Abstract
Krüppel-like factor 8 (KLF8) regulates critical gene transcription associated with cancer. The underlying mechanisms, however, remain largely unidentified. We have recently demonstrated that KLF8 expression enhances the activity but not expression of matrix metalloproteinase-2 (MMP2), the target substrate of MMP14. Here, we report a novel KLF8 to MMP14 signaling that promotes human breast cancer invasion and metastasis. Using cell lines for inducible expression and knockdown of KLF8, we demonstrate that KLF8 promotes MMP14 expression at the transcriptional level. Knocking down KLF8 expression inhibited the breast cancer cell invasion both in vitro and in vivo as well as the lung metastasis in mice, which could be rescued by ectopic expression of MMP14. Promoter reporter assays and oligonucleotide and chromatin immunoprecipitations determined that KLF8 activates the human MMP14 gene promoter by both directly acting on the promoter and indirectly via promoting the nuclear translocation of β-catenin, the expression of T cell factor-1 (TCF1) and subsequent activation of the promoter by the β-catenin/TCF1 complex. Inhibition of focal adhesion kinase (FAK) using pharmacological inhibitor, RNA interference or knockout showed that the cell surface presentation of active MMP14 downstream of KLF8 depends upon FAK expression and activity. Taken together, this work identified novel signaling mechanisms by which KLF8 and FAK work together to promote the extracellular activity of MMP14 critical for breast cancer metastasis.
Keywords: KLF8, FAK, MMP14, β-catenin/TCF1, metastasis, breast cancer
Introduction
Breast cancer metastasis remains the main hurdle of cure. In-depth understanding of the underlying mechanisms has been a mainstream focus of breast cancer research essential for targeting early stage metastatic cells to improve patient survival.
Krüppel-like factor 8 (KLF8) has recently emerged as an important cancer-promoting protein in various type of human cancer including breast cancer (1-8). KLF8 is a dual transcription factor found to target promoters of several oncogenes or tumor suppressor genes for transcriptional activation or repression (1, 2, 4, 6, 9-14). Role of KLF8 in cancer was initially determined when it was identified as a focal adhesion kinase (FAK) downstream effector (13) and subsequently found to be capable of transforming (2, 7). Like FAK, a critical driving force of breast cancer invasion and metastasis (15), KLF8 is highly overexpressed in invasive human cancers including breast cancer and promotes breast cancer cell invasion and metastasis (1, 4, 6) by propelling the cell cycle progression (5, 11-13, 16), transformation (7), and epithelial to mesenchymal transition (EMT) (1, 6). Given these important roles of it, KLF8 is tightly regulated at its transcriptional and post-translational levels as well as its nuclear localization (1, 12, 16-18). The molecular and signaling mechanisms by which KLF8 promotes human breast cancer metastasis have not been investigated. Matrix metalloproteinase (MMP)-promoted extracellular matrix (ECM) degradation or remodeling is known to play a critical role for tumor invasion and metastasis. Among the MMP family proteins, MMP2 and MMP9 have been demonstrated to function downstream of FAK in promoting tumor metastasis (15). Our recent work has identified MMP9 as a direct target of transcriptional activation by KLF8 and a mediator of KLF8-promoted breast cancer cell invasion (4). Along with that finding, we found that KLF8 also upregulates MMP2 enzymatic activity without affecting its expression levels.
In this report, we show evidence supporting an important role of MMP14 downstream of KLF8 in promoting breast tumor invasion and metastasis. We also provide a novel mechanistic model showing how KLF8 works together with FAK to effectively enrich a high level of expression and activity of MMP14 at the cell surface required for breast tumor invasion and metastasis.
Results
KLF8 upregulation of MMP14 is critical for breast cancer cell invasiveness
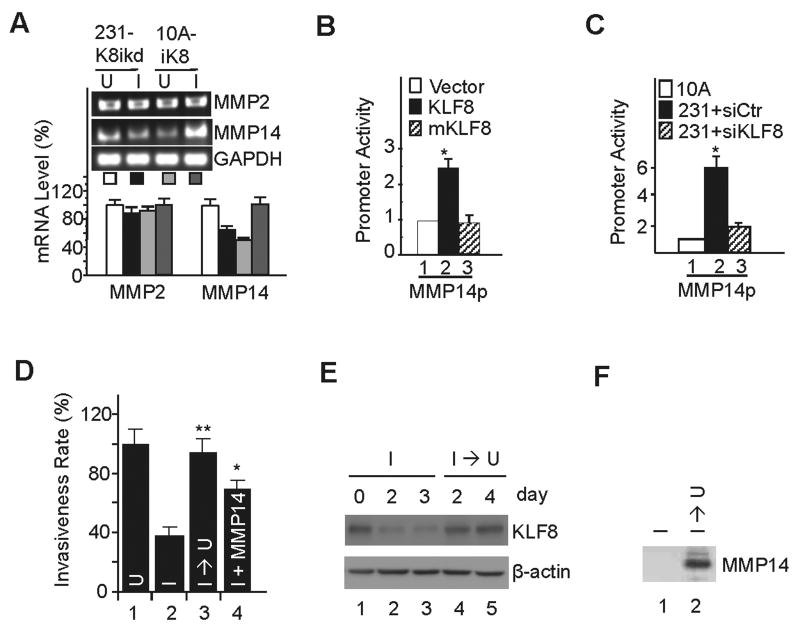
We have recently demonstrated that overexpression of KLF8 in the MCF-10A cells promotes invasion and knockdown of KLF8 in the MDA-MB-231 cells inhibits invasion (6). We also identified MMP9 as a transcriptional activating target of KLF8 in the cells that contributes partially to KLF8-dependent invasion (4). Interestingly, MMP2 activity but not expression is upregulated by KLF8 (4), and the message expression of MMP2-activating enzyme MMP14 was found to be upregulated by KLF8 in both of the cell lines as determined by microarray analysis of KLF8-dependent gene expression profiles (data not shown). We first attempted to verify these results by RT-PCR and qRT-PCR. We found that indeed, overexpression of KLF8 in the 10A-iK8 cells (a MCF-10A line that expresses inducible KLF8) upregulated the message expression of MMP14 but not MMP2, and knockdown of KLF8 expression in the 231-K8ikd cells (a MDA-MB-231 line that expresses inducible KLF8 shRNA) caused a decrease in MMP14 message expression without affecting MMP2 message expression (Figure 1A). These results suggested that MMP14 could be a transactivation target of KLF8 and the KLF8-dependent increase in the expression of MMP14 (and thus the activation of MMP2) may be another mechanism contributing to KLF8-promoted cell invasion.
Figure 1.
