Abstract
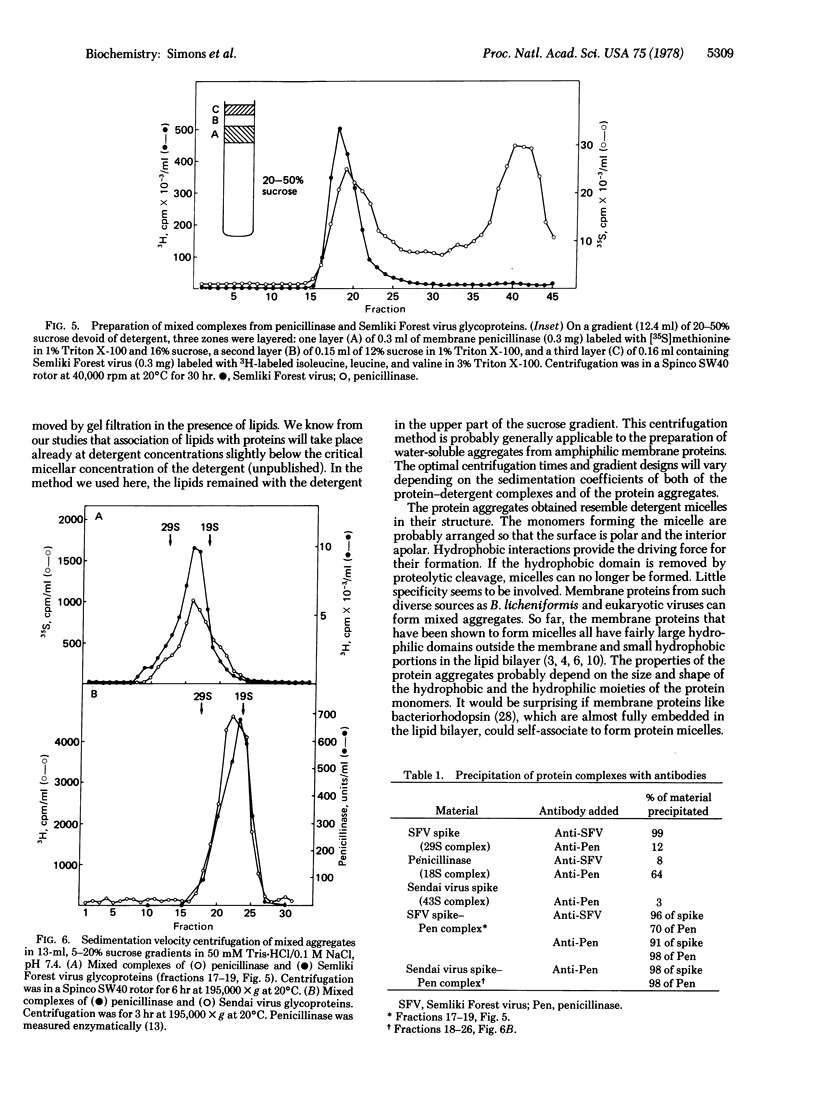
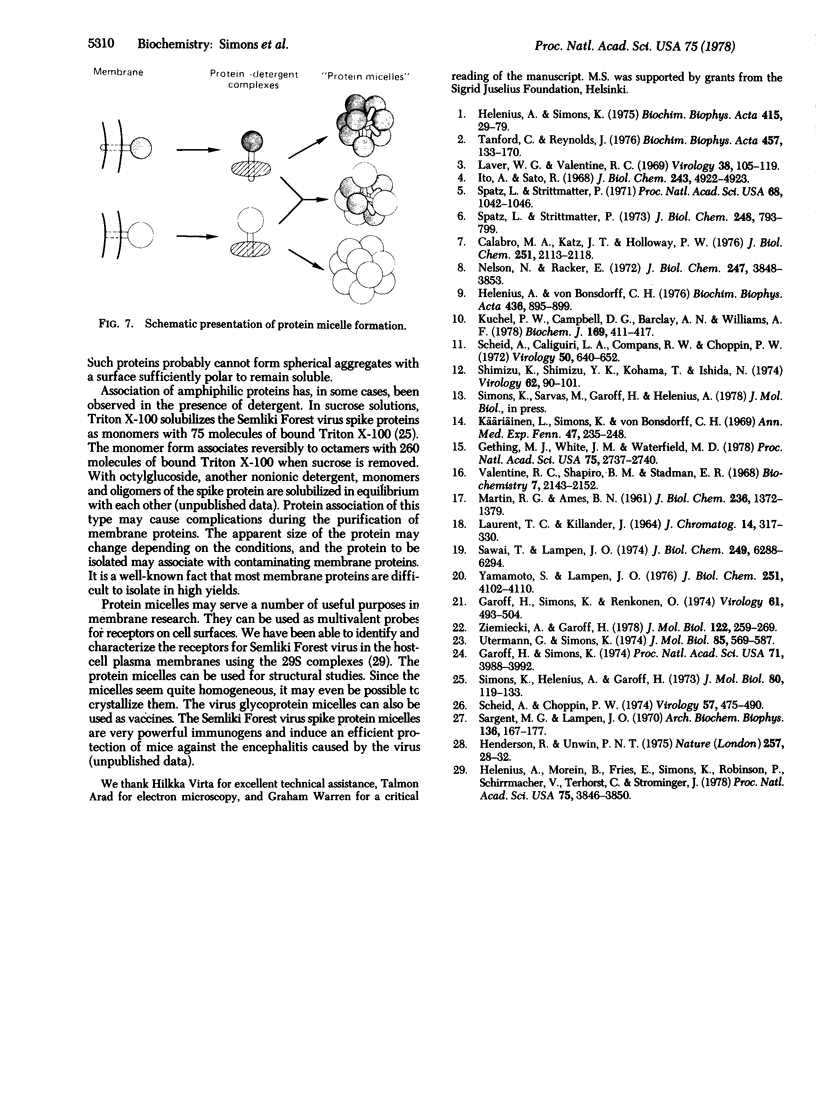
The membrane penicillinase (penicillin amido-beta-lactamhydrolase, EC 3.5.2.6) from Bacillus licheniformis, the Semliki Forest virus spike proteins, and the Sendai virus glycoproteins have each been isolated as soluble protein aggregates that are virtually free of lipid and detergent. The sedimentation coefficients of the complexes were 18 S, 29 S, and 43 S, respectively. Mixed aggregates containing both the virus glycoproteins and the penicillinase could also be formed. Such protein micelles may serve a number of useful purposes in membrane research.
Full text
PDF
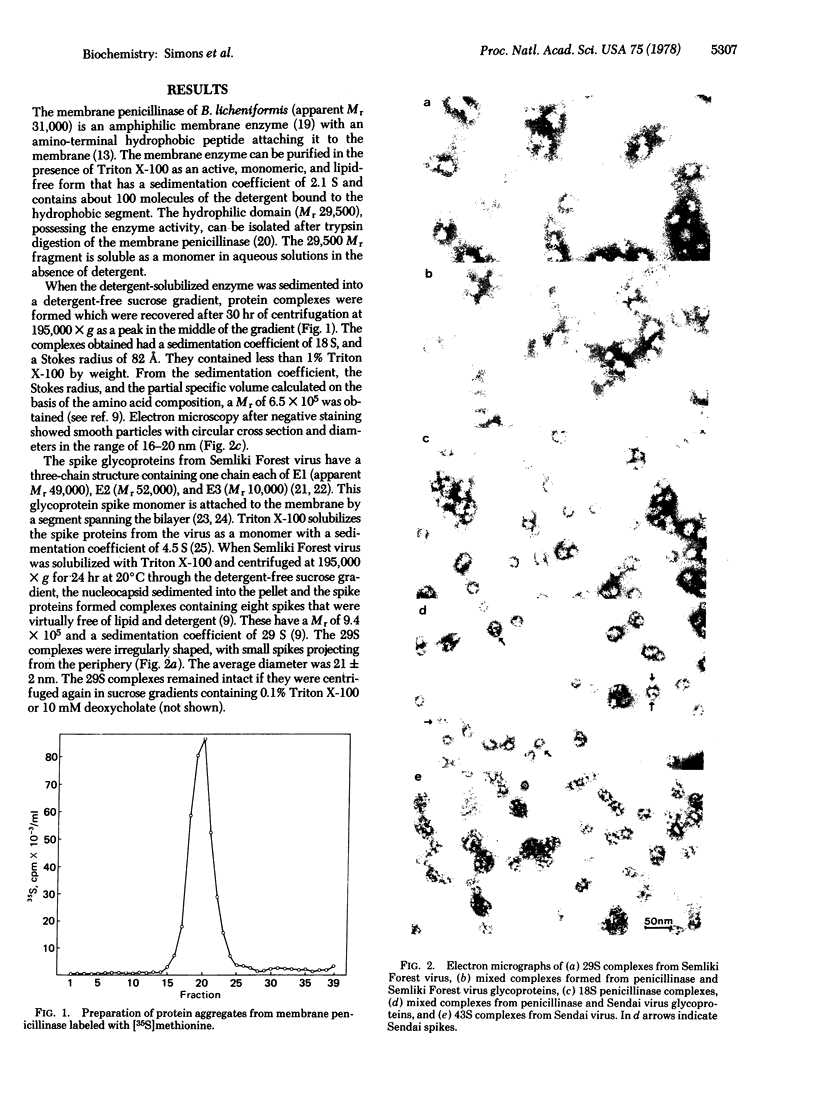
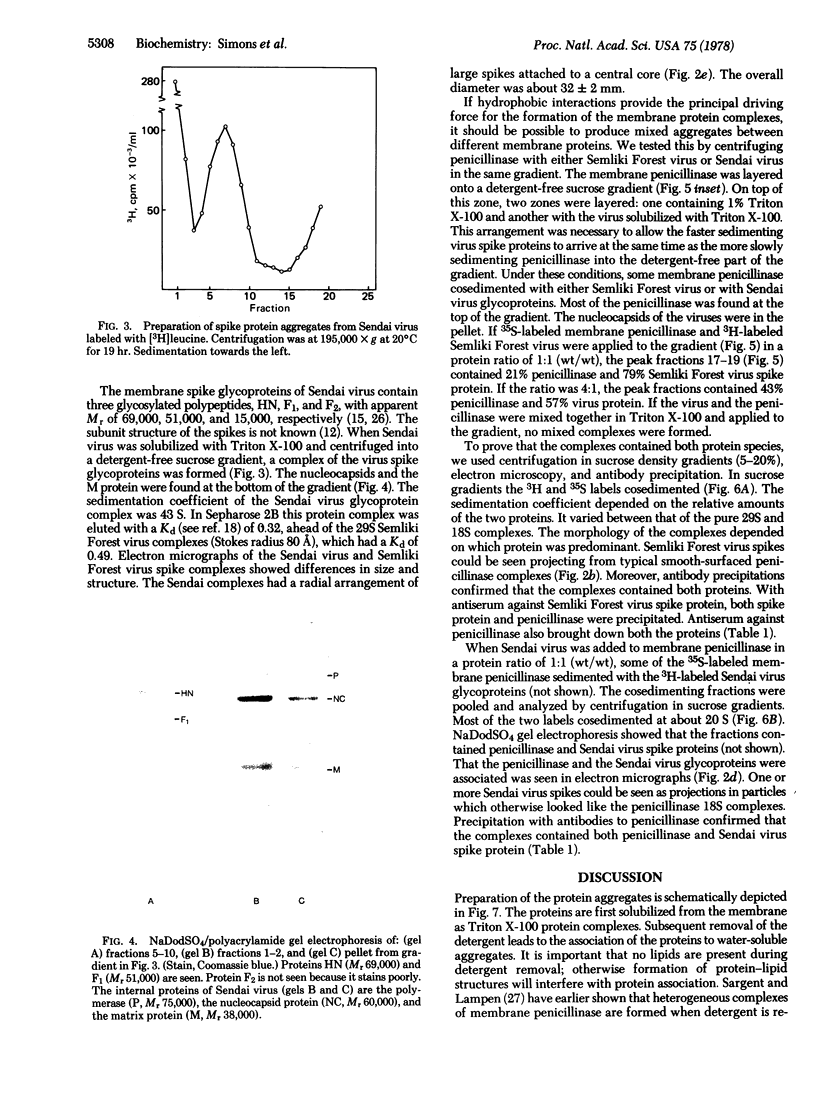
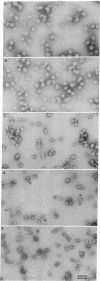
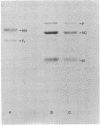
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calabro M. A., Katz J. T., Holloway P. W. Self-association of cytochrome b5 in aqueous solution. Gel filtration and ultracentirfugational studies. J Biol Chem. 1976 Apr 10;251(7):2113–2118. [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Simons K., Renkonen O. Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology. 1974 Oct;61(2):493–504. doi: 10.1016/0042-6822(74)90285-2. [DOI] [PubMed] [Google Scholar]
