Abstract
Long term survivors of allo-SCT have increased risk of cardiovascular disease. We retrospectively studied cardiovascular risk factors (CVRF) in 109 SCT survivors (62 males, 47 females; median age 34 years) ≥5 years after bone-marrow (15) or T-cell-depleted peripheral blood (94) SCT for CML (56), acute leukemia (29), MDS (13), and others (11). One death and 2 cardiovascular events were reported. At 5 and 10 years post-SCT respectively, 44% and 52% had abnormal lipid profiles. 23% of 5-year survivors met the Adult Treatment Panel III threshold for dyslipidemia treatment, which is substantially higher than the age-matched general population. There were significant increases in prevalence of hypertension (p<0.001), diabetes (p=0.018) and body mass index (p=0.044) post-SCT compared to baseline. The Framingham general cardiovascular risk score (FGCRS) in males at 5 years post-SCT projected a doubling (median 10.4% vs. 5.4%) in the 10-year risk of cardiovascular events. Females received HRT post-SCT and none had increased FGCRS. Chronic GVHD and C-reactive protein were not associated with CVRF at any time point. All CVRF stabilized between 5 and 10 years post-SCT. Thus, SCT survivors have sustained elevations in CVRF. Males have a significantly increased risk of cardiovascular events in their second and third decade post-SCT.
Keywords: allo-SCT, cardiovascular risks, long term survivors
Introduction
As increasing numbers of patients have received hematopoietic stem cell transplants (SCT) and survival rates have improved, the population of individuals surviving decades after SCT has expanded. After allogeneic transplantation, 25% of mortality in the first decade is attributed to treatment related causes [1]. While relapse and chronic graft versus host disease (cGVHD) remain the most common causes of mortality, survivors are also faced with endocrine dysfunction, infertility, osteoporosis, pulmonary, renal and immune dysfunction, gynecologic and ophthalmologic problems for many years post SCT [2–7]. Cardiovascular disease, the leading cause of mortality in the general population, is an increasing concern in long term survivors. Due to disease and transplantation related radiation, chemotherapy, immunosuppression, prolonged hospitalizations and deconditioning, the risk of premature death from cardiac complications has been reported to be 2.3 times higher among SCT survivors [1, 8].
Cardiovascular disease is a chronic condition with a long latency between developing vascular damage and acute cardiovascular events. Symptomatic cardiovascular disease may manifest decades after transplantation, making assessment of the relationship of cardiovascular events to SCT difficult. Alternatively, cardiovascular risk factors are detectible earlier and predict the course of cardiovascular disease. In post-transplant survivors, the well-established risk factors (smoking, hypertension, obesity, diabetes, dyslipidemia, family history, sedentary lifestyle) couple with transplant related factors to increase cardiovascular disease risk [9]. These factors include cumulative exposure to chest irradiation, anthracyclines, transplant conditioning regimen (myeloablative or non-myeloablative), GVHD, endocrine dysfunction and prolonged immunosuppressive therapy.
This study was conducted to evaluate the cardiovascular disease risk in a cohort of long-term allogeneic hematopoietic stem cell transplant survivors and to understand better the trends and associations between the various risk factors with time after transplant.
Patients and Methods
Patients & Study design
We evaluated the cardiovascular risk profiles in 109 individuals who underwent allogeneic SCT at the National Institutes of Health between 1993 and 2006. All patients surviving beyond a 3-year post-transplant landmark gave written informed consent to long-term evaluation and follow-up on a natural history protocol (NHLBI 05-H-0130; ClinicalTrials.gov Identifier NCT00106925). The survivors are followed up at regular clinic visits scheduled at years 3, 5, 7, 10, 15 and 20 post-SCT beginning in 2005. We conducted cross-sectional analyses at 5 and 10 years post-transplant to estimate the burden of cardiovascular risk in this population; when most survivors are off transplantation-related medications. At the time of analysis, 64 survivors were at the 5-years post-SCT time-point; 10 survivors had been followed-up starting at 10-years post-SCT and 35 survivors had both the 5 and 10-year visits recorded. Median ages at transplant, 5 and 10 year follow-up were 34, 40 and 46 years, respectively. All females received hormone replacement therapy post-SCT. Patient and transplant characteristics with outcomes are detailed in Table 1. Overall median follow up was 10.2 years.
Table 1.
