Abstract
A disulfide-linked conjugate of concanavalin A (Con A) and fragment A from diphtheria toxin has been synthesized and shown to be toxic for HeLa (human), Chinese hamster ovary (CHO), and SV3T3 (murine) cells. The conjugate was constructed by first coupling cystamine to Con A with a carbodiimide reagent and then reacting the modified Con A with reduced fragment A under conditions promoting disulfide interchange. The desired conjugate, obtained in nearly 50% yield relative to input of fragment A, was purified by affinity chromatography on Sephacryl S-200 and NAD-Sepharose; on analysis, it gave an average of 1.4 molecules of fragment A per tetrameric Con A molecule. The conjugate proved to be about equally active in inhibiting protein synthesis in HeLa, CHO, or SV3T3 cells in culture but was inactive relative to controls in a toxin-resistant strain of CHO cells containing altered elongation factor 2, the target protein of fragment A. With toxin-sensitive strains the conjugate was 100- to 1000-fold more active than controls, including fragment A, cystaminyl-Con A, and mixtures thereof, but was 1/50th to 1/500th as toxic as diphtheria toxin itself. Similar activity relative to controls was observed after intradermal inoculations in rabbits, and intravenous injections of the conjugate were lethal for mice. The activity of the conjugate in tissue culture was inhibited by Con A or α-methylmannoside but not by galactose. This and similar conjugates should be useful in studying mechanisms of entry of biologically active proteins into cells.
Keywords: crosslinking, affinity chromatography, protein synthesis
Full text
PDF

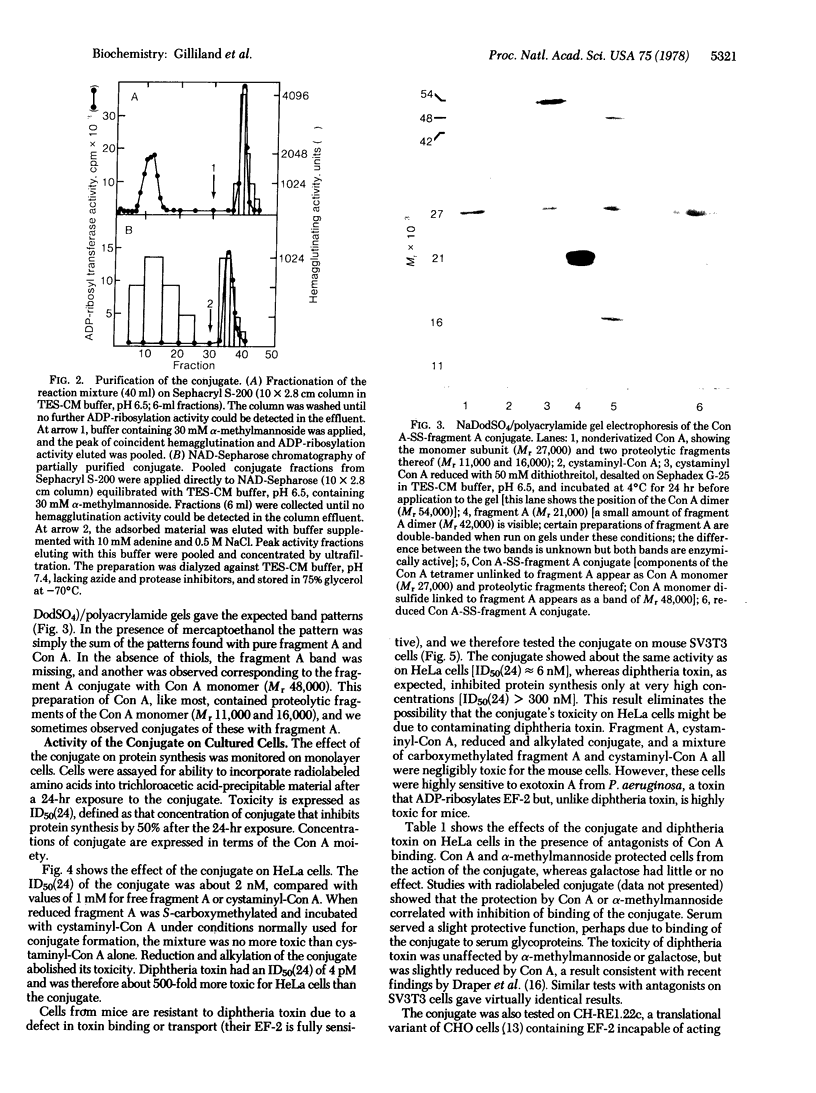
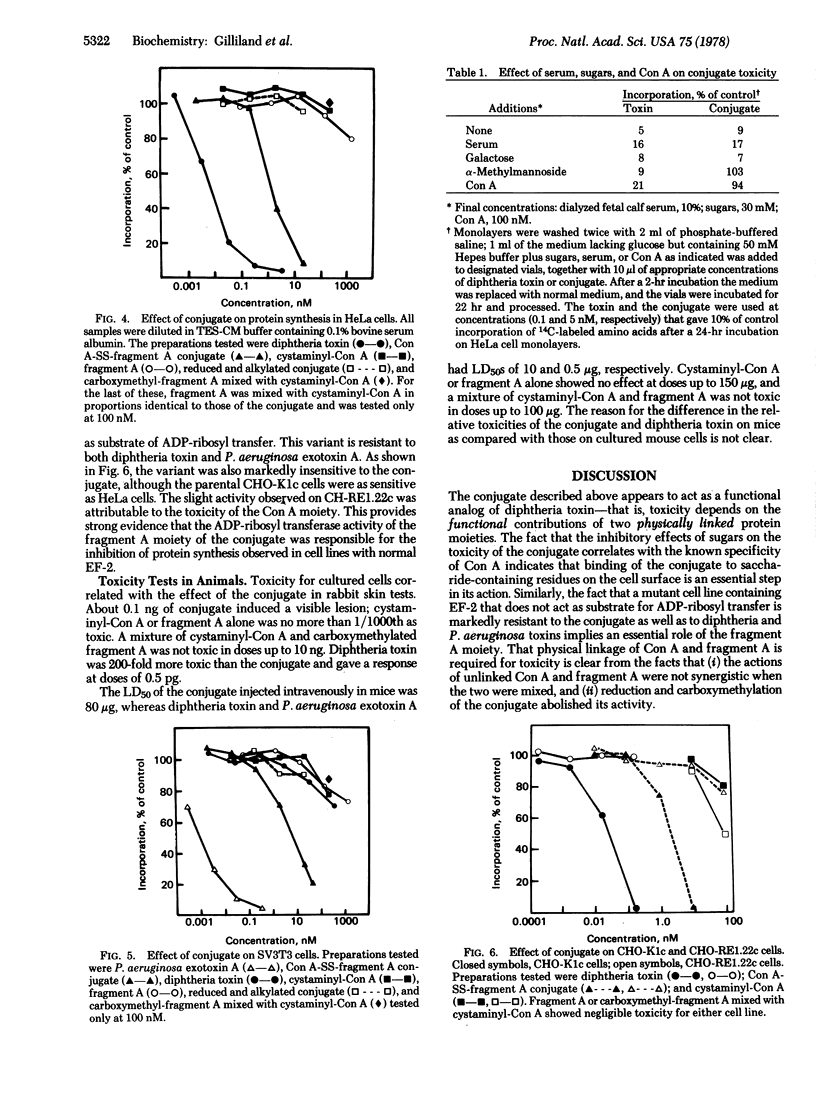

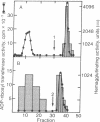
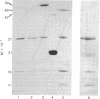
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boquet P., Silverman M. S., Pappenheimer A. M., Jr, Vernon W. B. Binding of triton X-100 to diphtheria toxin, crossreacting material 45, and their fragments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4449–4453. doi: 10.1073/pnas.73.12.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. M., Dazord A., Neville D. M., Jr Artificial hybrid protein containing a toxic protein fragment and a cell membrane receptor-binding moiety in a disulfide conjugate. II. Biochemical and biologic properties of diphtheria toxin fragment A-S-S-human placental lactogen. J Biol Chem. 1977 Feb 25;252(4):1515–1522. [PubMed] [Google Scholar]
