2005;3:47. doi: 10.1186/1477-7827-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, Zelinski MB. Fibrin promotes development of primate primary, but not secondary, follicles during the 3-dimensional culture including steroidogenesis, paracrine factor production, and oocyte maturation. Hum Reprod. 2013 doi: 10.1093/humrep/det093. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peluffo M, Stouffer RL, Hennebold JD, Zelinski MB. Cumulus oocyte complexes from small antral follicles during the early follicular phase of spontaneous cycles in rhesus monkeys can expand and yield oocytes capable of maturation in vitro. Biol Reprod. 2010;83:525–32. doi: 10.1095/biolreprod.110.084418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guzman L, Orgega-Hreppich C, Albuz FK, Verheyen G, Devroey P, Smitz J, De Vos M. Developmental capacity of in vitro-matured human oocyte retrieved from polycystic ovary syndrome ovaries containing no follicles larger than 6 mm. Fertil Steril. 2012;98:503–7. e1–2. doi: 10.1016/j.fertnstert.2012.01.114. [DOI] [PubMed] [Google Scholar]
- 66.Nogueira D, Sadeu JC, Montagut J. In vitro oocyte maturation: current status. Semin Reprod Med. 2012;30:199–213. doi: 10.1055/s-0032-1311522. [DOI] [PubMed] [Google Scholar]
- 67.Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev. 1995;42:437–42. doi: 10.1002/mrd.1080420410. [DOI] [PubMed] [Google Scholar]
- 68.Sirard MA. Follicle environment and quality of in vitro matured oocytes. J Assist Reprod Genet. 2011;28:483–8. doi: 10.1007/s10815-011-9554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banwell KM, Thompson JG. In vitro maturation of Mammalian oocytes: outcomes and consequences. Semin Reprod Med. 2008;26:162–74. doi: 10.1055/s-2008-1042955. [DOI] [PubMed] [Google Scholar]
- 70.Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, Kacchi M, Hoshi H, Takenouchi N. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod. 2004;70:83–91. doi: 10.1095/biolreprod.103.021238. [DOI] [PubMed] [Google Scholar]
- 71.Smitz J, Cortvindt R. The earliest stages of folliculogenesis in-vitro. Reproduction. 2002;123:185–202. doi: 10.1530/rep.0.1230185. [DOI] [PubMed] [Google Scholar]
- 72.Anckaert E, De Rycke M, Smitz J. Culture of oocytes and risk of imprinting defects. Hum Reprod Update. 2013;19:52–66. doi: 10.1093/humupd/dms042. [DOI] [PubMed] [Google Scholar]
- 73.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–52. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 74.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. Synthetic polymers improve vitrification outcomes of macaque ovarian tissue as assessed by histological integrity and the in vitro development of secondary follicles. Cryobiology. 2012;65:1–11. doi: 10.1016/j.cryobiol.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, Zelinski MB. Morphological and functional preservation of preantral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod. 2013 doi: 10.1093/humrep/det032. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campos J, Ting AY, Yeoman RR, Lawson MS, Rosa-e-Silva ACJS, Zelinski MB. Cryopreservation of isolated secondary follicles from nonhuman primate using a closed system and quartz capillaries. J Assist Reprod Genet. 2011;28:997–8. [Google Scholar]
- 77.Shikanov A, Xu M, Woodruff TK, Shea L. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–85. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials. 2011a;32:2524–31. doi: 10.1016/j.biomaterials.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, Shea LD. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tiss Eng. 2011b;17:3095–104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]