- Gething M. J., White J. M., Waterfield M. D. Purification of the fusion protein of Sendai virus: analysis of the NH2-terminal sequence generated during precursor activation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2737–2740. doi: 10.1073/pnas.75.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Morein B., Fries E., Simons K., Robinson P., Schirrmacher V., Terhorst C., Strominger J. L. Human (HLA-A and HLA-B) and murine (H-2K and H-2D) histocompatibility antigens are cell surface receptors for Semliki Forest virus. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3846–3850. doi: 10.1073/pnas.75.8.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Helenius A., von Bonsdorff C. H. Semlike Forest virus membrane proteins. Preparation and characterization of spike complexes soluble in detergent-free medium. Biochim Biophys Acta. 1976 Jul 15;436(4):895–899. doi: 10.1016/0005-2736(76)90421-1. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Ito A., Sato R. Purification by means of detergents and properties of cytochrome b5 from liver microsomes. J Biol Chem. 1968 Sep 25;243(18):4922–4923. [PubMed] [Google Scholar]
- Kuchel P. W., Campbell D. G., Barclay A. N., Williams A. F. Molecular weights of the Thy-1 glycoproteins from rat thymus and brain in the presence and absence of deoxycholate. Biochem J. 1978 Feb 1;169(2):411–417. doi: 10.1042/bj1690411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Simons K., von Bonsdorff C. H. Studies in subviral components of Semliki Forest virus. Ann Med Exp Biol Fenn. 1969;47(4):235–248. [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nelson N., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. X. Purification of spinach cytochrome f and its photooxidation by resolved photosystem I particles. J Biol Chem. 1972 Jun 25;247(12):3848–3853. [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. Organization of the membrane-bound penicillinases of Bacillus licheniformis. Arch Biochem Biophys. 1970 Jan;136(1):167–177. doi: 10.1016/0003-9861(70)90338-3. [DOI] [PubMed] [Google Scholar]
- Sawai T., Lampen J. O. Purification and characteristics of plasma membrane penicillinase from Bacillus licheniformis 749-C. J Biol Chem. 1974 Oct 10;249(19):6288–6294. [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Shimizu Y. K., Koama T., Ishida N. Isolation and characterization of two distinct types of HVJ (Sendai virus) spikes. Virology. 1974 Nov;62(1):90–101. doi: 10.1016/0042-6822(74)90305-5. [DOI] [PubMed] [Google Scholar]
- Simons K., Helenius A., Garoff H. Solubilization of the membrane proteins from Semliki Forest virus with Triton X100. J Mol Biol. 1973 Oct 15;80(1):119–133. doi: 10.1016/0022-2836(73)90236-2. [DOI] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem. 1973 Feb 10;248(3):793–799. [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Utermann G., Simons K. Studies on the amphipathic nature of the membrane proteins in Semliki Forest virus. J Mol Biol. 1974 Jan 5;85(4):569–587. doi: 10.1016/0022-2836(74)90316-7. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. The hydrophobic membrane penicillinase of Bacillus licheniformis 749/C. Characterization of the hydrophilic enzyme and phospholipopeptide produced by trypsin cleavage. J Biol Chem. 1976 Jul 10;251(13):4102–4110. [PubMed] [Google Scholar]
- Ziemiecki A., Garofff H. Subunit composition of the membrane glycoprotein complex of Semliki Forest virus. J Mol Biol. 1978 Jul 5;122(3):259–269. doi: 10.1016/0022-2836(78)90189-4. [DOI] [PubMed] [Google Scholar]