Patient characteristics at SCT and outcomes
| Total number of survivors, n | 109 | ||
| Median age in years (range) | At SCT | 34 | (7–66) |
| Gender, n (%) | Males | 62 | (57%) |
| Females | 47 | (43%) | |
| Diagnosis at SCT, n (%) | CML | 56 | (51%) |
| Acute leukemia | 29 | (27%) | |
| MDS | 13 | (12%) | |
| Others | 11 | (10%) | |
| Conditioning intensity, n (%) | Fully ablative | 99 | (91%) |
| Reduced intensity | 10 | (9%) | |
| Graft source, n (%) | Bone marrow | 15 | (14%) |
| Peripheral blood | 94 | (86%) | |
| No. of survivors at follow-up, n (%) | 5 years post-SCT | 64 | (59%) |
| 10 years post-SCT | 45 | (41%) | |
| 5 and 10 years post-SCT | 35 | (32%) | |
| Median age in years (range) | 5 years post-SCT | 40 | (12–71) |
| 10 years post-SCT | 46 | (17–66) | |
| Outcomes at time of analysis, n (%) | Alive | 97 | (89%) |
| Deaths | 12 | (11%) | |
| Causes of death, n (%) | Relapse | 4 | (33%) |
| cGVHD with multi-organ failure | 2 | (17%) | |
| Secondary malignancy | 2 | (17%) | |
| Infection/sepsis | 2 | (17%) | |
| Brain hemorrhage | 1 | (8%) | |
| Respiratory failure | 1 | (8%) |
Data was collected from electronic medical records and retrospective chart review. Only informative subjects were included in the analyses, subjects with incomplete or missing data were excluded. Since an age-matched control group was not available, we used general population based studies as comparators [10–13]. Standard clinical definitions were used to diagnose hypertension[14] and diabetes[15]. Obesity was defined as BMI >30 kg/m2 [16]. High sensitivity assays for C-reactive protein (CRP) were performed after 2009. The general cardiovascular 10-year risk calculator available online through the Framingham Heart Study[17] was used to calculate the cardiovascular risk score and heart/vascular age. Framingham risk score calculation was limited to survivors older than 30 years. Dyslipidemia estimation using the Adult Treatment Panel III guidelines was limited to survivors older than 20 years [18].
Statistical Analysis
The categorical risk factors: hypertension, diabetes and chronic GVHD were analyzed using the Chi-square or Fischer exact test and the continuous risk factors: CRP, low density lipoprotein (LDL), high density lipoprotein (HDL) levels, Body Mass Index (BMI) and Framingham risk score were analyzed using the t-test or 1-way ANOVA. Paired t-tests were used to compare the 5-year and 10-year post-transplant observations to the pre-transplant baseline and the cardiovascular risk score to age and sex matched normal values, obtained from the Framingham general cardiovascular risk calculator. The Spearman test was used for correlation. Statistical significance was considered when p<0.05. All statistical analyses were performed using Prism 5.03 (GraphPad Software, Inc. La Jolla, CA, USA).
RESULTS
Cardiovascular events
Three cardiovascular events had been reported at the time of analysis (incidence 2.8%). Out of a total of twelve deaths, only one was due to a cardiovascular cause. This male patient, with a family history of maternal death from myocardial infarction and a history of coronary artery disease requiring percutaneous coronary intervention (PCI) on two occasions 11 and 12 years post-SCT, died of hemorrhagic stroke 16 years post SCT.
Two other male survivors required PCI for coronary artery disease prior to 10 years post SCT. There were no other cardiac or vascular events.
Serial electrocardiograms revealed some new changes compared to the baseline pre-transplant EKG in 27 survivors at 5 years post SCT [prolonged QTc (7), sinus bradycardia (5), intraventricular conduction delay (4), accelerated AV conduction (2), accessory pathway (1), ventricular bigeminy (1), ectopic atrial rhythm (1), possible infarct (3) and borderline ST elevation (3)] and in 7 survivors at 10 years post SCT [sinus bradycardia (2), prolonged QTc (2), intraventricular conduction delays (2), atrial fibrillation (1)].
Serial echocardiograms were used to estimate the left ventricular ejection fraction (LVEF). LVEF at 5 years post-SCT vs. baseline showed significant improvement (p<0.001) which can be attributed to the pre-transplant disease status and chemotherapy. LVEF at 10 years showed no worsening compared to pre-transplant baseline (p=0.17) or 5 years post-SCT (p=0.97).
