Advanced parental age at childbirth has been identified as a risk factor for a number of developmental disorders, including Down’s syndrome,1 Marfan’s syndrome,2 achondroplasia3 and even some cancers.4 There has been considerable recent interest in the potential role of parental age, particularly paternal age, as a risk factor for autism5 and for schizophrenia.6 While claims of an association of parental age and risk for schizophrenia have been made for over 50 years, the issue attracted serious attention after a report by Malaspina et al.6 in a sample of 638 Israeli patients with schizophrenia, which found that the age of their fathers at childbirth significantly increased the odds of their being ill, by upwards of 1.2-fold per decade of advancing age. A number of subsequent studies have provided additional evidence for this association, which in a recent meta-analysis has been found to represent an overall increase of risk of ~1.3-fold, especially for fathers who are 55 years or older at childbirth.7 Maternal age tends not to be significantly associated with risk for schizophrenia.6 The popular interpretation of these data has been that de novo mutations, which are known to become more abundant in sperm of older men,2 are the mechanism. Indeed, a recent high-profile publication by Kong et al.8 demonstrated, in a sample of 78 families, a linear relationship between paternal age and the number of de novo mutations in offspring, with approximately two de novo mutations per year of advancing paternal age. Kong et al.8 interpret their data in the context of evidence that older fathers have increased risk of offspring with developmental disorders such as autism and schizophrenia, suggesting that they are causally linked.
There has been considerable front-page mass media attention to the possible conclusion that the two associations (that is, paternal age and risk for schizophrenia and paternal age and de novo mutations) are linked causally, including suggestions that younger males should consider sperm cryopreservation for later parenting (for example, NY Times, 22 August 2012). Despite the intuitive appeal of this causal link, it is conceivable that these two associations, though each valid, may be unrelated. In fact, they have not been linked in the same data, and the conclusion of a causal link is still premature. Indeed, other possibilities for the association with paternal age merit consideration. For example, a recent study of over 2.2 million people in Denmark replicated the paternal age effect on risk for schizophrenia, but suggested that de novo mutations could not account for it.9 These investigators found that the association was explained by the age of the father at the birth of his first child, not by the age at which the father had a child who developed schizophrenia. It is thus conceivable that some fathers have inherited traits that are linked both to higher risk for schizophrenia and to difficulty or slowness in becoming married, or perhaps to parenting. Moreover, there is evidence that very young paternal age also increases risk for schizophrenia, a finding that would not be explained by age-associated de novo mutations.7
Another approach to elucidate the potential role of age-related de novo mutations and risk for schizophrenia is to explore birth order within families. If de novo mutations inherited from fathers are a causative factor for schizophrenia, affected individuals would be more likely to be later born children, particularly in families with older fathers. Interestingly, for schizophrenia, epidemiological studies do not support an overall birth-order effect.10 We tested the association of paternal age and birth order of offspring with schizophrenia in 463 families with an affected offspring and multiple biological siblings (range 2–13). Overall, 155 offspring with schizophrenia were born first and 188 were born last. A null univariate effect of birth order in families with two offspring—the subject with schizophrenia was first born in 97 and second born in 106 (P = 0.575)—echo those of earlier epidemiological studies but are clearly of limited power in this subsample of our family data set.
However, statistical models that incorporate father’s age at the birth of an affected offspring in all the families suggest a paternal age association with birth order. Categorizing paternal age groups consistent with prior literature suggests that birth-order patterns between paternal aged groups are significantly different (Table 1A, P = 1.92×10−4). More generally, the overall correlation in the entire sample between birth order of affected offspring and paternal age, corrected for family size, is highly significant (P = 1.26×10−13). Our results are tempered by sample size and potentially unknown ascertainment biases, but they are consistent with the expected effects of de novo mutations on risk for schizophrenia within families.
Table 1.
Parental age versus birth order
| Birth order |
Total | |||
|---|---|---|---|---|
| First | Middle | Last | ||
| A | ||||
| 30 years or younger | 95 | 45 | 54 | 194 |
| 31–40 years | 27 | 41 | 78 | 146 |
| Older than 40 years | 5 | 7 | 21 | 33 |
| Total | 127 | 93 | 153 | 373 |
| B | ||||
| 25 years or younger | 57 | 32 | 20 | 109 |
| 26–35 years | 43 | 45 | 93 | 181 |
| Older than 35 years | 2 | 10 | 24 | 36 |
| Total | 102 | 87 | 137 | 326 |
Categorized (A) paternal age and (B) maternal age versus birth order. χ2-tests for youngest versus oldest categories and first versus last born (gray cells, thus reducing to a 2 × 2 table) suggest a birth-order effect in both tables (A: P = 1.92 × 10−4, B: P = 1.32 × 10−8).