KLF8 upregulation of MMP14 is critical for breast cancer cell invasiveness. A, KLF8 promotes mRNA expression of MMP14 but not MMP2. The 231-K8ikd or 10A-iK8 cells were grown under uninduced (U) or induced (I) conditions for 24 h and RNA was prepared for qRT-PCR and RT-PCR (inset). GAPDH was used as an internal control. B, KLF8 activates MMP14 gene promoter (MMP14p). MCF-10A cells were co-transfected with MMP14p luciferase reporter with wild-type KLF8, its activation domain-defective mutant (mKLF8) or control vector. After 16 hr, luciferase activity was measured. C, KLF8 knockdown reduces MMP14 promoter activity in MDA-MB-231 cells. The MDA-MB-231 cells were transfected with control siRNA or KLF8 siRNA for 48 hrs. The MMP14p reporter was then transfected for reporter assays. MCF-10A cells transfected with the MMP14p was included as a normalizing control. D-F, MMP14 mediates KLF8-induced invasiveness. The 231-K8ikd cells were grown for 24 hours under uninduced conditions (U), induced conditions (I), induced conditions followed by uninduced conditions for 4 days (I → U) or induced conditions plus ectopic expression of MMP14 (I + MMP14). The cells were then used for Matrigel invasion assays (see Materials and Methods) (D). **P < 0.01 and *P < 0.05 compared to lane 2. Effective KLF8 knockdown and recovery (E) and MMP14 overexpression (F) were confirmed by Western blotting. Data represent the mean ± S.D. of at least three independent experiments.
To test these possibilities, we performed MMP14 promoter luciferase reporter (MMP14p, (19)) assays. We found that the promoter activity was significantly increased when KLF8 was co-transfected into the MCF-10A cells compared to the vector control (Figure 1B, compare lanes 2 to 1). However, when the transactivation-defective mutant mKLF8 (11) was co-transfected, the promoter activity remained at the control level (Figure 1B, compare lanes 3 to 1). The promoter activity was 5-times higher in the MDA-MB-231 cells that express significantly higher levels of endogenous KLF8 than MCF-10A cells (6, 7) and knockdown of KLF8 in the cells almost completely abolished the promoter activity (Figure 1C, lane 3). This result suggested that activation of MMP14 transcription by KLF8 contributes to the cancer cell invasiveness. To determine the role of MMP14 in the regulation of 231-K8ikd cell invasiveness downstream of KLF8, we performed Matrigel invasion assays. Consistent with previous findings (4, 6), knockdown of KLF8 expression dramatically reduced the cell invasiveness (Figure 1D, compare columns 2 to 1) and this reduction was restored by switching off the KLF8 knockdown (Figure 1D, compare columns 3 to 2). When ectopic MMP14 was overexpressed, the cell invasiveness was also markedly rescued, although not to a full extent, regardless of the KLF8 knockdown (Figure 1D, compare columns 4 to 2 and 3, Figure 1E and 1F).
Taken together these results indicated that KLF8 activation of MMP14 transcription plays a critical role in the promotion of the cell invasion in vitro.
MMP14 is required for KLF8-promoted the lung metastasis of breast cancer
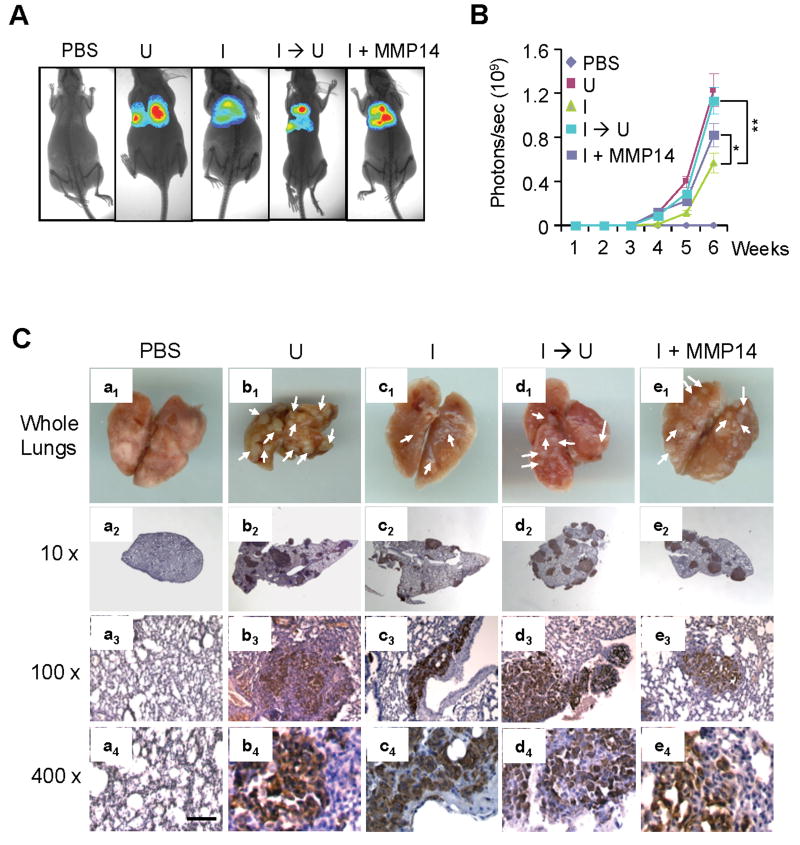
We then determined the role of MMP14 downstream of KLF8 in the lung metastasis of the 231-K8ikd cells (Figure 2). The cells where the expression of KLF8 and MMP14 was genetically modified were injected into the tail veins and the lung metastasis of the cells was monitored and quantified by bioluminescent imaging (BLI) (Figure 2A & 2B). Consistent with previous report (4), when KLF8 knockdown was induced (I), the lung metastasis rate 6 weeks after the injection was inhibited by ∼50% compared to the cells in which the knockdown of KLF8 expression was not induced (U). And this reduction was completely rescued if KLF8 knockdown was switched off at the time of the cell injection (I → U). When ectopic MMP14 was overexpressed, the lung metastasis was markedly recovered, though in part, from the inhibition by KLF8 knockdown (I + MMP14). These results were verified by both macroscopic observation of the whole mount lungs (Figure 2C, a1 – e1) and immunohistochemical (IHC) staining of the lung metastases using anti-human vimentin (Figure 2C, a2 – e4). The expression or knockdown of KLF8 or MMP14 in the tumor metastases was confirmed by IHC staining (Figure S1). Taken together, these results strongly suggested an important role of MMP14 in mediating KLF8-promoted lung metastasis.
Figure 2.
MMP14 is required, at least partially, for KLF8-dependent lung metastasis of breast cancer. A & B, Bioluminescence imaging (BLI) analysis of the lung metastasis. The 231-K8ikd or 231-K8ikd/MMP14 cells were incubated for 3 days under uniduced (U) or induced (I) conditions. The cells were then injected into the tail veins of nude mice. The mice were fed with Dox Diet (I, KLF8 knockdown induced) or Control Diet (U, KLF8 knockdown uninduced). At the indicated weeks, the BLI images of the lung metastasis were taken (A) and quantitatively analyzed (B) as described in the Materials and Methods. **P < 0.01 and *P < 0.05. C, IHC analysis of the lung metastasis. The lung tissue samples were prepared from the mice 6 weeks post-implantation for IHC staining. a1 – e1, whole mount lungs with metastatic nodules pointed to by white arrows. a2 – e4, IHC staining of the lung sections with human vimentin antibody (brown). The nuclei were counter-stained (blue). The expression of KLF8 and MMP14 in the tumors was verified by IHC staining (see Figure S1).