Cardiovascular Risk Factors
Smoking
Nineteen subjects had a documented history of smoking prior to transplant. At the 5-year post-SCT follow-up, six of these continued smoking.
Hypertension
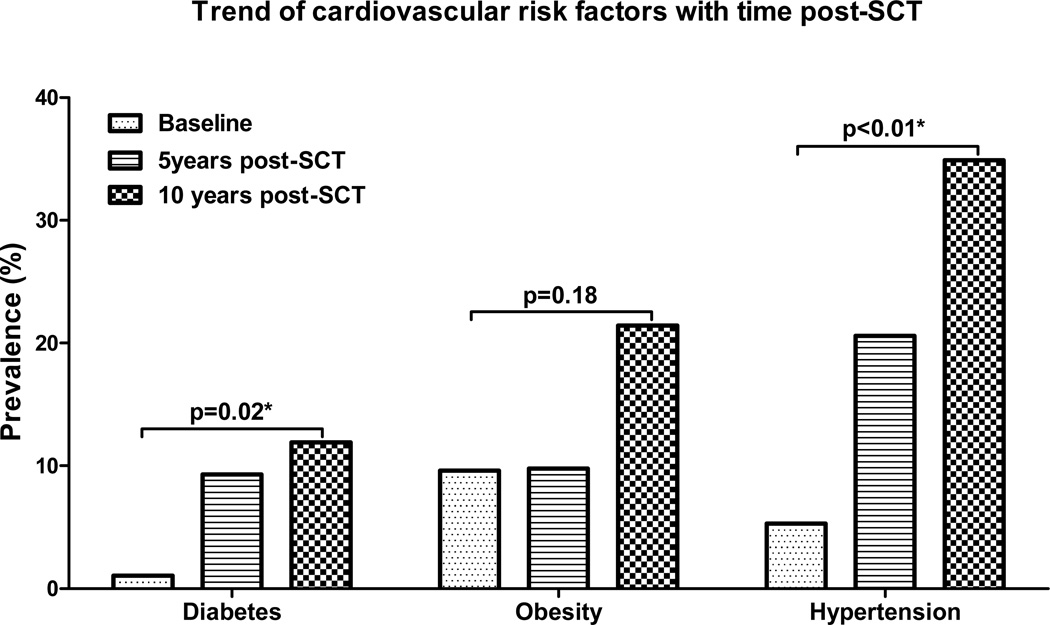
Five of 95 informative subjects (5.3%) had a history of hypertension prior to transplant and four of these were on antihypertensive medications pre-transplant. At 5-years post-SCT, 20 of 97 survivors (20.6%) had hypertension, 17 of whom were receiving antihypertensives. The prevalence of hypertension was 34.9% at the 10-years post-SCT time-point (15 of 43 informative subjects), with 14 survivors were on treatment. The increases in prevalence at 5 and 10 years post-SCT were significant (p<0.01) when compared to baseline [Figure 1] but the prevalence of hypertension did not change significantly between the 5 and 10-year time points (p=0.09). The increased prevalence of hypertension in transplant survivors was comparable to the age-matched normal population [10].
Figure 1.
Prevalence trends of cardiovascular risk factors at pre-transplant baseline, 5 and 10 years post-transplant. *Statistically significant.
Diabetes
Only one of the 95 informative patients had diabetes mellitus pre-transplant while 9 out of 97 (9.3%) and 5 out of 42 (11.9%) survivors had diabetes at 5 and 10-years post-SCT follow up respectively (p=0.02) [Figure 1]. However, the increase in diabetes prevalence was not significantly different than the age-matched normal population [12].
Body Mass Index and Obesity
Mean BMIs (95% CI) at pre-transplant baseline, 5 and 10-years post-SCT were 24.8 kg/m2 (23.7 kg/m2–25.8 kg/m2), 25.2 kg/m2 (24.2 kg/m2–26.3 kg/m2), 27.34 kg/m2 (25.2 kg/m2–29.5 kg/m2), respectively. BMI at 5 and 10 years was significantly increased compared to baseline (p=0.04). The prevalence of obesity (BMI>30kg/m2) was 9.6% pre-transplant, 9.8% at 5 years post-SCT and 21.4% at 10 years post-SCT [Figure 1]. This increase with time was not significantly different from the normal population [11, 19].