Because paternal and maternal ages are correlated in our families (r = 0.70, P < 2 × 10−16), we next considered the possibility that parity and illness status are primarily linked to maternal age, which would implicate a cause of birth-order risk other than de novo mutations. Categorizing maternal age into three typical bins resulted in birth-order patterns that are highly significantly different (Table 1B, P = 1.33 × 10−8). Moreover, overall proband birth order was much more highly correlated with maternal age (P < 2.16 × 10−16) than paternal age, and when both are entered into a multiple regression (adjusting for family size), only maternal age has a significant effect (P = 7.53 × 10−16); paternal age does not (P = 0.995). This statistical approach is the de facto method for untangling highly correlated maternal and paternal age effects in the literature.8
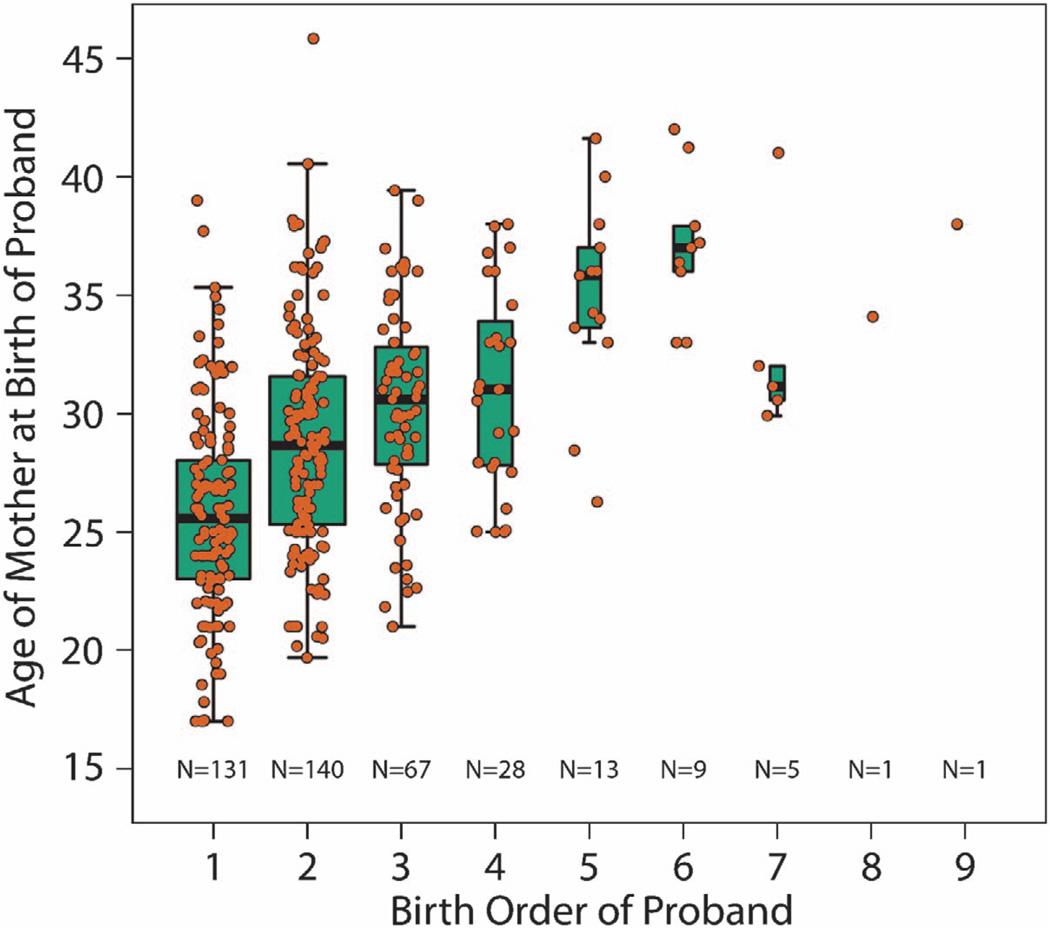
The strong maternal age association might suggest that parity adversely influences intrauterine development, and obstetrical complications (OCs) are also a risk factor for schizophrenia.11 Quantitative ratings of OCs were available for 197 probands, and were actually associated with lower birth order (P = 6.84 × 10−5), consistent with the generally greater frequency of complications in first pregnancies and perhaps a factor in the increased risk of illness in the first-born children of younger mothers. Still, increasing maternal age was independently associated with higher proband birth order even in this smaller subset of subjects (Figure 1, P = 1.81 × 10−15).