MMP14 is required for the invasive growth of breast cancer in vivo
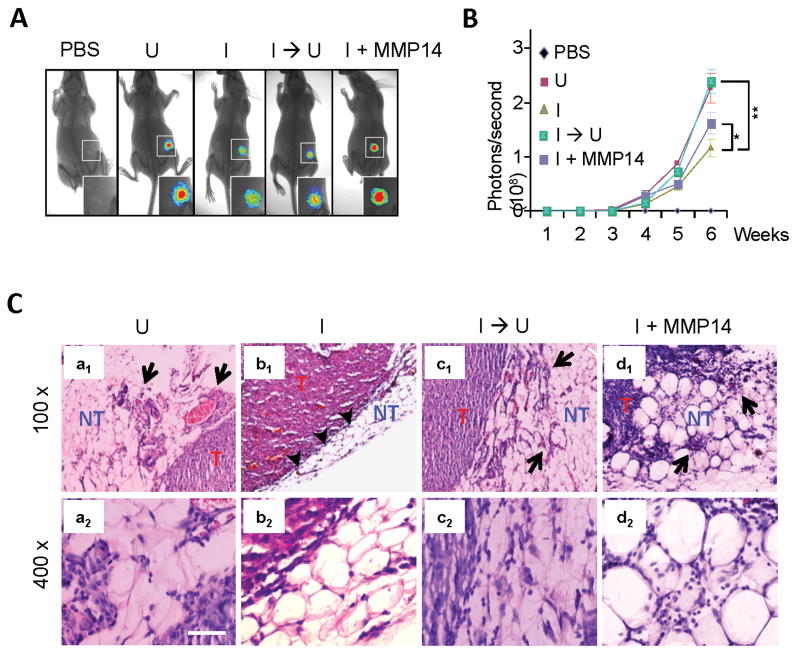
We next determined the role of MMP14 downstream of KLF8 in promoting the invasive tumor growth after orthotopic implantation of the 231-K8ikd cells into the mammary fat pads (Figure 3). BLI analysis indicated that KLF8 knockdown (I) inhibited the tumor growth and this inhibition was restored by switching KLF8 knockdown off (I → U). Regardless of KLF8 knockdown, overexpression of ectopic MMP14 recovered the tumor growth by ∼40% (I + MMP14). Hematoxylin and eosin (H&E) staining revealed that when without KLF8 knockdown (U) the cells were highly invasive as indicated by the tumor nodules breaching the basement membrane and spreading into the non-tumor tissues (Figure 3C, panels a1 & a2). In contrast, KLF8 knockdown (I) protected the integrity of the basement membrane that kept the tumor from spreading (Figure 3C, panels b1 & b2). However, this protection failed if the KLF8 knockdown was switched back off (I → U) (Figure 3C, panels c1 & c2) or ectopic MMP14 was overexpressed (I + MMP14) (Figure 3C, panels d1 & d2). The expression or knockdown of KLF8 or MMP14 in the tumor metastases was confirmed by IHC staining (Figure S2). These results suggested that MMP14 plays a critical role downstream of KLF8 in promoting the invasive breast tumor growth.
Figure 3.
Overexpression of MMP14 restores the invasiveness of breast cancer in vivo. A & B, BLI analysis of the tumor growth. The cell culture was carried out identically as described in Figure 2. The cells were then injected into the mammary fat pads of nude mice as described in the Materials and Methods. The mice were fed and the BLI analysis was done as described in Figure 2. *P < 0.05; **P < 0.01. C, H&E analysis of the mammary tumors. T, tumor tissue; NT, non-tumor tissue; Arrows point to tumor tissue invading the non-tumor tissue; Arrowheads point to the basement membrane separating the tumor from the surrounding non-tumor tissue. The expression of KLF8 and MMP14 in the tumors was verified by IHC staining (see Figure S2).
KLF8 activates MMP14 transcription directly as well as indirectly via β-catenin/TCF1 transactivation complex
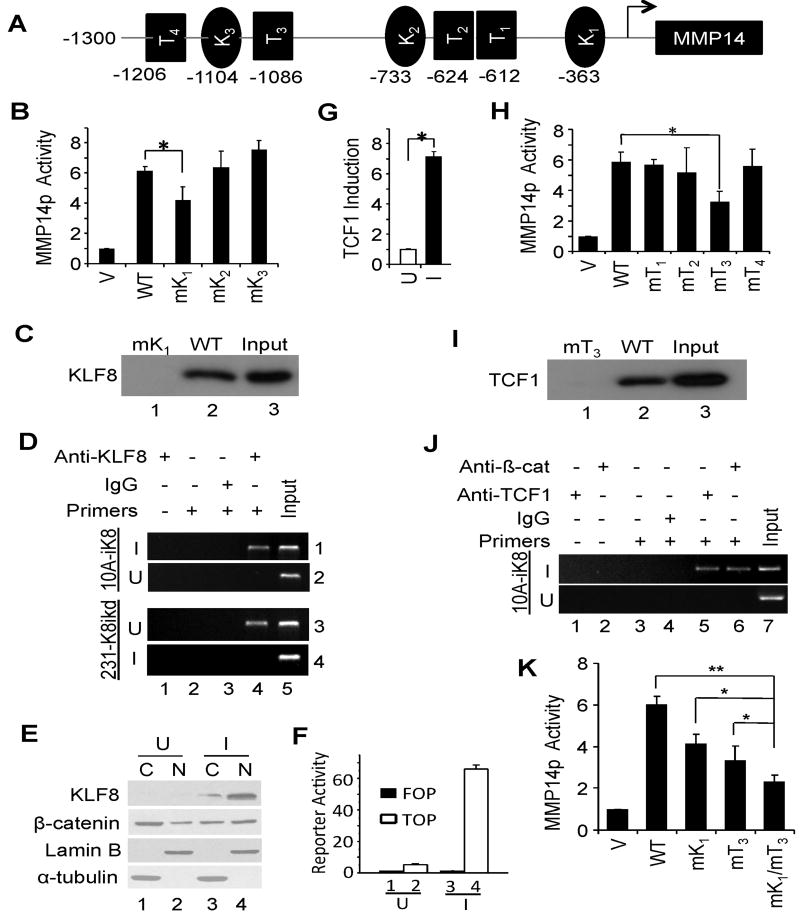
As described above in Figure 1, KLF8 positively promotes MMP14 expression at the transcriptional level. To determine the molecular mechanisms, we first searched the MMP14 promoter sequence for potential KLF8 binding sites and identified three GT-boxes (Figure 4A, K1 - K3). To test if any of them is a functional KLF8 binding site, we disrupted each of them individually by site-directed mutagenesis and then examined the promoter activation by KLF8 (Figure 4B). We found that mutation of the GT-box 1, but not the other two GT-boxes, inhibited the promoter activation by KLF8 by ∼30%. This result suggested that the GT-box 1 could be a functional KLF8 binding site that partially contributes to the activation of the promoter by KLF8. Indeed, BOP (Figure 4C) and ChIP) assays (Figure 4D) revealed that KLF8 could interact specifically with the promoter at the GT-box 1 site given that mutation of the GT-box 1 (Figure 4C, lane 1) or lack of KLF8 expression (Figure 4D, panel 2 or 4, lane 4) abolished the interaction.