Dyslipidemia
Using the Adult Treatment Panel III (ATP III) criteria, individuals with one or more of the following: LDL>130 mg/dl, HDL<40 mg/dl or total cholesterol >200 mg/dl were considered to have an abnormal lipid profile. Forty-four percent of the survivors at 5-years and 52% at 10-years post-SCT had abnormal lipid profiles. Since pre-transplant baseline lipid values were not available, comparisons to baseline could not be made. The ATP III treatment guidelines were used to identify individuals having dyslipidemia meeting the threshold for lipid-lowering drug therapy. At 5-years post-transplant, 23% of survivors [median age 41 (range 20–71) years] had dyslipidemia requiring therapy. We compared the findings in our post-transplant population to dyslipidemia prevalence in persons free of known clinical cardiovascular disease at baseline as reported by the Multi-Ethnic Study of Atherosclerosis (MESA). In the 45–54 year age group, 27.3% of survivors had dyslipidemia requiring therapy which was substantially higher when compared to a prevalence of 15.5% reported by MESA in the same age group.[13] At 10-years post-transplant, of the 35 patients evaluated, 17% had dyslipidemia meeting the ATP III lipid-lowering drug treatment threshold.
Predicted 10-year risk of cardiovascular events
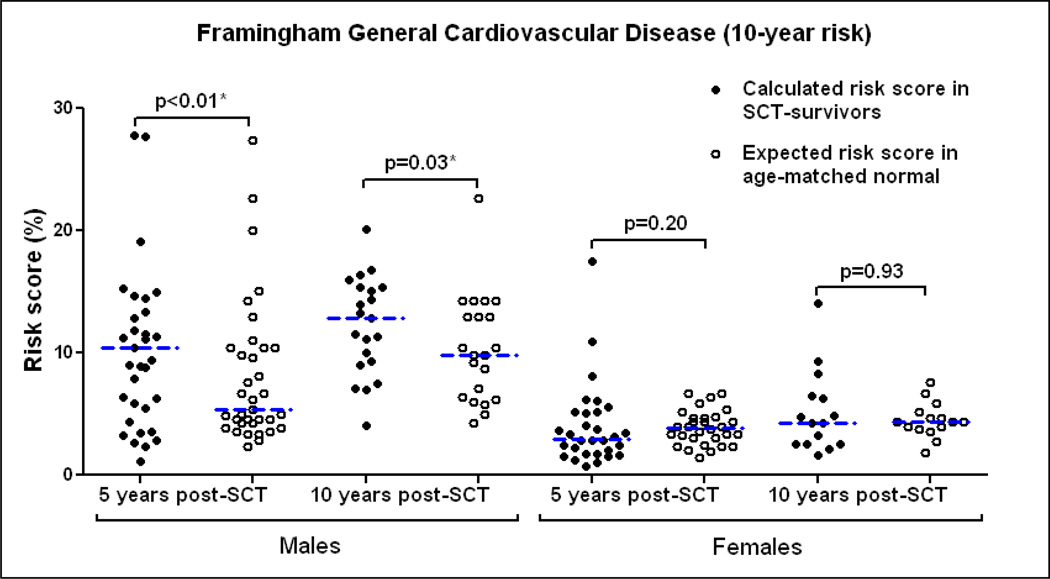
The Framingham Heart Study’s General Cardiovascular Disease (10-year risk) calculator is an online tool predicting the risk of cardiovascular disease [17]. The model uses input variables (age, diabetes, smoking, treated and untreated systolic blood pressure, total cholesterol and HDL cholesterol) to calculate the 10-year risk of developing a cardiovascular event (cardiac: coronary death, myocardial infarction, coronary insufficiency, angina or heart failure, CNS: ischemic stroke, hemorrhagic stroke and transient ischemic attack, and peripheral arterial disease) in individuals between 30 and 74 years of age without cardiovascular disease at baseline. Results are represented as the individual’s risk and calculated heart/vascular age as well as the expected normal risk [20].
At 5-years post-SCT, the median (range) 10-year general cardiovascular risk score for males was found to be 10.4% (1.1%– 56%) vs. 5.4% (2.3–27.4%) expected normal score in individuals matched for age and sex (p<0.01). The calculated risk score for females 3% (0.7%– 17.5%) was comparable to the expected normal score 3.9% (1.4%– 6.7%) (p=0.20). At 10-years post-SCT, the calculated risk score for males [12.8% (4.1%–20.1%)] continued to be higher than expected [9.8% (4.3%– 22.6%)] (p=0.03) while the females did not show an increased risk compared to normal [4.3% (1.6%–14.1%) vs. 4.4% (1.8%–7.6%)] (p=0.93) [Figure 2]. Older age at transplant (>40 years) led to higher cardiovascular risk score post SCT (p<0.001) but the elevation in cardiovascular risk scores compared to normal was not affected by age (p=0.82).