Figure 1.
Plot of maternal age at birth of proband versus his or her birth order. Each point (orange) represents the proband from one family. The boxplots demonstrate the interquartile range of maternal ages for all families that contain a proband born at that particular order. There is a strong linear relationship between age of the mother at the birth of the schizophrenic proband within her family and the birth order of that child, even after adjusting for the number of children in the family (P < 2.16 × 10−16).
Our results in families, while not implicating a specific cause of the paternal age association with risk for schizophrenia, support the Danish study9 in suggesting that de novo mutations do not play a prominent role. It is important to emphasize that our data do not speak to the validity of the paternal age association with risk for schizophrenia, which is assumed to be valid. The parental age effects may, for instance, increase risk for schizophrenia through social factors related to parenting,9 or more generally, to epigenetic mechanisms associated with the developmental environment, both intrauterine and postnatal. Our data suggest, however, that the principal mechanism of this association is not de novo mutations. Clearly, more work is needed to determine the degree to which the association of schizophrenia with paternal age is due to paternally derived de novo mutations or to a cause linked to other factors related to childbearing in later middle age or to a combination of both.
ONLINE METHODS
Data were from 463 multiple offspring families studied by the Weinberger Lab at the National Institutes of Health between 1996 and 2011, in which birth order was known.12 These families were part of the larger Clinical Brain Disorders Branch (CBDB)/NIMH Sibling Study (DR Weinberger, PI). Briefly, patients with a history of schizophrenia, their full siblings and normal comparison subjects who had no first-degree relatives with schizophrenia were recruited from local and national sources based on referrals and national advertisement. Additional details on ascertainment of the NIMH Sibling Study are provided in the studies by Huffaker et al.12 and Egan et al.13 Of the 463 families included in this study, there were 371 families with known father’s age at proband birth and 395 with mother’s age. A binomial test assessed the difference between the first and last born in families with two children. Fisher’s exact test compared proportions of the first and last born in defined paternal and maternal age groups, and linear regression adjusting for family size was used for proband birth order versus parental age. OCs were derived from maternal history using the McNeil Scale as previously described.11
ACKNOWLEDGEMENTS
We thank Sally Cheung for her tireless help with assembling the birth-order data. This study was supported by the Lieber Institute for Brain Development and NIMH IRP funding of the Weinberger Lab.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
DRW and AEJ planned the study; DRW and SM collected the data; all authors contributed to the data analysis and writing of the manuscript.
REFERENCES
- 1.Erickson JD. Ann Hum Genet. 1978;41:289–298. doi: 10.1111/j.1469-1809.1978.tb01896.x. [DOI] [PubMed] [Google Scholar]
- 2.Risch N, Reich EW, Wishnick MM, McCarthy JG. Am J Hum Genet. 1987;41:218–248. [PMC free article] [PubMed] [Google Scholar]
- 3.Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, et al. Nature. 1994;371:252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 4.Johnson KJ, Carozza SE, Chow EJ, Fox EE, Horel S, McLaughlin CC, et al. Epidemiology. 2009;20:475–483. doi: 10.1097/EDE.0b013e3181a5a332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, et al. Arch Gen Psychiatry. 2006;63:1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 6.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, et al. Arch Gen Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 7.Miller B, Messias E, Miettunen J, Alaräisänen A, Järvelin MR, Koponen H, et al. Schizophr Bull. 2011;37:1039–1047. doi: 10.1093/schbul/sbq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, et al. Nature. 2012;488:471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen L, Mortensen PB, Pedersen CB. Am J Psychiatry. 2011;168:82–88. doi: 10.1176/appi.ajp.2010.10020252. [DOI] [PubMed] [Google Scholar]
- 10.Kemppainen L, Veijola J, Jokelainen J, Hartikainen AL, Järvelin MR, Jones P, et al. Acta Psychiatr Scand. 2001;104:148–152. doi: 10.1034/j.1600-0447.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- 11.Nicodemus KK, Marenco S, Batten AJ, Vakkalanka R, Egan MF, Straub RE, et al. Mol Psychiatry. 2008;13:873–877. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- 12.Huffaker SJ, Chen J, Nicodemus KK, Sambataro F, Yang F, Mattay V, et al. Nat Med. 2009;15:509–518. doi: 10.1038/nm.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]