Figure 4.
KLF8 activates MMP14 transcription both directly and indirectly via β-catenin/TCF1. A, Potential KLF8- and TCF1-binding sites on MMP14 gene promoter. K, KLF8 binding GT-box, i.e., caccc or gggtg; T, TCF binding sites, i.e., g/cctttga/ta/t or a/ta/tcaaagc/g. B-D, KLF8 directly activates MMP14 promoter by binding to the GT-box 1. Wild-type MMP14p reporter (WT), its mutants with each of the GT-boxes mutated (mK1-mK3) or control vector (V) were co-transfected with KLF8 to NIH3T3 cells for luciferase reporter assays (B. *, P < 0.05). KLF8 binding to the GT-box 1 was determined by BOP assays using lysate prepared from KLF8 transfected 293 cells (C) and ChIP assay using genomic DNA prepared from the indicated cells grown under the U or I conditions for 72 h (D). E. KLF8 induces β-catenin nuclear translocation. Western blotting for β-catenin in cytoplasm (C) or nucleus (N) of the 10A-iK8 cells grown in induced (I) or uninduced (U) conditions for 3 days. Lamin B or α-tubulin was used as a marker for nucleus or cytoplasm. The data was quantitated and verified by immunofluorescent staining of β-catenin in the cells (see Figure S3). F, KLF8 enhances β-catenin nuclear activity. The 10A-iK8 U- or I-cells were tested for β-catenin activity on TopFlash promoter reporter (TOP). FOPFlash (FOP) was used as negative control. **P < 0.01 compared to lanes 2 or 3. G, KLF8 upregulates TCF1 expression. Induction of TCF1 expression in the 10A-iK8 I-cells was verified by qRT-PCR that was initially identified by microarray as well as pathway-focused gene expression profiling using Human Signaling Pathway PCR Array (PAHS-014, SABiosciences) (Data not shown). H-J, TCF1 directly activates MMP14 promoter by binding to the T3 site together with β-catenin. MMP14p luciferase reporter assays (H), BOP assays (I) and ChIP assay (J) were performed similarly as in B-D except that the TCF1 binding to the promoter was examined. K, Both KLF8 and TCF1 binding to the promoter are required for maintaining MMP14 promoter activity. The 10A-iK8 cells were transfected with the wild-type MMP14p reporter (WT), its mutants defective for binding KLF8 (mK1), TCF1 (mT3), or both (mK1/mT3) or control vector (V). Reporter assays were carried out after the cells were grown for 72 hours under the induced conditions. *, P < 0.05; **, P < 0.01.
Previously we demonstrated that during the KLF8-induced EMT β-catenin showed obvious nuclear translocation from the plasma membrane (6). This result was confirmed by western blotting of β-catenin in the cytoplasmo-nuclear fractions of the 10A-iK8 cells (Figure 4E and S3A) and immunofluorescent staining of β-catenin (Figure S3B). The nuclear translocation of β-catenin is known to mediate the transcriptional activity of β-catenin. To test if this is true for the 10A-iK8 cells during EMT, we transfected the β-catenin target promoter reporter TOPFlash (TOP) into the cells and determined the promoter activity in response to the induction of KLF8 expression (Figure 4F). We found that the TOP activity was increased by > 60 times in the induced cells (I) compared to that in the uninduced cells (U) (Figure 4F, compare columns 4 to 2) whereas the mutant promoter FOPFlash (FOP) showed no activity (Figure 4F, compare columns 3 to 4). These results suggested that KLF8 promotes the nucleur translocation of β-catenin to regulate its indirect target genes.
It is known that in the nucleus β-catenin activates gene transcription by complexing with the TCF transcription factor family proteins. Interestingly, microarray and Human Signaling Pathway PCR Array showed the upregulation of TCF1 mRNA in the 10A-iK8 cells upon the induction of the KLF8 expression (Data not shown). These results were verified by qRT-PCR (Figure 4G). A revisit to the MMP14 promoter sequence revealed four potential β-catenin/TCF1 binding sites (Figure 4A, T1-T4). To test if any of them is a functional site, we performed similar site-specific promoter reporter assays (Figure 4H) along with BOP (Figure 4I) and ChIP (Figure 4J) assays. Mutation of the T3 site, but not the other three, blocked the promoter activation by KLF8 by ∼50% (Figure 4H, compare columns mT3 and WT) and completely abolished the interaction between TCF1 and the promoter segment (Figure 4I, compare lanes 1 to 2). The ChIP assays indicated that both β-catenin and TCF1 bind to the native promoter region containing this T3 site in a KLF8 expression-dependent manner (Figure 4J). These assays together identified the T3 site as a likely one that responds to β-catenin/TCF1 to activate the MMP14 promoter downstream of KLF8. When both the GT-box 1 and T3 site were disabled, the promoter activation by KLF8 was inhibited by ∼70% (Figure 4K. Compare columns mK1/mT3 and WT).
Altogether, these results supported the notion that KLF8 activates MMT14 promoter directly as well as indirectly by promoting the nuclear expression and function of the β-catenin/TCF1 complex.