Figure 2.
Framingham General Cardiovascular Disease 10-year risk: Comparison between SCT-survivors and age-matched normal scores predicting an increased risk of cardiovascular events in males. *Statistically significant.
The calculated heart/vascular age was also found to be significantly higher compared to the chronological age for males at 5-years (median 50 vs. 42 years, p<0.001) and 10-years (median 54 vs. 49 years, p=0.04) post-SCT while it was not significantly different for females at 5 or 10 years post-SCT. Thus, at 5-years post-SCT, male survivors had an approximate doubling in the 10-year risk of developing a cardiovascular event and their heart/vascular age was 8 years more than their actual age.
Indicators of inflammation
Chronic GVHD
Survivors were classified according to the presence or absence of chronic GVHD. Ninety four survivors (86.2%) developed cGVHD including 36 (33%) who had significant cGVHD defined as cGVHD requiring immunosuppressive treatment for more than 3-years post-SCT [4–6]. Stratification by cGVHD did not reveal any significant differences in individual cardiovascular risk factors at 5 and 10-years post-SCT: hypertension (p=0.34), diabetes (p=0.72), CRP (p=0.44, p=0.58), HDL (p=0.02, p=0.72) and LDL (p=0.69, p=0.06). Presence or absence of cGVHD and/or significant cGVHD did not affect the 10-year risk of cardiovascular events (p=0.53 at 5-years post-SCT, p=0.87 at 10-years post-SCT).
C - reactive protein
CRP is an inflammatory marker potentially linked to underlying atherosclerosis and is considered an adjunct to screening for traditional cardiovascular risk factors. For the determination of cardiovascular risk, low, average, and high risk CRP values are defined as <1, 1 to 3, and >3 mg/L [21]. Mean CRPs (95% CI) at baseline, 5-years and 10-years were 5.3 mg/L (4.8 mg/L – 5.8 mg/L), 5.7 mg/L (3.8 mg/L–7.6 mg/L) and 4.5 mg/L (2.6 mg/L–6.4 mg/L), respectively. There was no significant difference between the CRP values at pre-transplant baseline, 5 and 10-years post-SCT (p=0.13). The CRP values did not show any association with the individual cardiovascular risk factors or calculated cardiovascular risk score.
Discussion
Extrapolating from data available from the Center for International Blood and Marrow Transplant Research (CIBMTR) [22], a conservative estimate would suggest that there will be more than 500,000 SCT recipients worldwide surviving a decade or more post SCT. This growing population of individuals surviving the initial morbidities and mortality associated with SCT deserves increasing attention and supervision for less well characterized long-term complications. Our study showed that there is a persistent elevation in the cardiovascular risk profile in long term survivors of allogeneic hematopoietic stem cell transplantation even at 10-years post-SCT [Figures 1 and 2]. Although, the risk factors stabilized between the 5 and 10-year post-SCT time points and few cardiovascular events occurred, the increased cardiovascular risk scores indicate that if risk factors are not well controlled, more events are likely with longer follow-up. Male survivors are significantly more at risk. The prevalence of dyslipidemia meeting drug treatment thresholds is higher and occurs earlier in the post-SCT survivors compared to the normal population and is likely the major driver for the elevation of the Framingham general cardiovascular risk scores. cGVHD and CRP did not seem to influence any of the risk factors. The long follow-up duration, the serial comparison between 5 and 10 years, and the utilization of the Framingham risk score are unique aspects of this study.
Cardiovascular risk factors are not static and prevalence is expected to increase with age. Survivor studies on transplant populations need to take into account this natural progression of cardiovascular risk factors. Our estimates of increasing prevalence of hypertension, diabetes and obesity reflect a significant worsening compared to baseline but are not surprising when compared to normal population estimates [10–12]. This suggests that caution must be exercised in interpreting time-dependent conditions in the post-transplant population. However, the increase in dyslipidemia was substantially higher than the normal population.