- Chang T. M., Neville D. M., Jr Artificial hybrid protein containing a toxic protein fragment and a cell membrane receptor-binding moiety in a disulfide conjugate. I. Synthesis of diphtheria toxin fragment A-S-S-human placental lactogen with methyl-5-bromovalerimidate. J Biol Chem. 1977 Feb 25;252(4):1505–1514. [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. The mechanism of ADP-ribosylation of elongation factor 2 catalyzed by fragment A from diphtheria toxin. Biochim Biophys Acta. 1977 Aug 11;483(2):248–257. doi: 10.1016/0005-2744(77)90053-5. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Draper R. K., Chin D., Simon M. I. Diphtheria toxin has the properties of a lectin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):261–265. doi: 10.1073/pnas.75.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Wang J. L. Binding and functional properties of concanavalin A and its derivatives. III. Interactions with indoleacetic acid and other hydrophobic ligands. J Biol Chem. 1978 May 10;253(9):3016–3022. [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Interaction of diphtheria toxin and its active subunit, fragment A, with toxin-sensitive and toxin-resistant cells. Infect Immun. 1976 May;13(5):1426–1432. doi: 10.1128/iai.13.5.1426-1432.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Selection and characterization of cells resistant to diphtheria toxin and pseudomonas exotoxin A: presumptive translational mutants. Cell. 1977 Jun;11(2):447–454. doi: 10.1016/0092-8674(77)90063-0. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Ultrastructural analysis of toxin binding and entry into mammalian cells. Nature. 1974 Oct 18;251(5476):628–630. doi: 10.1038/251628a0. [DOI] [PubMed] [Google Scholar]
- Refsnes K., Olsnes S., Pihl A. On the toxic proteins abrin and ricin. Studies of their binding to and entry into Ehrlich ascites cells. J Biol Chem. 1974 Jun 10;249(11):3557–3562. [PubMed] [Google Scholar]
- Thorpe P. E., Ross W. C., Cumber A. J., Hinson C. A., Edwards D. C., Davies A. J. Toxicity of diphtheria toxin for lymphoblastoid cells is increased by conjugation to antilymphocytic globulin. Nature. 1978 Feb 23;271(5647):752–755. doi: 10.1038/271752a0. [DOI] [PubMed] [Google Scholar]
- Waxdal M. J., Konigsberg W. H., Henley W. L., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. II. Isolation and characterization of the cyanogen bromide fragments. Biochemistry. 1968 May;7(5):1959–1966. doi: 10.1021/bi00845a046. [DOI] [PubMed] [Google Scholar]
- van der Bosch J., McConnell M. Fusion of dipalmitoylphosphatidylcholine vesicle membranes induced by concanavalin A. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4409–4413. doi: 10.1073/pnas.72.11.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]