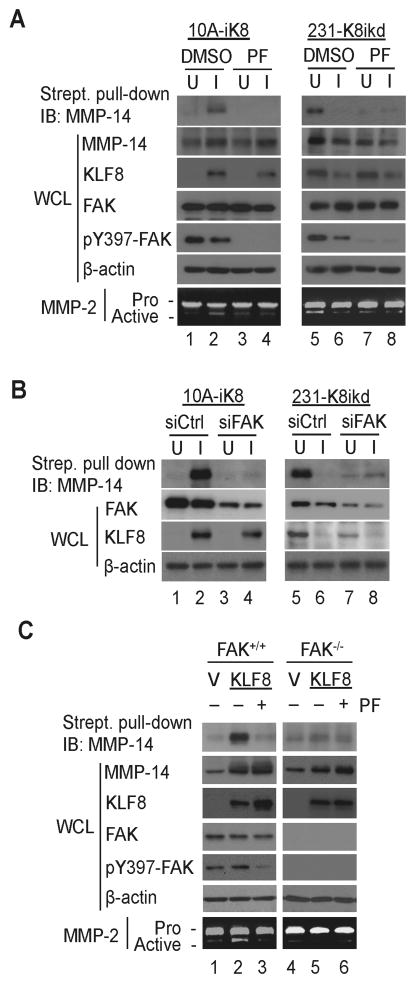
Active FAK function is required for the cell surface presentation and function of MMP14 protein downstream of KLF8
It is known that MMP14 must be presented to the cell surface in order for it to activate its target proteins including MMP2 outside of the cell. It has been reported that FAK function is essential for the cell surface presentation of active mmp14 by inhibiting its endocytosis in mouse embryonic fibroblast (MEF) cells (20). We asked if FAK plays a similar role supporting the presentation and activity of KLF8-upregulated MMP14 proteins on the cell surface in human breast cells. The 10A-iK8 and 231-K8ikd cells grown under induced (I) or uninduced (U) conditions were treated with the FAK specific inhibitor that blocks the phosphorylation at the Y397 of FAK (pY397-FAK), the cell surface expression of MMP14 protein and the MMP2 activation in the cell culture media were compared (Figure 5A). We found that the cell surface expression of MMP114 protein was significantly higher which was correlated with the activation of MMP2 when the cells expressed higher levels of KLF8 (Figure 5A, compare lanes 2 to 1 or lanes 5 to 6, and Figures S4A). When FAK was inhibited, as indicated by the loss of pY397-FAK, the cell surface presentation of MMP14 proteins and the MMP2 activation were almost completely abolished regardless of the high expression of KLF8 in the cells (Figure 5A, compare lanes 4 to 2 or lanes 7 to 5, and Figures S4B). These results were confirmed by independent experiments by FAK specific knockdown in the cells (Figure 5B and S4C). Consistent with previous reports on cells other than breast cancer cells (5, 13), the FAK knockdown caused a partial decrease in the expression of KLF8 in the 231-K8ikd cells (Figure 5B, compare lanes 5 and 7, and Figure S4C). Similar results were further obtained using the MEF cells expressing wild type or null FAK along with ectopic KLF8 (Figure 5C and Figure S4D and S4E). Taken together, these results clearly demonstrated that both KLF8-upregulated MMP14 expression inside the cell and FAK-promoted enrichment of active MMP14 on the outside surface of the cell are critical for the invasive growth and possibly metastasis of the breast tumors. The results also highlighted an important pathological loop of FAK-KLF8-MMP14 signaling in the cancer cells.
Figure 5.
Active FAK is required for cell surface expression and activity of KLF8-upregulated MMP14 protein. A, Inhibition of FAK reduces the cell surface expression of KLF8-induced MMP14. The 10A-iK8 or 231-K8ikd cells were incubated for 2 hours under uninduced (U) or induced (I) conditions. The cells were then serum-deprived for 16 hours in the absence or presence of the FAK inhibitor PF573228. The media were collected for analysis of MMP2 activity by zymography. The cells were then subject to either whole cell lysate (WCL) preparation for western blotting or biotinylation of surface proteins for streptavidin-based pull-down of cell surface MMP14 as described in the Materials and Methods. B, knockdown of FAK impairs KLF8-induced cell surface expression of MMP14. The uninduced 10A-iK8 or 231-K8ikd cells were transfected with FAK-specific siRNA (siFAK) or control siRNA (siCtrl) for 24 hours. After incubated under unindiced (U) or induced (I) conditions for 24 hours followed by serum starvation for 16 hours, the cells were processed for western blotting and analysis of cell surface MMP14 as described above. C, Deletion of FAK in MEFs inhibits KLF8-dependent cell surface enrichment for active MMP14. The FAK+/+ MEFs and FAK-/- MEFs were transfected with KLF8 or vector alone for 24 hours. The cells were then serum-deprived for 16 hours in the absence or presence of PF573228. Zymography of MMP2, western blotting and analysis of cell surface MMP14 were conducted similarly as described above in A. (see Figure S4 for quantitation of the data.)
Collectively, these results strongly suggest that KLF8-activated MMP14 transcription and FAK-dependent enrichment of MMP14 at the cell surface and subsequent activation of MMP2 activity towards the remodeling of extracellular matrix contribute to a critical part of the metastatic progression of breast cancer.
Discussion
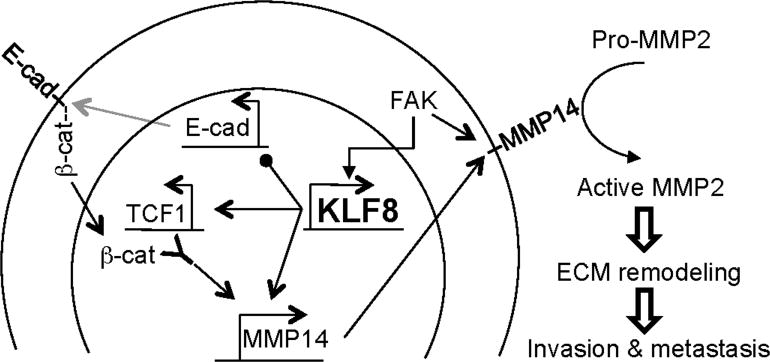
In this report, we identified a novel KLF8 and FAK to MMP14 signaling mechanism for the invasive growth and lung metastasis of human breast cancer (Figure 6). In this pathologic signaling model, the aberrant high expression of KLF8 in the cancer cells ensures the high levels of MMP14 expression. On the one hand, KLF8 transactivates the MMP14 gene transcription by directly binding to the gene promoter. On the other hand, KLF8 helps enrich for β-catenin in the nucleus by repressing E-cadherin expression and subsequent translocation of β-catenin to the nucleus during EMT (6) and upregulates TCF1 expression. The high levels of both β-catenin and TCF1 in the nucleus result in further activation of the MMP14 gene transcription downstream of KLF8 via the interaction of the β-catenin/TCF1 transcriptional activating complex with the MMP14 gene promoter. Once the high levels of MMP14 protein are produced in the cells, it requires active FAK to assist in maintaining the highly expressed, active MMP14 protein on the cell surface via perhaps preventing the internalization and subsequent degradation of the MMP14 protein (20). Altogether, these mechanisms guarantee the maximum function of MMP14 on the cell surface to activate its substrates including MMP2 required for the re-modeling of the ECM and subsequent tumor invasion and metastasis.
Figure 6.
Mechanism of action model for KLF8/FAK-MMP14 signaling loop in promotion of invasion and metastasis. KLF8 directly activates MMP14 promoter. On the other hand, KLF8 promotes nuclear presence of β-catenin presumably by repressing E-cadherin expression and upregulates TCF1 expression resulting in β-catenin/TCF1-dependent activation of the MMP14 promoter. The KLF8- upregulated MMP14 protein is enriched by FAK on the cell surface where it catalyzes the activation of MMP2 and subsequent ECM degradation required for invasion and metastasis.