Accurate estimates of cardiovascular events post-SCT are methodologically constrained by the requirement for large data sets, biases in ascertainment, complicated choice of controls and the requirement for long term follow-up. A retrospective cohort study found an increased cardiovascular death rate of 3.6 per 1000 patient years in 1491 transplant survivors using a Washington State database[23]. Bhatia, et al. have shown a 2.3-fold risk of premature death due to cardiac complications in 854 allogeneic SCT survivors compared with the general population at a median follow-up of 9.5 years [1]. Tichelli et al. reported a 3.6% incidence of cardiovascular events in at least one arterial territory at a median age of 54 years in a study of 548 patients from the European Group for Blood and Marrow Transplantation. The cumulative incidence of a first arterial event 15 years after hematopoietic stem cell transplantation was 6% [24]. In comparison, the incidence of cardiovascular events in our cohort was 2.8% which can be explained by the relatively younger age of the patient population and smaller sample size. More events are likely to occur with longer follow-up.
A comparative study of cardiovascular risk in recipients of allogeneic vs. autologous hematopoietic stem cell transplantation reported a higher risk (6.92-fold increased relative risk) of premature arterial vascular disease in allogeneic transplant survivors. The relative risk for an arterial event was significantly higher for allogeneic HSCT, older age at time of transplant and higher cardiovascular risk factor score, but not gender [25]. In our study, the few events that were reported occurred in male survivors and the calculated cardiovascular risk score indicated that males were more likely to develop cardiovascular events compared to females. Hormone replacement therapy in females may have influenced their cardiovascular risk. This suggests that underlying endocrine insufficiencies may play a role in the heightened cardiovascular risk profiles in males [26, 27].
Established cardiovascular risk factors (obesity, dyslipidemia, hypertension and diabetes) in the general population also impact cardiovascular events in long-term post-SCT survivors [28]. We show that risk factor prevalence increases significantly in the first decade post-SCT and sometimes to a greater extent than in the age-matched general population. Though not seen in our cohort, higher age-BMI-adjusted risks of diabetes and hypertension in transplant survivors compared to their siblings have been reported [29, 30]. Allogeneic transplant related factors such as chest radiation and steroid use may therefore significantly enhance cardiovascular risk in the background beyond what is detectable by the Framingham risk scores. We found that cGVHD did not have an impact on cardiovascular risk as demonstrated previously[23–25, 28–30] and CRP which is considered to be an important biomarker predicting risk of cardiovascular events did not correlate with the heightened cardiovascular risk profiles. We also show that the elevation of cardiovascular risk remains sustained for a prolonged period. Our findings emphasize the need for continued monitoring beyond the early transplant period during which fluctuations in blood pressure and diabetes are frequently seen [31].
There are limitations inherent in our retrospective study of mostly younger transplant subjects. Data on other known risk factors such as family history and physical inactivity was unavailable for all patients and history of smoking may have been under-reported. The majority of our patients received full dose total body irradiation (TBI) based conditioning; therefore the association of cardiovascular risk with conditioning intensity could not be evaluated. We did not have data from age matched controls; hence analysis was done using general population study results as the comparison group. Patients who have undergone a complex therapeutic procedure such as SCT are more likely to have access to medical care and systemic biases may have affected our study with longer follow-up of survivors with more severe health issues.
In conclusion, there are persistent and prolonged elevations in cardiovascular risk factors in long term survivors of allogeneic hematopoietic stem cell transplantation without a significant change between the 5 and 10-year post-SCT time points. The few cardiovascular events noted and elevations in cardiovascular risk score indicate that even longer follow-up and tight lipid control is necessary in this patient population. Males are at a higher risk of cardiovascular disease compared to females. In view of the lack of association with cGVHD and CRP, further studies are necessary to identify the pathogenic mechanisms responsible in this setting.