Considering the critical role of MMP14 in the invasive tumor growth and metastasis, there are likely multiple mechanisms that ensure constant activation of its transcription. Thus, that KLF8 activates MMP14 transcription both directly and indirectly is to maximize its regulation of MMP14 expression in the cells. This is consistent with such a role of KLF8 in regulating other target genes (13). Prevention of the formation of E-cadherin/β-catenin complex under the plasma membrane is one of the mechanisms to maintain the nuclear presence of β-catenin. One way for this to happen is the loss of E-cadherin expression at the plasma membrane. E-cadherin has been demonstrated to be a major transcriptional repression target of KLF8 in MCF-10A cells during EMT as well as in the MDA-MB-231 cancer cells (6). Therefore, it is likely that KLF8 maintains the high levels of nuclear β-catenin by inhibiting the membrane expression of E-cadherin and subsequently promoting the nuclear translocation of β-catenin. The high levels of nuclear β-catenin agree with the role of KLF8 in participation in the maintenance of the mesenchymal phenotype of the invasive cancer cells (4, 6). The molecular mechanisms by which KLF8 upregulates the expression of TCF1 are not known and under investigation. Other TCF family proteins such as TCF4 has been reported to partner with β-catenin to regulate MMP14 gene transcription in non-breast cancer cells including colorectal cancer and fibrosarcoma (21-23). Thus, the KLF8-promoted nuclear presence of β-catenin may further increase chances for the activation of MMP14 transcription by additional TCF family member proteins such as TCF4.
Effective function of MMP14 out of the cell depends upon its effective presentation to the cell surface. FAK is reported to inhibit the endocytosis of MMP14 from the cell surface for cytoplasimc degradation by promoting Src phosphorylation of endophilin A2 (20), which may be what happens to the MMP14 proteins downstream of KLF8 in the breast cancer cells described here. Interestingly, KLF8 was initially identified as an effector protein downstream of FAK (5, 13). This suggests that sufficient cells surface expression of MMP14 requires the cooperation of KLF8-promoted expression and FAK-inhibited endocytosis of MMP14. Notably, however, KLF8 expression is undetectable in some of the normal or non-tumorigenic cells including MCF-10A that do express quite abundant FAK (see Figure 5B) (7). These results highlight a potentially important pathological signaling loop consisting of FAK and KLF8 upstream of MMP14 and suggest that KLF8 may be a more tumor-specific therapeutic target than FAK.
It is well known that active MMP2 plays a critical role downstream of MMP14 for tumor invasion by remodeling the tumor microenvironment through cleavage of extracellular matrix proteins such as collagen. In addition to proMMP2, however, many other MMP14 substrate proteins have recently been identified that contribute to tumor invasion and metastasis (24). These other substrates of MMP14 include the extracellular proteins (24) including collagen, glycoproteins, proteoglycans, proMMP8, proMMP13, TGF-β, mannose-binding lectin, stromal cell-derived factor (SDF-1) and monocyte chemo-attractant protein-3 (MCP-3); cell surface proteins including integrins, cadherins, CD44, the low density lipoprotein receptor-related protein (LRP), receptor activator of NF-kB ligand (RANKL), semaphorin 4D, transglutaminase, mucin, and extracellular matrix metalloproteinase inducer (EMMPRIN); and the intracellular protein pericentrin. It is likely that some of these MMP14 substrate proteins in addition to MMP2 also contribute to the MMP14-promoted breast cancer invasion and metastasis downstream of KLF8.
The fact that overexpression of MMP14 was not sufficient to fully rescue the invasive growth and metastasis from the effect of KLF8 knockdown (see Figure 2 & 3) suggests that the KLF8-dependent cell invasion and metastasis in vivo of the breast cancer cells are partially contributed to by MMP14 and other KLF8 targets such as MMP9 and E-cadherin (4, 6) may also likely play a significantly contributing role. Recent studies have demonstrated that the shedding of E-cadherin ectodomain is done by MMP9, MMP14 or MMP2 in lung or skin cancer cells during EMT (25-27). On the other hand, treatment with soluble E-cadherin, the shedding product of E-cadherin ectodomain, in turn upregulates the expression of MMPs (28) and promotes the cell invasion (27). Additionally, overexpression of full-length E-cadherin can reduce MMP14 expression and MMP2 activity in the cells (28, 29). The blood level of the soluble E-cadherin has been correlated with tumor metastasis potential in cancer patients (30). These lines of evidence and our results described in this report support potential functional interactions between the MMPs and E-cadherin that mediate KLF8-promoted metastatic progression of the breast cancer cells. Experiments are in progress testing this interesting possibility.
In summary, we have identified a novel signaling loop between KLF8 and FAK that regulates MMP14 at both transcriptional expression and post-translational activation essential for the KLF8-dependent progression of breast cancer metastasis. Given the barely detectable expression of KLF8, unlike FAK, MMP14 and MMP9, in normal epithelial cells (4, 6, 7), KLF8 could be explored as a favorable target for breast cancer intervention.
Materials and Methods
Antibodies, plasmids and cell culture
Antibodies used are anti-HA (F-7), anti-MMP14 (sc-30074), anti-FAK (sc-557) and ant-TCF1 (sc-101170) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-pY397-FAK (Invitrogen, 44625G, Carlsbad, CA, USA); anti-human vimentin (550513) and anti-β-catenin (610153) (BD Pharmingen, San Jose, CA, USA). Anti-KLF8 antibody was described previously (4, 6, 13). The mammalian expression plasmids pKH3, pKH3-KLF8 and pKH3-mKLF8 were previously described (11). pKH3-TCF1 was constructed by transferring the human TCF1 cDNA from pCMV-TCF1 vector (GeneCopoeia Inc, ID # FL14658, Rockville, MD, USA). The human MMP14 gene promoter luciferase reporter plasmid (pGL3-MMP14p) was described previously (19). GT-box or TCF site specific mutants of the promoter were constructed by site-directed mutagenesis PCR (31). The E-cadherin/TCF complex-responsive promoter reporter TOPFlash and its TCF-binding defective mutant FOPFlash were purchased from Addgene (ID # 12456 and 12457, Cambridge, MA, USA) (32). To construct the lentiviral vector pLVZP-MMP14, we sub-cloned MMP14 cDNA from EX-M0327-Lv125 vector (GeneCopoeia Inc. Rockville, MD) into a lentiviral vector pLVZP. The MCF-10A, MDA-MB-231, the MCF-10A that expresses inducible KLF8 (10A-iK8), the MDA-MB-231 that expresses inducible KLF8 shRNA (231-K8ikd), and FAK-/- and FAK+/+ mouse embryonic fibroblast (MEF) cells were described previously (4, 6, 7, 20). The 231-K8ikd cell line expressing ectopic MMP14 (231-K8ikd/MMP14) was generated by infecting the 231-K8ikd cells with the pLVZP-MMP14 lentivirus followed by puromycin selection. These cells were maintained in DMEM/F-12 or DMEM with 10% fetal bovine serum. The inducible cell lines were maintained under uninduced (U, in the absence of doxycycline) or induced (I, in the presence of doxycycline) conditions depending on the experimental requirement.
RNA interference, zymography and Matrigel invasion assays
These assays were performed as previously described (4, 6, 18). The human FAK specific siGENOME siRNAs (D-003164-05; D-003164-07; D-003164-08; D-003164-09) and control siRNAs were purchased from Dharmacon (Lafayette, CO, USA). The siRNAs were delivered into the cells by Oligofectamine-mediated transfection according to manufacturer's instructions (Invitrogen, Grand Island, NY, USA).
Analysis of cell surface expression of MMP14 protein
Cell surface protein biotinylation was performed as previously described (20) with slight modifications. Sub-confluent 10A-iK8, 231-K8ikd, FAK−/− MEF or FAK+/+ MEF cells grown on 60-mm dishes were washed twice with ice-cold phosphate-buffered saline (PBS) and incubated for 15 min in ice-cold PBS. Biotinylation was performed by incubating cells in PBS containing 0.5 mg/ml of the EZ-Link Sulfo-NHS-SS-Biotin (Thermo Scientific, Prod# 21331, Rockford, IL, USA) for 30 min at 4°C. To determine cell surface levels of MMP14 protein, the cells were lysed and the biotinylated surface proteins were precipitated with High Capacity Streptavidin Agarose Resin (Thermo Scientific, Prod# 20359, Rockford, IL, USA) and blotted with anti-MMP14 antibody. To test the role of FAK activity on the cell surface presentation of MMP14, a change in the MMP14 levels on the cell surface was examined when the FAK activity was inhibited with its specific inhibitor PF573228 (1 μM, Sigma-Aldrich, St. Louis, MO, USA), the FAK expression was knocked down or the FAK gene was knocked out.
Bioluminescence imaging (BLI) analysis of tumor growth and metastasis
Female Balb/c nude mice (Harlan Laboratories, Harlan, KY, USA) of 4 - 6 weeks old were used for all xenografting studies. The mice were housed and maintained in specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture, United States Department of Health and Human Services, and the National Institute of Health. Animal care and use was approved by the Institutional Animal Care and Use Committee. Human care of the mice was thoroughly considered. For lung metastasis formation, 2 × 106 cells were washed and harvested in 0.1 ml PBS and injected into the lateral tail vein. For mammary fat pad injection, 5 × 105 cells were washed and harvested in 0.05 ml PBS and injected into mammary fat pads. The mice were fed with diet (Bio Servs, Frenchtown, NJ, USA) supplemented with doxycycline or Dox Diet (product # S3888) to maintain the ‘on’-state of the KLF8 knockdown or with the Control Diet not containing doxycycline (product # S4207) to maintain the ‘off’-state of the KLF8 knockdown. After injection, mice were monitored every weekly. For BLI, mice were anaesthetized and injected intraperitoneally with 150 mg/kg of D-luciferin (15 mg/ml in PBS). BLI was then completed between 2 and 5 min using a Kodak In Vivo Imaging System coupled to Molecular Imaging Software (Carestream, Rochester, NY, USA) followed by X-ray imaging. Photon flux (photons/s/cm2 per steradian) was measured with a region of interest drawn around the bioluminescence signal encompassing the thorax or the mammary fat pad. A background value was obtained from a D-luciferin-injected control mouse and subtracted.
Pathological Analysis
Hematoxylin and Eosin (H&E) and immunohistochemical (IHC) analyses of the mammary tumors and lung metastases were performed as described (2).
Quantitative real-time PCR (qRT-PCR) and western blotting
These assays were done as previously described (6). Primers for human MMP14 are 5′-GCA GAA GTT TTA CGG CTT GCA A (forward) and 5′-CCT TCG AAC ATT GGC CTT GAT (reverse). Primers for human MMP2 are 5′-TGG CAA GTA CGG CTT CTG TC (forward) and 5′-TTC TTG TCG CGG TCG TAG TC (reverse). Primers for human TCF1 are 5′-ACC AGC GGC ATG TAC AAA GAG-3′ (forward), 5′-TTC AGG TTG CGG TCG AAG GGC (reverse). Sub-cellular fractionation was described previously (5).
Promoter reporter assays, chromatin immunoprecipitation (ChIP) and biotinylated oligonucleotide precipitation (BOP)
These assays were performed as previously described (6). For reporter assays, cells were co-transfected with the pGL3-MMP14p (wild-type or mutant), TOPFlash or FOPFlash vector with pKH3-KLF8 (or induced expression of KLF8 in the case of 10A-iK8 cells) or pKH3-TCF1 vector into NIH 3T3 cells prior to analysis of the promoter activity. ChIP assays were conducted using antibody for KLF8, TCF1, or β-catenin and genomic DNA prepared from the 10A-iK8 or 231-K8ikd cells grown under the uninduced or induced conditions for 72 h. BOP assays were performed using HEK293 cells and the following oligos. GT-box 1 site: 5′-TCC TCG TTG CCC CTA GCC ACA TAG CCC CCA ATA ATT CCC ACC CTG AGG TGA GAC AAA TGC TGA ATA CCA GAG GAA TCA AGC CAC TC (sense, wild-type) and 5′-TCC TCG TTG CCC CTA GCC ACA TAG CCC CCA ATA ATT CCa aac aTG AGG TGA GAC AAA TGC TGA ATA CCA GAG GAA TCA AGC CAC TC (sense, mutant). TCF1: 5′-TAC CTG ACC TAA TTT CTG TCC ACC CTC CTC TAT TCC TTC CTT TGC TTT CTT CTC CCT TCC TCC TCG CAC TAC CTC TGT CCT CTC TC (sense, wild-type) and 5′-TAC CTG ACC TAA TTT CTG TCC ACC CTC CTC TAT TCC TTA CGT CGC TTT CTT CTC CCT TCC TCC TCG CAC TAC CTC TGT CCT CTC TC (sense, mutant).
Statistical Analysis
Data is presented as mean +/- the standard deviation (SD) with a minimum of three observations per group. Unpaired, paired or single sample Student's t-test with the Bonferroni correction for the multiple comparisons was applied as appropriate. Significance was determined by the alpha level of 0.05
Supplementary Material
Acknowledgments
We appreciate Dr. Jouko Lohi of University of Helsinki for kindly providing the human MMP14 gene promoter reporter vectors. This work was supported by grants from NCI (CA132977) and Susan G. Komen for Cure breast cancer foundation (KG090444 and KG080616) to J.Z.