Acknowledgments
Support:
This work was supported by the intramural research program of the NIH Clinical Center and NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Declaration:
Priyanka A Pophali, MD1 - None
Jeffrey K Klotz, MD1- None
Sawa Ito, MD1- None
Eleftheria Koklanaris, RN, BSN1- None
Natasha A Jain, MD1- None
Robert Q Le, MD, PhD1- None
Christopher S. Hourigan, MD, DPhil1- None
Bipin N. Savani, MD2- None
Kamna Chawla, MD, MS1- None
Sujata Shanbhag, MD3- None
A. John Barrett, MD1- None
Minoo Battiwalla, MD, MS1- None
Authorship Contributions: P.A.P., A.J.B. and M.B. designed the study; P.A.P., K.C., J.K.K., R.Q.L and B.N.S collected data; P.A.P, A.J.B. and M.B. analyzed and interpreted the data; J.K.K., S.I., E.K., R.Q.L., B.N.S., A.J.B and M.B. took care of patients; P.A.P., C.S.H., S.S., A.J.B. and M.B. wrote the manuscript. P.A.P. and M.B. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savani BN, Griffith ML, Jagasia S, Lee SJ. How I treat late effects in adults after allogeneic stem cell transplantation. Blood. 2011;117:3002–3009. doi: 10.1182/blood-2010-10-263095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savani BN, Donohue T, Kozanas E, et al. Increased risk of bone loss without fracture risk in long-term survivors after allogeneic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:517–520. doi: 10.1016/j.bbmt.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 4.Savani BN, Koklanaris EK, Le Q, Shenoy A, Goodman S, Barrett AJ. Prolonged chronic graft-versus-host disease is a risk factor for thyroid failure in long-term survivors after matched sibling donor stem cell transplantation for hematologic malignancies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:377–381. doi: 10.1016/j.bbmt.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savani BN, Montero A, Srinivasan R, et al. Chronic GVHD and pretransplantation abnormalities in pulmonary function are the main determinants predicting worsening pulmonary function in long-term survivors after stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12:1261–1269. doi: 10.1016/j.bbmt.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savani BN, Stratton P, Shenoy A, Kozanas E, Goodman S, Barrett AJ. Increased risk of cervical dysplasia in long-term survivors of allogeneic stem cell transplantation--implications for screening and HPV vaccination. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:1072–1075. doi: 10.1016/j.bbmt.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rovo A, Tichelli A, Passweg JR, et al. Spermatogenesis in long-term survivors after allogeneic hematopoietic stem cell transplantation is associated with age, time interval since transplantation, and apparently absence of chronic GvHD. Blood. 2006;108:1100–1105. doi: 10.1182/blood-2006-01-0176. [DOI] [PubMed] [Google Scholar]
- 8.Savani BN. How can we improve life expectancy and quality of life in long-term survivors after allogeneic stem cell transplantation? Seminars in hematology. 2012;49:1–3. doi: 10.1053/j.seminhematol.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Rovo A, Tichelli A. Cardiovascular complications in long-term survivors after allogeneic hematopoietic stem cell transplantation. Seminars in hematology. 2012;49:25–34. doi: 10.1053/j.seminhematol.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie M C, Kuklina EV, MD, PhD, Briss PA, MD, Blair NA, MPH, Hong Y., MD, PhD Vital signs: prevalence, treatment, and control of hypertension--United States, 1999–2002 and 2005–2008. MMWR Morbidity and mortality weekly report. 2011;60:103–108. [PubMed] [Google Scholar]
- 11.Flegal K, Carroll M, Kit B, Ogden C. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA (Chicago, Ill) 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 12.Percentage of Civilian, Noninstitutionalized Population with Diagnosed Diabetes, by Age, United States, 1980–2010. 2010 [Google Scholar]
- 13.Goff DC, Jr, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–656. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BCWHO; http://www.who.int/bmi/index.jsp?introPage=intro_3.html) [Google Scholar]
- 17.Framingham Heart Study: General Cardiovascular Disease (10-year risk) [Google Scholar]
- 18.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 19.Williams PT, Wood PD. The effects of changing exercise levels on weight and age-related weight gain. International journal of obesity. 2006;30:543–551. doi: 10.1038/sj.ijo.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 21.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 22.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Annals of internal medicine. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 24.Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 25.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 26.Gunasekaran U, Agarwal N, Jagasia MH, Jagasia SM. Endocrine complications in long-term survivors after allogeneic stem cell transplant. Seminars in hematology. 2012;49:66–72. doi: 10.1053/j.seminhematol.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. European journal of endocrinology / European Federation of Endocrine Societies. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 28.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rovo A, Daikeler T, Halter J, et al. Late altered organ function in very long-term survivors after allogeneic hematopoietic stem cell transplantation: a paired comparison with their HLA-identical sibling donor. Haematologica. 2011;96:150–155. doi: 10.3324/haematol.2010.030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majhail NS, Challa TR, Mulrooney DA, Baker KS, Burns LJ. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1100–1107. doi: 10.1016/j.bbmt.2009.05.010. [DOI] [PubMed] [Google Scholar]