This work was supported by grants from NCI (CA132977) and Susan G. Komen for the Cure Breast Cancer Foundation (KG090444 and KG080616) to JZ.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Lahiri SK, Zhao J. Kruppel-like factor 8 emerges as an important regulator of cancer. Am J Transl Res. 2012;4(3):357–63. Epub 2012/09/01. eng. [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H, Wang X, Urvalek AM, Li T, Xie H, Yu L, et al. Transformation of human ovarian surface epithelial cells by Kruppel-like factor 8. Oncogene. 2012 Dec 10; doi: 10.1038/onc.2012.545. Epub 2012/12/12. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Hu L, Li T, Lahiri S, Shen C, Wason MS, et al. A Novel Role of Kruppel-like Factor 8 in DNA Repair in Breast Cancer Cells. J Biol Chem. 2012 Dec 21;287(52):43720–9. doi: 10.1074/jbc.M112.418053. Epub 2012/10/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Lu H, Urvalek AM, Li T, Yu L, Lamar J, et al. KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene. 2011 Apr 21;30(16):1901–11. doi: 10.1038/onc.2010.563. Epub 2010/12/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Urvalek AM, Liu J, Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem. 2008 May 16;283(20):13934–42. doi: 10.1074/jbc.M709300200. Epub 2008/03/21. eng. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Zheng M, Liu G, Xia W, McKeown-Longo PJ, Hung MC, et al. Kruppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res. 2007 Aug 1;67(15):7184–93. doi: 10.1158/0008-5472.CAN-06-4729. Epub 2007/08/03. eng. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007 Jan 18;26(3):456–61. doi: 10.1038/sj.onc.1209796. Epub 2006/07/13. eng. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci U S A. 2012 Jun 12;109(24):9623–8. doi: 10.1073/pnas.1121606109. Epub 2012/05/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnell O, Romagna A, Jaehnert I, Albrecht V, Eigenbrod S, Juerchott K, et al. Kruppel-like factor 8 (KLF8) is expressed in gliomas of different WHO grades and is essential for tumor cell proliferation. PLoS One. 2012;7(1):e30429. doi: 10.1371/journal.pone.0030429. Epub 2012/01/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urvalek AM, Lu H, Wang X, Li T, Yu L, Zhu J, et al. Regulation of the oncoprotein KLF8 by a switch between acetylation and sumoylation. Am J Transl Res. 2011 Feb;3(2):121–32. Epub 2011/03/19. eng. [PMC free article] [PubMed] [Google Scholar]
- 11.Urvalek AM, Wang X, Lu H, Zhao J. KLF8 recruits the p300 and PCAF co-activators to its amino terminal activation domain to activate transcription. Cell Cycle. 2010 Feb 1;9(3):601–11. doi: 10.4161/cc.9.3.10606. Epub 2010/01/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei H, Wang X, Gan B, Urvalek AM, Melkoumian ZK, Guan JL, et al. Sumoylation delimits KLF8 transcriptional activity associated with the cell cycle regulation. J Biol Chem. 2006 Jun 16;281(24):16664–71. doi: 10.1074/jbc.M513135200. Epub 2006/04/18. eng. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell. 2003 Jun;11(6):1503–15. doi: 10.1016/s1097-2765(03)00179-5. Epub 2003/06/25. eng. [DOI] [PubMed] [Google Scholar]
- 14.van Vliet J, Turner J, Crossley M. Human Kruppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 2000 May 1;28(9):1955–62. doi: 10.1093/nar/28.9.1955. Epub 2000/04/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009 Jun;28(1-2):35–49. doi: 10.1007/s10555-008-9165-4. Epub 2009/01/27. eng. [DOI] [PubMed] [Google Scholar]
- 16.Mehta TS, Lu H, Wang X, Urvalek AM, Nguyen KH, Monzur F, et al. A unique sequence in the N-terminal regulatory region controls the nuclear localization of KLF8 by cooperating with the C-terminal zinc-fingers. Cell Res. 2009 Sep;19(9):1098–109. doi: 10.1038/cr.2009.64. Epub 2009/06/03. eng. [DOI] [PubMed] [Google Scholar]
- 17.Afsar NA, Ufer M, Haenisch S, Remmler C, Mateen A, Usman A, et al. Relationship of drug metabolizing enzyme genotype to plasma levels as well as myelotoxicity of cyclophosphamide in breast cancer patients. Eur J Clin Pharmacol. 2012 Apr;68(4):389–95. doi: 10.1007/s00228-011-1134-0. Epub 2011/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Wang X, Li T, Urvalek AM, Yu L, Li J, et al. Identification of poly (ADP-ribose) polymerase-1 (PARP-1) as a novel Kruppel-like factor 8-interacting and -regulating protein. J Biol Chem. 2011 Jun 10;286(23):20335–44. doi: 10.1074/jbc.M110.215632. Epub 2011/04/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohi J, Lehti K, Valtanen H, Parks WC, Keski-Oja J. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000 Jan 25;242(1-2):75–86. doi: 10.1016/s0378-1119(99)00549-1. eng. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Gan B, Yoo Y, Guan JL. FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell. 2005 Aug;9(2):185–96. doi: 10.1016/j.devcel.2005.06.006. eng. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002 Aug 29;21(38):5861–7. doi: 10.1038/sj.onc.1205755. eng. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Tillo E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci U S A. 2011 Nov 29;108(48):19204–9. doi: 10.1073/pnas.1108977108. Epub 2011/11/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Yang J, Pei J, Pei D, Wilson MJ. Regulation of MT1-MMP activity by beta-catenin in MDCK non-cancer and HT1080 cancer cells. J Cell Physiol. 2010 Nov;225(3):810–21. doi: 10.1002/jcp.22292. Epub 2010/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: Substrate diversity in pericellular proteolysis. Semin Cell Dev Biol. 2008 Feb;19(1):24–33. doi: 10.1016/j.semcdb.2007.06.008. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covington MD, Burghardt RC, Parrish AR. Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14) Am J Physiol Renal Physiol. 2006 Jan;290(1):F43–51. doi: 10.1152/ajprenal.00179.2005. eng. [DOI] [PubMed] [Google Scholar]
- 26.Dwivedi DJ, Pino G, Banh A, Nathu Z, Howchin D, Margetts P, et al. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am J Pathol. 2006 Jan;168(1):69–79. doi: 10.2353/ajpath.2006.041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007 Mar 1;67(5):2030–9. doi: 10.1158/0008-5472.CAN-06-2808. eng. [DOI] [PubMed] [Google Scholar]
- 28.Nawrocki-Raby B, Gilles C, Polette M, Bruyneel E, Laronze JY, Bonnet N, et al. Upregulation of MMPs by soluble E-cadherin in human lung tumor cells. Int J Cancer. 2003 Jul 20;105(6):790–5. doi: 10.1002/ijc.11168. eng. [DOI] [PubMed] [Google Scholar]
- 29.Ara T, Deyama Y, Yoshimura Y, Higashino F, Shindoh M, Matsumoto A, et al. Membrane type 1-matrix metalloproteinase expression is regulated by E-cadherin through the suppression of mitogen-activated protein kinase cascade. Cancer Lett. 2000 Sep 1;157(2):115–21. doi: 10.1016/s0304-3835(00)00494-8. eng. [DOI] [PubMed] [Google Scholar]
- 30.Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol. 2006 Jun;33(3 Suppl 9):S9–14. doi: 10.1053/j.seminoncol.2006.03.016. eng. [DOI] [PubMed] [Google Scholar]
- 31.Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998 Dec 28;143(7):1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003 Apr 15;13(8):680–5. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.